Abstract
DNA methylation is a crucial epigenetic modification in the establishment of cell-type-specific characteristics. However, how DNA methylation is selectively reprogrammed at adipocyte-specific loci during adipogenesis remains unclear. Here, we show that the transcription factor, C/EBPδ, and the DNA methylation eraser, TET3, cooperatively control adipocyte differentiation. We perform whole-genome bisulfite sequencing to explore the dynamics and regulatory mechanisms of DNA methylation in adipocyte differentiation. During adipogenesis, DNA methylation selectively decreases at adipocyte-specific loci carrying the C/EBP binding motif, which correlates with the activity of adipogenic promoters and enhancers. Mechanistically, we find that C/EBPδ recruits a DNA methylation eraser, TET3, to catalyse DNA demethylation at the C/EBP binding motif and stimulate the expression of key adipogenic genes. Ectopic expression of TET3 potentiates in vitro and in vivo adipocyte differentiation and recovers downregulated adipogenic potential, which is observed in aged mice and humans. Taken together, our study highlights how targeted reprogramming of DNA methylation through cooperative action of the transcription factor C/EBPδ, and the DNA methylation eraser TET3, controls adipocyte differentiation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
WGBS of 3T3-L1 preadipocytes and adipocytes (GEO accession no. GSE186340), Bulk RNA-seq data of 3T3-L1 preadipocytes and adipocytes (GEO accession no. GSE185493), Bulk RNA-seq data of 3T3-L1 (ref. 21, GEO accession no. GSE95533), scRNA-seq data of ASCs (BioProject accession no. PRJNA708350), scRNA-seq data of SVCs (ref. 29; GEO accession no. GSE160729), PCHi-C (ref. 21, GEO accession no. GSE95533), ChIP–seq data73, GEO accession no. GSE56872), GTEx data (GTEX, https://www.gtexportal.org/home/). All scripts and codes are found under https://github.com/dohlee/adipogenesis-methylation. Source data are provided with this paper.
References
Razin, A. & Riggs, A. D. DNA methylation and gene function. Science 210, 604–610 (1980).
Jones, P. A. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 13, 484–492 (2012).
Tirado-Magallanes, R., Rebbani, K., Lim, R., Pradhan, S. & Benoukraf, T. Whole-genome DNA methylation: beyond genes silencing. Oncotarget 8, 5629–5637 (2017).
Greenberg, M. V. C. & Bourc’his, D. The diverse roles of DNA methylation in mammalian development and disease. Nat. Rev. Mol. Cell Biol. 20, 590–607 (2019).
Wu, H. & Zhang, Y. Reversing DNA methylation: mechanisms, genomics, and biological functions. Cell 156, 45–68 (2014).
Guo, J. U., Su, Y., Zhong, C., Ming, G. L. & Song, H. Hydroxylation of 5-methylcytosine by TET1 promotes active DNA demethylation in the adult brain. Cell 145, 423–434 (2011).
Zhu, J. K. Active DNA demethylation mediated by DNA glycosylases. Annu. Rev. Genet. 43, 143–166 (2009).
Smith, Z. D. & Meissner, A. DNA methylation: roles in mammalian development. Nat. Rev. Genet. 14, 204–220 (2013).
Suelves, M., Carrió, E., Núñez-Álvarez, Y. & Peinado, M. A. DNA methylation dynamics in cellular commitment and differentiation. Brief. Funct. Genomics 15, 443–453 (2016).
Okano, M., Bell, D. W., Haber, D. A. & Li, E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 99, 247–257 (1999).
Li, E., Bestor, T. H. & Jaenisch, R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell 69, 915–926 (1992).
An, J. et al. Acute loss of TET function results in aggressive myeloid cancer in mice. Nat. Commun. 6, 10071 (2015).
Liao, J. et al. Targeted disruption of DNMT1, DNMT3A and DNMT3B in human embryonic stem cells. Nat. Genet. 47, 469–478 (2015).
Chen, J. et al. The combination of Tet1 with Oct4 generates high-quality mouse-induced pluripotent stem cells. Stem Cells 33, 686–698 (2015).
Costa, Y. et al. NANOG-dependent function of TET1 and TET2 in establishment of pluripotency. Nature 495, 370–374 (2013).
Sardina, J. L. et al. Transcription factors drive Tet2-mediated enhancer demethylation to reprogram cell fate. Cell Stem Cell 23, 727–741 (2018).
Liu, H. et al. Systematic identification and annotation of human methylation marks based on bisulfite sequencing methylomes reveals distinct roles of cell-type-specific hypomethylation in the regulation of cell identity genes. Nucleic Acids Res. 44, 75–94 (2016).
Kim, A. Y. et al. Obesity-induced DNA hypermethylation of the adiponectin gene mediates insulin resistance. Nat. Commun. 6, 7585 (2015).
Park, Y. J. et al. DNMT1 maintains metabolic fitness of adipocytes through acting as an epigenetic safeguard of mitochondrial dynamics. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.2021073118 (2021).
Madsen, J. G. S. et al. Highly interconnected enhancer communities control lineage-determining genes in human mesenchymal stem cells. Nat. Genet. 52, 1227–1238 (2020).
Siersbaek, R. et al. Dynamic rewiring of promoter-anchored chromatin loops during adipocyte differentiation. Mol. Cell 66, 420–435 (2017).
Fujiki, K. et al. PPARγ-induced PARylation promotes local DNA demethylation by production of 5-hydroxymethylcytosine. Nat. Commun. 4, 2262 (2013).
Londono Gentile, T. et al. DNMT1 is regulated by ATP-citrate lyase and maintains methylation patterns during adipocyte differentiation. Mol. Cell. Biol. 33, 3864–3878 (2013).
Guo, W., Chen, J., Yang, Y., Zhu, J. & Wu, J. Epigenetic programming of Dnmt3a mediated by AP2α is required for granting preadipocyte the ability to differentiate. Cell Death Dis. 7, e2496 (2016).
Chen, Y. S. et al. Inhibiting DNA methylation switches adipogenesis to osteoblastogenesis by activating Wnt10a. Sci. Rep. 6, 25283 (2016).
Cakouros, D. et al. Specific functions of TET1 and TET2 in regulating mesenchymal cell lineage determination. Epigenetics Chromatin 12, 3 (2019).
Liu, X. S. et al. Editing DNA methylation in the mammalian genome. Cell 167, 233–247 (2016).
Santiago, M. et al. Tet3 regulates cellular identity and DNA methylation in neural progenitor cells. Cell. Mol. Life Sci. 77, 2871–2883 (2020).
Sárvári, A. K. et al. Plasticity of epididymal adipose tissue in response to diet-induced obesity at single-nucleus resolution. Cell Metab. 33, 437–453 (2021).
Nahmgoong, H. et al. Distinct properties of adipose stem cell subpopulations determine fat depot-specific characteristics. Cell Metab. https://doi.org/10.1016/j.cmet.2021.11.014 (2022).
Merrick, D. et al. Identification of a mesenchymal progenitor cell hierarchy in adipose tissue. Science https://doi.org/10.1126/science.aav2501 (2019).
Shao, M. et al. Pathologic HIF1α signaling drives adipose progenitor dysfunction in obesity. Cell Stem Cell 28, 685–701 (2021).
Palmer, A. K. & Kirkland, J. L. Aging and adipose tissue: potential interventions for diabetes and regenerative medicine. Exp. Gerontol. 86, 97–105 (2016).
De Carvalho, F. G., Justice, J. N., Freitas, E. C., Kershaw, E. E. & Sparks, L. M. Adipose tissue quality in aging: how structural and functional aspects of adipose tissue impact skeletal muscle quality. Nutrients https://doi.org/10.3390/nu11112553 (2019).
Mancuso, P. & Bouchard, B. The impact of aging on adipose function and adipokine synthesis. Front. Endocrinol. 10, 137 (2019).
Melamed, P., Yosefzon, Y., David, C., Tsukerman, A. & Pnueli, L. Tet enzymes, variants and differential effects on function. Front. Cell Dev. Biol. 6, 22 (2018).
Siersbaek, R. et al. Extensive chromatin remodelling and establishment of transcription factor ‘hotspots’ during early adipogenesis. EMBO J. 30, 1459–1472 (2011).
An, J., Rao, A. & Ko, M. TET family dioxygenases and DNA demethylation in stem cells and cancers. Exp. Mol. Med. 49, e323 (2017).
Gu, T. P. et al. The role of Tet3 DNA dioxygenase in epigenetic reprogramming by oocytes. Nature 477, 606–610 (2011).
Li, T. et al. Critical role of Tet3 in neural progenitor cell maintenance and terminal differentiation. Mol. Neurobiol. 51, 142–154 (2015).
Orlanski, S. et al. Tissue-specific DNA demethylation is required for proper B cell differentiation and function. Proc. Natl Acad. Sci. USA 113, 5018–5023 (2016).
Lim, Y. C. et al. Dynamic DNA methylation landscape defines brown and white cell specificity during adipogenesis. Mol. Metab. 5, 1033–1041 (2016).
Yoo, Y. et al. TET-mediated hydroxymethylcytosine at the Ppargamma locus is required for initiation of adipogenic differentiation. Int J. Obes. 41, 652–659 (2017).
Guo, W. et al. Adipogenesis licensing and execution are disparately linked to cell proliferation. Cell Res. 19, 216–223 (2009).
Jensen, M. D. Role of body fat distribution and the metabolic complications of obesity. J. Clin. Endocrinol. Metab. 93, S57–63 (2008).
Wollina, U., Wetzker, R., Abdel-Naser, M. B. & Kruglikov, I. L. Role of adipose tissue in facial aging. Clin. Inter. Aging 12, 2069–2076 (2017).
Abate, N., Garg, A., Peshock, R. M., Stray-Gundersen, J. & Grundy, S. M. Relationships of generalized and regional adiposity to insulin sensitivity in men. J. Clin. Invest. 96, 88–98 (1995).
Ibrahim, M. M. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes. Rev. 11, 11–18 (2010).
Lee, Y. S. & Olefsky, J. Chronic tissue inflammation and metabolic disease. Genes Dev. 35, 307–328 (2021).
Choe, S. S., Huh, J. Y., Hwang, I. J., Kim, J. I. & Kim, J. B. Adipose tissue remodeling: its role in energy metabolism and metabolic disorders. Front. Endocrinol. 7, 30 (2016).
Fox, C. S. et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 116, 39–48 (2007).
Caso, G. et al. Peripheral fat loss and decline in adipogenesis in older humans. Metabolism 62, 337–340 (2013).
Alt, E. U. et al. Aging alters tissue-resident mesenchymal stem cell properties. Stem Cell Res. 8, 215–225 (2012).
Nguyen, H. P. et al. Aging-dependent regulatory cells emerge in subcutaneous fat to inhibit adipogenesis. Dev. Cell 56, 1437–1451 (2021).
Krueger, F. & Andrews, S. R. Bismark: a flexible aligner and methylation caller for bisulfite-seq applications. Bioinformatics 27, 1571–1572 (2011).
Su, J. et al. CpG_MPs: identification of CpG methylation patterns of genomic regions from high-throughput bisulfite sequencing data. Nucleic Acids Res. 41, e4 (2013).
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010).
Kuleshov, M. V. et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 44, W90–97 (2016).
Li, B. & Dewey, C. N. RSEM: accurate transcript quantification from RNA-seq data with or without a reference genome. BMC Bioinformatics 12, 323 (2011).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Gao, T. & Qian, J. EnhancerAtlas 2.0: an updated resource with enhancer annotation in 586 tissue/cell types across nine species. Nucleic Acids Res. 48, D58–D64 (2020).
Wingett, S. et al. HiCUP: pipeline for mapping and processing Hi-C data. F1000Res 4, 1310 (2015).
Cairns, J. et al. CHiCAGO: robust detection of DNA looping interactions in capture Hi-C data. Genome Biol. 17, 127 (2016).
Schoenfelder, S. et al. The pluripotent regulatory circuitry connecting promoters to their long-range interacting elements. Genome Res. 25, 582–597 (2015).
Nahmgoong, H. et al. Distinct properties of adipose stem cell subpopulations determine fat depot-specific characteristics. Cell Metab. 34, 458–472 (2022).
Jeon, Y. G. et al. RNF20 functions as a transcriptional coactivator for PPARγ by promoting NCoR1 degradation in adipocytes. Diabetes 69, 20–34 (2020).
Electrophoretic mobility shift assays. Nat. Methods 2, 557–558 (2005).
Angueira, A. R. et al. Defining the lineage of thermogenic perivascular adipose tissue. Nat. Metab. 3, 469–484 (2021).
Ampomah, P. B. et al. Macrophages use apoptotic cell-derived methionine and DNMT3A during efferocytosis to promote tissue resolution. Nat. Metab. 4, 444–457 (2022).
Shamsi, F. et al. Vascular smooth muscle-derived Trpv1+ progenitors are a source of cold-induced thermogenic adipocytes. Nat. Metab. 3, 485–495 (2021).
Park, J. et al. Activation of invariant natural killer T cells stimulates adipose tissue remodeling via adipocyte death and birth in obesity. Genes Dev. 33, 1657–1672 (2019).
Siersbæk, R. et al. Transcription factor cooperativity in early adipogenic hotspots and super-enhancers. Cell Rep. 7, 1443–1455 (2014).
Acknowledgements
We thank K. Fujiki and M. Murata for providing the TET2 and TET3 vectors. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT; no. NRF-2018R1A5A1024340 to J.B.K. and J.P., 2020R1A3B2078617 to J.B.K. and NRF-2018R1A5A2024425 to S.H.C.).
Author information
Authors and Affiliations
Contributions
J.P. conceptualized the study, performed the experiments, analysed the data and wrote the manuscript. D.H.L., S.J.H., T.Y.R. and S.K. analysed the WGBS and RNA-seq data. D.H.L. performed a combined analysis of WGBS, PCHi-C and RNA-seq data. J.O. and J.P. provided the adipocyte lineage-tracing mice. J.R.N. and C.H.L. provided aged mice. Y.K.L. and S.H.C. performed the experiments on human adipose tissue. Y.J.P., G.L., S.M.H., J.S.H., Y.Y.K., Y.G.J., H.N., K.C.S. and S.M.K., conducted the animal experiments. J.B.K. supervised the study and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Evan Rosen, Peter Tessarz and the other, anonymous, reviewer for their contribution to the peer review of this work. Primary Handling Editor: Christoph Schmitt, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1
a, FPKM of Dlk1 in pre-AD and AD (n = 3). b, Microscopic images of pre-AD and AD used for RNA-seq and WGBS analyzes. c,d, The degrees of DNA methylation of pre-AD and AD were analyzed using several criteria. The median is shown as the center line in the boxes. The first or third quartile where 25% or 75% of the data are below is represented by the line at the bottom or top of the box. DNA methylation was analyzed based on SINE, LINE, LTR (c), satellite, RNA, and other regions (d). The median is shown as the center line in the boxes (c,d). SINE, n = 884915; LINE n = 625222; LTR n = 597813; DNA, n = 95333. Satellite, n = 9599; RNA n = 8908; Other, n = 11517. The first or third quartile where 25% or 75% of the data are below is represented by the line at the bottom or top of the box (c,d). e, Absolute changes in DNA methylation of hypo-DMRs (green) and hyper-DMRs (brown). f, Kyoto Encyclopedia of Genes and Genomes pathway associated with DNA methylation changes. All data represent mean ± SEM (a). Two tailed Student’s t-test (a).
Extended Data Fig. 2 The mean DNA methylation around several motifs. Related to Fig. 2.
a,b, The levels of DNA methylation in promoters and enhancers around several transcription factor binding motifs. The X-axis is the distance from the center of each motif. The Y-axis represents the mean of DNA methylation. c, Examples of genes having interactions between promoters and enhancers with hypo-DMRs and the C/EBP binding motif.
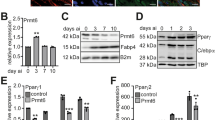
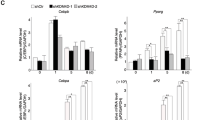
Extended Data Fig. 3 Transcript and DNA methylation changes associated with DNA methylation enzymes in several models of AD. Related to Fig. 3.
a, Relative mRNA levels of the DNA methyltransferase (DNMT) family in 3T3-L1 pre-AD and AD (n = 3). b, Relative mRNA expression of adipogenic genes, TET family, and DNMT family in fibro-adipogenic progenitors (FAPs) and AD from published scRNA-seq data (Sarvari et al., 2021, GSE160729). c–g, Expression levels of Pparg2 (c), Cd36 (d), Dnmt1 (e), Dnmt3a (f), and Dnmt3b (g) on a UMAP plot from published scRNA-seq data (Sarvari et al., 2021, GSE160729). h–j, Representative genome browser view showing DNA methylation Pparg2 (h), Adipoq (i), and Fabp4 (j) in AD of WT (violet) and AD-specific Dnmt1 KO male mice (Dnmt AKO) (purple). k, Relative mRNA of the TET family in 3T3-L1 pre-AD and AD during adipogenesis. Data from GSE56872 (n = 2). All data represent mean ± SEM. Two-way ANOVA with Tukey post hoc test (a,k).
Extended Data Fig. 4 The roles of TET protein in adipogenic gene regulation. Related to Fig. 3.
a, Relative enrichment of TET3 binding to the Glut1 promoter (n = 4). b–d, Relative mRNA levels of Tet3 (b), Pparg2 (c), and Cebpa (d) (n = 3). e–g, Relative enrichment of TET2 binding at Pparg2 (e), Adipoq (f), and Glut1 (g) promoters in 3T3-L1 AD. h,i, Relative mRNA levels of TET family (h) and adipogenic genes (i) such as Pparg2, Adipoq, Cebpa, Cd36, and Fabp4 in mock or TET2 overexpressed 3T3-L1 pre-AD (n = 6). All data represent mean ± SEM. n.s., not significant. Two-tailed Student’s t-test (b–d, h–i).
Extended Data Fig. 5 The relationship between C/EBPβ, TET3, and methylated DNA. Related to Fig. 4.
a, ChIP-qPCR of C/EBPα at the Pparg2 promoter during adipogenesis (n = 2). b, Co-immunoprecipitation. HEK293T cells were transfected with TET3 and/or C/EBPδ expression vectors. Co-immunoprecipitation and western blotting were performed with the indicated antibodies. IP, immunoprecipitation. c, The mRNA levels of Cebpd and Tet3 (n = 4). d, 3T3-L1 preadipocytes were transfected with siRNAs. After 2 days of contact inhibition, ChIP-qPCR was performed. Relative enrichment of TET3 at the Pparg2 and Adipoq promoters (n = 3). e, Relative mRNA levels of Cebpd after treatment with dexamethasone (DEXA) (n = 3). f, Knockdown efficiency of Cebpb (n = 3). g, Relative enrichment of TET3 at the Pparg2 and Adipoq promoters (n = 3). h, Electrophoretic mobility shift assay in which radioisotope-labeled hot DNA (C/EBP response element, C/EBP RE) and C/EBPβ were co-incubated. Unlabeled CH3-CEBP RE, Srebp1c RE (SRE), and PPAR RE (PPRE) were used as binding competitors for C/EBPβ as cold DNA. All data represent mean ± SEM. n.s., not significant. Two-tailed Student’s t-test (a, c–g). The blue dotted line is the IgG control.
Extended Data Fig. 6 scRNA data of ASCs isolated from eWAT and iWAT. Related to Fig. 5.
Single-cell RNA sequencing was performed in adipose stem cells (ASCs; CD31−/CD45−) isolated from eWAT and iWAT (PRJNA708350). a, Transcript levels of the adipogenic genes of ASCs in eWAT and iWAT. b, Transcript levels of the TET family of ASCs in eWAT and iWAT. c, Transcript levels of the DNMT family of ASCs in eWAT and iWAT. d, The levels of 5mC in CD45+ cells from pgWAT and iWAT of male and female mice (n = 4). n.s., not significant. Significance was determined using the Wilcoxon rank sum test in ‘FindMarkers’ function of Seurat (PMID: 31178118) (a–c). All data represent mean ± SEM (d). Two-way ANOVA with Tukey post hoc test (d).
Extended Data Fig. 7 The effects of Tet3 suppression in iWAT.
a, Experimental scheme. siNC or siTet3 was injected into the left or right fat pads of 8-week-old WT male mice, respectively. b–d, The mRNA levels of Tet3 (b), adipogenic genes such as Cebpa, Adipoq, Plin1, Cebpb, and Cebpd (c,d) (n = 4). e, Histological analysis of iWAT after siNC or siTet3 administration (siNC n = 4, siTet3 n = 4). Scale bar, 100 mm. n.s., not significant (Student’s t-test). f, Quantification of adipocyte size of siNC or siTet3-administrated iWAT (siNC n = 1,310, siTet3 n = 1,523). Two adipose tissue images from each mouse were quantified. Two-tailed Student’s t-test (b–d, f).
Extended Data Fig. 8 During human aging, adipogenic potential is reduced in subcutaneous adipose tissue. Related to Fig. 6.
The GTEx data (https://gtexportal.org) were used to analyze transcript changes according to human aging. Human subcutaneous adipose tissue and visceral adipose tissue data were reprocessed with age. a–c, Transcript changes in adipogenic genes, such as PPARG (a), CEBPA (b), and FABP4 (c) in subcutaneous adipose tissue. 20–29, n = 32; 30–39, n = 38, 40–49, n = 74; 50–59, n = 149; 60–69, n = 136; 70–79, n = 13. d–f, Transcript changes in adipogenic genes, such as PPARG (d), CEBPA (e), and FABP4 (f) in visceral adipose tissue. 20–29, n = 25; 30–39, n = 28, 40–49, n = 57; 50–59, n = 124; 60–69, n = 109; 70–79, n = 12. All data are presented as the mean ± SEM. n.s., not significant (one-way ANOVA with Tukey’s post hoc test).
Extended Data Fig. 9 Several phenotypes of aged male mice, compared to young male mice. Related to Fig. 6.
a, Body weights of young and aged male mice (n = 6). b,c, Mass of subcutaneous (b) and visceral adipose tissue (c) (n = 6). d, e, iWAT (d) and eWAT mass (e) of aged male mice overexpressing mock or TET3 (n = 9). f, Insulin-independent glucose uptake. All data represent mean ± SEM. n.s., not significant. Two-tailed Student’s t-test (a–c).
Extended Data Fig. 10 TET2 overexpression does not recover the decrease in adipogenic capacity with aging. Related to Fig. 6.
a, Experimental scheme for TET2 overexpression in C57BL/6J aged male mice. TET2 was overexpressed in the right fat pad of aged mice, and the left fat pad was injected with the mock vector as a control. b, Picture of subcutaneous adipose tissue of aged mice overexpressing mock or TET2. c,d, The transcript levels of Tet2 (c) and adipogenic genes (d) in iWAT of TET2 overexpressed mice (mock n = 5, TET3 n = 6). All data are presented as the mean ± SEM. n.s., not significant. Two-tailed Student’s t-test (c–d).
Supplementary information
Supplementary Information
Supplementary Fig. 1 FACS gating strategy
Supplementary Tables 1 and 2
Gene list and nucleotide sequences
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 4
Unprocessed western blots and gels.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 5
Unprocessed western blots and gels.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
About this article
Cite this article
Park, J., Lee, D.H., Ham, S. et al. Targeted erasure of DNA methylation by TET3 drives adipogenic reprogramming and differentiation. Nat Metab 4, 918–931 (2022). https://doi.org/10.1038/s42255-022-00597-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-022-00597-7
This article is cited by
-
A negative feedback loop between TET2 and leptin in adipocyte regulates body weight
Nature Communications (2024)
-
Ubiquitin ligase RNF20 coordinates sequential adipose thermogenesis with brown and beige fat-specific substrates
Nature Communications (2024)
-
DNA methylation restricts coordinated germline and neural fates in embryonic stem cell differentiation
Nature Structural & Molecular Biology (2024)