Abstract
Nitrogen oxide (NO) and sulfur dioxide (SO2) are the major environmental pollutants generated from the steel industry. The sintering process of iron ores accounts for more than 40 and 70 pct of the total emission of NO and SO2 from the steel industry. The current study aims to clarify the effects of the hearth layer used in the sinter bed on the reduction of NO and SO2. It was attempted to examine the potential of several materials in reducing NO and SO2 such as the hearth layer commonly applied in the steel plants, reagent-grade FeO, mill-scale, reagent-grade CaO, and calcined dolomite. The fractional reduction of NO (\({\eta }_{\text{NO}}\)) was directly proportional to the FeO content in the materials. The effects of experimental variables such as temperature, specimen arrangement, and oxygen addition to inlet gas mixture on the fractional reduction of NO (\({\eta }_{\text{NO}}\)) were evaluated. In case the sample was placed perpendicular to the flow of gas at high temperatures, the reduction of NO (\({\eta }_{\text{NO}}\)) was improved. However, the increase of oxygen in the inlet gas decreased the reduction of NO. Reagent-grade FeO and mill-scale were effective at 423 K (150 °C) for reducing NO in coal combustion, while reagent-grade CaO and calcined dolomite facilitate the reduction of SO2 at 773 K (500 °C). Based on the results, it was suggested to replace the hearth layer in the sinter bed with mill-scale in the low-temperature zone and calcined dolomite in the high-temperature zone, which would provide the best reduction ratio of NO and SO2.

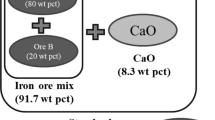
(Adapted from Ref. [17])















Similar content being viewed by others
References
X. Fan, Z. Yu, M. Gan, X. Chen, and Y. Huang: Ironmak. Steelmak., 2016, vol. 43, pp. 403–10.
H. Di, Z.J. He, J.H. Zhang, and Q.H. Pang: Metalurgija, 2018, vol. 57, pp. 27–30.
Y.S. Kang, S.S. Kim, H.D. Lee, J.K. Kim, and S.C. Hong: Appl. Chem. Eng., 2011, vol. 22, pp. 219–23.
W. Lv, X. Fan, X. Min, M. Gan, X. Chen, and Z. Ji: ISIJ Int., 2018, vol. 58, pp. 236–43.
K. Morioka, S. Inaba, M. Shimizu, K. Ano, and T. Sugiyama: ISIJ Int., 2000, vol. 40, pp. 280–85.
M. Gan, X. Fan, X. Chen, Z. Ji, W. Lv, Y. Wang, Z. Yu, and T. Jiang: ISIJ Int., 2012, vol. 52, pp. 1574–78.
H. Zhou, P. Ma, M. Zhou, Z. Lai and M. Cheng: J. Energy Inst., 2018, pp. 1–11.
G. Li, C. Liu, M. Rao, Z. Fan, Z. You, Y. Zhang, and T. Jiang: ISIJ Int., 2014, vol. 54, pp. 37–42.
H. Yu, C. Zhang, and H. Wang: ISIJ Int., 2015, vol. 55, pp. 1876–81.
Y. Chen, Z. Guo, and Z. Wang: Fuel Process. Technol., 2009, vol. 90, pp. 933–38.
H. Han, Y. Chen, C. Xie, D. Yuan, Y. Chen, and B. Wang: Adv. Mater. Res., 2013, vol. 781–784, pp. 2594–97.
D. Fernandez-Gonzalez, I. Ruiz-Bustinza, J. Mochon, C. Gonzalez-Gasca, and L.F. Verdeja: Miner. Process. Extr. Metall. Rev., 2017, vol. 38, pp. 215–27.
A.K. Biswas: Principles of Blast Furnace Ironmaking, Cootha Publishing House, Brisbane, 1981, p. 194.
Japan Industrial Standard (JIS): Iron Ores—Method for Determination of Acid Soluble Iron(II) Content (JIS Standard No. M8213:1995).
International Organization for Standardization (ISO): Iron Ores—Determination of Various Elements—Inductively Coupled Plasma Atomic Emission Spectrometric Method (ISO Standard No. 11535: 2006).
Nanjing Kesen Kenen Environment & Energy Co. (NKK), Sintering Flue Gas and Sinter Heat Recovery Power Generation Technology (2013). http://www.njkskn.com/cpjsShow.aspx?id=130&class=yjhy. Accessed 05 November 2020.
Steel Plantech, Maximizing Sintering Plant Heat Recovery (2012). https://steelplantech.com/wp-content/uploads/2013/11/201203_Maximizing-Sinter-Plant-Heat-Recovery.pdf. Accessed 25 May 2021.
H.G. Lee: Chemical Thermodynamics for Metals and Materials, Imperial College Press, London, 1999, p. 285.
S. Wu, T. Sugiyama, K. Morioka, E. Kasai, and Y. Omori: Tetsu-to-Hagane, 1994, vol. 80, pp. 276–81.
G.A. Simons, A.R. Garman, and A.A. Boni: AIChE J., 1987, vol. 33, pp. 211–17.
M.S. Lee and S.C. Shim: ISIJ Int., 2004, vol. 44, pp. 470–75.
R.T. Symonds, D.Y. Lu, V. Manovic, and E.J. Anthony: Ind. Eng. Chem. Res., 2012, vol. 51, pp. 7177–84.
K. Zhang, S. Yang, S. Liu, J. Shangguan, W. Du, Z. Wang, and Z. Chang: ACS Omega, 2020, vol. 5, pp. 3047–54.
C.W. Bale, P. Chartrand, S.A. Degterov, G. Eriksson, K. Hack, R. Ben Mahfoud, J. Melançon, A.D. Pelton, and S. Petersen: Calphad, 2002, vol. 26, pp. 189–228.
M. Arslan, C. Alkan, M. Cici, and M. Kaya: Resour. Conserv. Recycl., 1994, vol. 10, pp. 341–47.
E. Humeres, R.F.P.M. Moreira, and M.G.B. Peruch: Carbon, 2002, vol. 40, pp. 751–60.
M. Tsujimura, T. Furusawa, and D. Kunii: J. Chem. Eng. Jpn, 1983, vol. 16, pp. 132–36.
B. Olanders and D. Stromberg: Energy Fuels, 1995, vol. 9, pp. 680–84.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tomas da Rocha, L., Cho, S., Chung, BJ. et al. Effect of Replacing the Hearth Layer Used in the Sintering Process on the Reduction of NO and SO2. Metall Mater Trans B 53, 3071–3082 (2022). https://doi.org/10.1007/s11663-022-02587-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-022-02587-2