Abstract
Purpose
To explore the impact of a true half dose of [18F]-FDG on image quality in pediatric oncological patients undergoing total-body PET/CT and investigate short acquisition times with half-dose injected activity.
Methods
One hundred pediatric oncological patients who underwent total-body PET/CT using the uEXPLORER scanner after receiving a true half dose of [18F]-FDG (1.85 MBq/kg) were retrospectively enrolled. The PET images were first reconstructed using complete 600-s data and then split into 300-s, 180-s, 60-s, 40-s, and 20-s duration groups (G600 to G20). The subjective analysis was performed using 5-point Likert scales. Objective quantitative metrics included the maximum standard uptake value (SUVmax), SUVmean, standard deviation (SD), signal-to-noise ratio (SNR), and SNRnorm of the background. The variabilities in lesion SUVmean, SUVmax, and tumor-to-background ratio (TBR) were also calculated.
Results
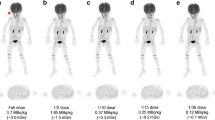
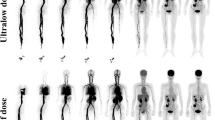
The overall image quality scores in the G600, G300, G180, and G60 groups were 4.9 ± 0.2, 4.9 ± 0.3, 4.4 ± 0.5, and 3.5 ± 0.5 points, respectively. All the lesions identified in the half-dose images were localized in the G60 images, while 56% of the lesions could be clearly identified in the G20 images. With reduced acquisition time, the SUVmax and SD of the backgrounds were gradually increased, while the TBR values showed no statistically significant differences among the groups (all p > 0.1). Using the half-dose images as a reference, the variability in the lesion SUVmax gradually increased from the G180 to G20 images, while the lesion SUVmean remained stable across all age groups. SNRnorm was highly negatively correlated with age.
Conclusion
Total-body PET/CT with a half dose of [18F]-FDG (1.85 MBq/kg, estimated whole-body effective dose: 1.76–2.57 mSv) achieved good performance in pediatric patients, with sufficient image quality and good lesion conspicuity. Sufficient image quality and lesion conspicuity could be maintained at a fast scanning time of 60 s with half-dose activity.






Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Code availability
The code applied during and/or analyzed during the current study is available from the corresponding author upon reasonable request.
References
Boellaard RD-BR, Oyen WJ, Giammarile F, Tatsch K, Eschner W, et al. European Association of Nuclear Medicine (EANM). FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:28–54. https://doi.org/10.1007/s00259-014-2961-x.
Fahey FHTS, Adelstein SJ. Minimizing and communicating radiation risk in pediatric nuclear medicine. J Nucl Med. 2011;52:1240–51. https://doi.org/10.2967/jnumed.109.069609.
Ricard F, Cimarelli S, Deshayes E, Mognetti T, Thiesse P, Giammarile F. Additional benefit of F-18 FDG PET/CT in the staging and follow-up of pediatric rhabdomyosarcoma. Clin Nucl Med. 2011;36:672–7. https://doi.org/10.1097/RLU.0b013e318217ae2e.
Fahey FH, Goodkind A, MacDougall RD, Oberg L, Ziniel SI, Cappock R, et al. Operational and dosimetric aspects of pediatric PET/CT. J Nucl Med. 2017;58:1360–6. https://doi.org/10.2967/jnumed.116.182899.
Turpin S, Martineau P, Levasseur MA, Meijer I, Décarie JC, Barsalou J, et al. 18F-Flurodeoxyglucose positron emission tomography with computed tomography (FDG PET/CT) findings in children with encephalitis and comparison to conventional imaging. Eur J Nucl Med Mol Imaging. 2019;46:1309–24. https://doi.org/10.1007/s00259-019-04302-x.
Chawla SC, Federman N, Zhang D, Nagata K, Nuthakki S, McNitt-Gray M, et al. Estimated cumulative radiation dose from PET/CT in children with malignancies: a 5-year retrospective review. Pediatr Radiol. 2010;40:681–6. https://doi.org/10.1007/s00247-009-1434-z.
Mercolini F, Zucchetta P, Jehanno N, Corradini N, Van Rijn RR, Rogers T, et al. Role of (18)F-FDG-PET/CT in the staging of metastatic rhabdomyosarcoma: a report from the European paediatric Soft tissue sarcoma Study Group. Eur J cancer (Oxford, England: 1990). 2021;155:155–62. https://doi.org/10.1016/j.ejca.2021.07.006.
Nievelstein RA, Quarlesvanufford HM, Kwee TC, Bierings MB, Ludwig I, Beek FJ, et al. Radiation exposure and mortality risk from CT and PET imaging of patients with malignant lymphoma. Eur Radiol. 2012;22:1946–54. https://doi.org/10.1007/s00330-012-2447-9.
Alessio AM, Farrell MB, Fahey FH. Role of reference levels in nuclear medicine: a report of the SNMMI Dose Optimization Task Force. J Nucl Med. 2015;56:1960–4. https://doi.org/10.2967/jnumed.115.160861.
Poli GL, Torres L, Coca M, Veselinovic M, Lassmann M, Delis H, et al. Paediatric nuclear medicine practice: an international survey by the IAEA. Eur J Nucl Med Mol Imaging. 2020;47:1552–63. https://doi.org/10.1007/s00259-019-04624-w.
Treves ST, Gelfand MJ, Fahey FH, Parisi MT. 2016 Update of the North American Consensus Guidelines for Pediatric Administered Radiopharmaceutical Activities. J Nucl Med. 2016;57:15n-n18.
Lassmann M, Biassoni L, Monsieurs M, Franzius C. The new EANM paediatric dosage card: additional notes with respect to F-18. Eur J Nucl Med Mol Imaging. 2008;35:1666–8. https://doi.org/10.1007/s00259-008-0799-9.
Cherry SR, Jones T, Karp JS, Qi J, Moses WW, Badawi RD. Total-body PET: maximizing sensitivity to create new opportunities for clinical research and patient care. J Nucl Med. 2018;59:3–12. https://doi.org/10.2967/jnumed.116.184028.
Badawi RD, Shi H, Hu P, Chen S, Xu T, Price PM, et al. First human imaging studies with the EXPLORER total-body PET scanner. J Nucl Med. 2019;60:299–303. https://doi.org/10.2967/jnumed.119.226498.
Schmall JP, Surti S, Otero HJ, Servaes S, Karp JS, States LJ. Investigating low-dose image quality in whole-body pediatric (18)F-FDG scans using time-of-flight PET/MRI. J Nucl Med. 2021;62:123–30. https://doi.org/10.2967/jnumed.119.240127.
Gatidis S, Schmidt H, la Fougère C, Nikolaou K, Schwenzer NF, Schäfer JF. Defining optimal tracer activities in pediatric oncologic whole-body (18)F-FDG-PET/MRI. Eur J Nucl Med Mol Imaging. 2016;43:2283–9. https://doi.org/10.1007/s00259-016-3503-5.
Kertész H, Beyer T, London K, Saleh H, Chung D, Rausch I, Cal-Gonzalez J, Kitsos T, Kench PL. Reducing radiation exposure to paediatric patients undergoing [18F]FDG-PET/CT imaging. Mol Imaging Biol. 2021 ;23(5):775–86. https://doi.org/10.1007/s11307-021-01601-4.
Zhao YM, Li YH, Chen T, Zhang WG, Wang LH, Feng J, et al. Image quality and lesion detectability in low-dose pediatric (18)F-FDG scans using total-body PET/CT. Eur J Nucl Med Mol Imaging. 2021;48:3378–85. https://doi.org/10.1007/s00259-021-05304-4.
Liu G, Hu P, Yu H, Tan H, Zhang Y, Yin H, et al. Ultra-low-activity total-body dynamic PET imaging allows equal performance to full-activity PET imaging for investigating kinetic metrics of (18)F-FDG in healthy volunteers. Eur J Nucl Med Mol Imaging. 2021;48:2373–83. https://doi.org/10.1007/s00259-020-05173-3.
Tan H, Sui X, Yin H, Yu H, Gu Y, Chen S, et al. Total-body PET/CT using half-dose FDG and compared with conventional PET/CT using full-dose FDG in lung cancer. Eur J Nucl Med Mol Imaging. 2021;48:1966–75. https://doi.org/10.1007/s00259-020-05091-4.
Spinelli A, Buoncristiano M, Nardone P, Starc G, Hejgaard T, Júlíusson PB, et al. Thinness, overweight, and obesity in 6- to 9-year-old children from 36 countries: The World Health Organization European Childhood Obesity Surveillance Initiative-COSI 2015–2017. Obesity Rev. 2021;22(Suppl 6):e13214. https://doi.org/10.1111/obr.13214.
Daniels SR, Arnett DK, Eckel RH, Gidding SS, Hayman LL, Kumanyika S, et al. Overweight in children and adolescents: pathophysiology, consequences, prevention, and treatment. Circulation. 2005;111:1999–2012. https://doi.org/10.1161/01.Cir.0000161369.71722.10.
de Groot EH, Post N, Boellaard R, Wagenaar NR, Willemsen AT, van Dalen JA. Optimized dose regimen for whole-body FDG-PET imaging. EJNMMI Res. 2013;3:63. https://doi.org/10.1186/2191-219x-3-63.
Cox CPW, van Assema DME, Verburg FA, Brabander T, Konijnenberg M, Segbers M. A dedicated paediatric [(18)F]FDG PET/CT dosage regimen. EJNMMI Res. 2021;11:65. https://doi.org/10.1186/s13550-021-00812-8.
Meier JM, Alavi A, Iruvuri S, Alzeair S, Parker R, Houseni M, et al. Assessment of age-related changes in abdominal organ structure and function with computed tomography and positron emission tomography. Semin Nucl Med. 2007;37:154–72. https://doi.org/10.1053/j.semnuclmed.2007.02.001.
Yeung HW, Sanches A, Squire OD, Macapinlac HA, Larson SM, Erdi YE. Standardized uptake value in pediatric patients: an investigation to determine the optimum measurement parameter. Eur J Nucl Med Mol Imaging. 2002;29:61–6. https://doi.org/10.1007/s00259-001-0662-8.
Wegner EA, Barrington SF, Kingston JE, Robinson RO, Ferner RE, Taj M, et al. The impact of PET scanning on management of paediatric oncology patients. Eur J Nucl Med Mol Imaging. 2005;32:23–30. https://doi.org/10.1007/s00259-004-1645-3.
Montravers F, McNamara D, Landman-Parker J, Grahek D, Kerrou K, Younsi N, et al. [(18)F]FDG in childhood lymphoma: clinical utility and impact on management. Eur J Nucl Med Mol Imaging. 2002;29:1155–65. https://doi.org/10.1007/s00259-002-0861-y.
Sgouros G, Frey EC, Bolch WE, Wayson MB, Abadia AF, Treves ST. An approach for balancing diagnostic image quality with cancer risk: application to pediatric diagnostic imaging of 99mTc-dimercaptosuccinic acid. J Nucl Med. 2011;52:1923–9. https://doi.org/10.2967/jnumed.111.092221.
Alessio AM, Sammer M, Phillips GS, Manchanda V, Mohr BC, Parisi MT. Evaluation of optimal acquisition duration or injected activity for pediatric 18F-FDG PET/CT. J Nucl Med. 2011;52:1028–34. https://doi.org/10.2967/jnumed.110.086579.
Tatsumi M, Miller JH, Wahl RL. 18F-FDG PET/CT in evaluating non-CNS pediatric malignancies. J Nucl Med. 2007;48:1923–31. https://doi.org/10.2967/jnumed.107.044628.
Uslu L, Donig J, Link M, Rosenberg J, Quon A, Daldrup-Link HE. Value of 18F-FDG PET and PET/CT for evaluation of pediatric malignancies. J Nucl Med. 2015;56:274–86. https://doi.org/10.2967/jnumed.114.146290.
Büsing KA, Schönberg SO, Brade J, Wasser K. Impact of blood glucose, diabetes, insulin, and obesity on standardized uptake values in tumors and healthy organs on 18F-FDG PET/CT. Nucl Med Biol. 2013;40:206–13. https://doi.org/10.1016/j.nucmedbio.2012.10.014.
Sastre J, Pallardó FV, Plá R, Pellín A, Juan G, O’Connor JE, et al. Aging of the liver: age-associated mitochondrial damage in intact hepatocytes. Hepatology (Baltimore, MD). 1996;24:1199–205. https://doi.org/10.1002/hep.510240536.
Schmidkonz C, Krumbholz M, Atzinger A, Cordes M, Goetz TI, Prante O, et al. Assessment of treatment responses in children and adolescents with Ewing sarcoma with metabolic tumor parameters derived from (18)F-FDG-PET/CT and circulating tumor DNA. Eur J Nucl Med Mol Imaging. 2020;47:1564–75. https://doi.org/10.1007/s00259-019-04649-1.
Pijl JP, Kwee TC, Slart R, Yakar D, Wouthuyzen-Bakker M, Glaudemans A. Clinical implications of increased uptake in bone marrow and spleen on FDG-PET in patients with bacteremia. Eur J Nucl Med Mol Imaging. 2021;48:1467–77. https://doi.org/10.1007/s00259-020-05071-8.
Patel NH, Osborne MT, Teague H, Parel P, Svirydava M, Sorokin AV, et al. Heightened splenic and bone marrow uptake of (18)F-FDG PET/CT is associated with systemic inflammation and subclinical atherosclerosis by CCTA in psoriasis: An observational study. Atherosclerosis. 2021;339:20–6. https://doi.org/10.1016/j.atherosclerosis.2021.11.008.
Akamatsu G, Ishikawa K, Mitsumoto K, Taniguchi T, Ohya N, Baba S, et al. Improvement in PET/CT image quality with a combination of point-spread function and time-of-flight in relation to reconstruction parameters. J Nucl Med. 2012;53:1716–22. https://doi.org/10.2967/jnumed.112.103861.
Zhang YQ, Hu PC, Wu RZ, Gu YS, Chen SG, Yu HJ, et al. The image quality, lesion detectability, and acquisition time of (18)F-FDG total-body PET/CT in oncological patients. Eur J Nucl Med Mol Imaging. 2020;47:2507–15. https://doi.org/10.1007/s00259-020-04823-w.
Shammas A, Lim R, Charron M. Pediatric FDG PET/CT: physiologic uptake, normal variants, and benign conditions. Radiographics. 2009;29:1467–86. https://doi.org/10.1148/rg.295085247.
Halpern BS, Dahlbom M, Quon A, Schiepers C, Waldherr C, Silverman DH, et al. Impact of patient weight and emission scan duration on PET/CT image quality and lesion detectability. J Nucl Med. 2004;45:797–801.
Accorsi R, Karp JS, Surti S. Improved dose regimen in pediatric PET. J Nucl Med. 2010;51:293–300. https://doi.org/10.2967/jnumed.109.066332.
de Langen AJ, Vincent A, Velasquez LM, van Tinteren H, Boellaard R, Shankar LK, et al. Repeatability of 18F-FDG uptake measurements in tumors: a metaanalysis. J Nucl Med. 2012;53:701–8. https://doi.org/10.2967/jnumed.111.095299.
Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122s-s150. https://doi.org/10.2967/jnumed.108.057307.
Author information
Authors and Affiliations
Contributions
Conceptualization: Yingying Hu, Yizhuo Zhang, and Yumo Zhao; methodology: Wanqi Chen, Lei Liu, Yumo Zhao, and Zhijian Li; formal analysis and investigation: Yingying Hu, Wanqi Chen, Lei Liu, Zhijian Li, and Weiguang Zhang; writing—original draft preparation: Wanqi Chen, Lei Liu, and Yingying Hu; writing—review and editing: Yingying Hu, Yizhuo Zhang, Yumo Zhao, Runze Wu, Yinghe Li, Shatong Li, and Xu Zhang; technical support: Hongyan Sun, Debin Hu, Runze Wu and Yun Zhou; resources: Yingying Hu; and supervision: Yumo Zhao, Yizhuo Zhang, and Yingying Hu. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were conducted in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from legal guardians.
Consent for publication
Additional informed consent was obtained from all legal guardians for whom identifying information is included in this article.
Conflict of interest
Authors Runze Wu, Hongyan Sun, Debin Hu, and Yun Zhou are employees of United Imaging Research. The other authors working at Sun Yat-sen University Cancer Center have full control of the data and declare that they have no conflicts of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pediatric
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, W., Liu, L., Li, Y. et al. Evaluation of pediatric malignancies using total-body PET/CT with half-dose [18F]-FDG. Eur J Nucl Med Mol Imaging 49, 4145–4155 (2022). https://doi.org/10.1007/s00259-022-05893-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-022-05893-8