Abstract
Background
Previous observational studies utilizing administrative claims data have largely been unable to consider clinical factors that may be related to patterns of drug use among patients with rheumatoid arthritis (RA).
Objective
To understand predictors of treatment changes following initiation of a tumor necrosis factor inhibitor (TNFi) using nation-wide electronic health record (EHR) data in the USA.
Methods
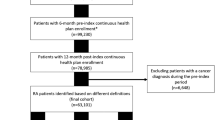
The Optum Immunology Condition EHR data (01/01/2011–09/30/2019) was used to identify a population of adult patients with RA initiating a TNFi as the first line biologic disease-modifying anti-rheumatic drug (DMARD). The primary outcome was any treatment change during the 1-year post-index period defined as cycling to a different TNFi or switching to non-TNFi biologic or targeted synthetic DMARDs. Secondary outcomes were the individual components of TNFi cycling and switching, examined separately. To identify predictors of DMARD treatment changes, we used a least absolute shrinkage and selection operator (LASSO) regression model. Model c-statistics and odds ratios (ORs, 95% confidence intervals (CIs)) of predictors were reported.
Results
We identified 24,871 patients with RA who initiated a TNFi. The mean age was 55.5 (± 13.7) years and 77.2% were female. Among the TNFi initiators, 22.2% experienced TNFi cycling or switching during the 1-year follow-up time. Predictors that are associated with higher likelihood of TNFi cycling or switching included female gender (OR: 1.26, 95% CI: 1.16–1.36) and glucocorticoid use (OR: 1.30, 95% CI: 1.21–1.40). In contrast, inflammatory bowel disease (OR: 0.62, 95% CI: 0.48–0.78), psoriasis (OR: 0.82, 95% CI: 0.70–0.95), recent use of methotrexate (OR: 0.89, 95% CI: 0.81–0.97), and vitamin D intake (OR: 0.92, 95% CI: 0.85–0.99) were negatively associated with TNFi cycling or switch.
Conclusions
Gender, glucocorticoid use, inflammatory bowel disease, psoriasis, and vitamin D intake were identified as significant predictors of TNFi cycling or switching for TNFi initiators in the RA population. Predicting treatment change remains challenging even with large detailed EHR data.
Plain Language Summary
This study aimed to identify key determinants of treatment changes among patients with rheumatoid arthritis (RA) initiating a tumor necrosis factor inhibitor (TNFi) as their first-line biologic disease-modifying antirheumatic drug (DMARD) in routine care settings using a US nation-wide longitudinal electronic health record (EHR). Among 24,871 patients with RA who initiated a TNFi, 22.2% experienced TNFi cycling or switching during the 1-year follow-up time. Female patients and those who used glucocorticoids were more likely to experience TNFi cycling or switching, whereas inflammatory bowel disease, psoriasis, recent methotrexate use, and vitamin D intake were negatively associated with the outcome. However, predicting treatment change remains challenging even with larger detailed EHR data potentially due to unmeasured factors such as prescriber’s preference or patient’s belief in the medication.





Similar content being viewed by others
References
Hunter TM, Boytsov NN, Zhang X, Schroeder K, Michaud K, Araujo AB. Prevalence of rheumatoid arthritis in the United States adult population in healthcare claims databases, 2004–2014. Rheumatol Int. 2017;37(9):1551–7.
Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheumatol. 2008;58(1):15–25.
Kim SC, Yelin E, Tonner C, Solomon DH. Changes in use of disease-modifying antirheumatic drugs for rheumatoid arthritis in the United States during 1983–2009. Arthritis Care Res (Hobok). 2013;65(9):1529–33.
Desai RJ, Solomon DH, Jin Y, Liu J, Kim SC. Temporal trends in use of biologic DMARDs for rheumatoid arthritis in the United States: a cohort study of publicly and privately insured patients. J Manag Care Spec Pharm. 2017;23(8):809–14.
Fraenkel L, Bathon JM, England BR, et al. 2021 American College of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res. 2021;73(7):924–39.
Uson J, Rodriguez-García SC, Castellanos-Moreira R, et al. EULAR recommendations for intra-articular therapies. Ann Rheum Dis. 2021;2021:annrheumdis-2021-220266.
Wei W, Knapp K, Wang L, et al. Treatment persistence and clinical outcomes of tumor necrosis factor inhibitor cycling or switching to a new mechanism of action therapy: real-world observational study of rheumatoid arthritis patients in the United States with prior tumor necrosis factor inhibitor therapy. Adv Ther. 2017;34(8):1936–52.
Meissner B, Trivedi D, You M, Rosenblatt L. Switching of biologic disease modifying anti-rheumatic drugs in patients with rheumatoid arthritis in a real world setting. J Med Econ. 2014;17(4):259–65.
Harnett J, Wiederkehr D, Gerber R, Gruben D, Koenig A, Bourret J. Real-world evaluation of TNF-inhibitor utilization in rheumatoid arthritis. J Med Econ. 2016;19(2):91–102.
Shahabi A, Shafrin J, Zhao L, et al. The economic burden of switching targeted disease-modifying anti-rheumatic drugs among rheumatoid arthritis patients. J Med Econ. 2019;22(4):350–8.
Kim G, Barner JC, Rascati K, Richards K. Factors associated with the initiation of biologic disease-modifying antirheumatic drugs in Texas Medicaid patients with rheumatoid arthritis. J Manag Care Spec Pharm. 2015;21(5):401–7.
Oladapo A, Barner JC, Lawson KA, et al. Medication effectiveness with the use of tumor necrosis factor inhibitors among Texas Medicaid patients diagnosed with rheumatoid arthritis. J Manag Care Spec Pharm. 2014;20(7):657–67.
Rashid N, Lin AT, Aranda G Jr, et al. Rates, factors, reasons, and economic impact associated with switching in rheumatoid arthritis patients newly initiated on biologic disease modifying anti-rheumatic drugs in an integrated healthcare system. J Med Econ. 2016;19(6):568–75.
Hetland ML. DANBIO: a nationwide registry of biological therapies in Denmark. Clin Exp Rheumatol. 2005;23(5 Suppl 39):S205-207.
van Vollenhoven RF, Askling J. Rheumatoid arthritis registries in Sweden. Clin Exp Rheumatol. 2005;23(5 Suppl 39):S195-200.
Kremer JM. The Corrona US registry of rheumatic and autoimmune diseases. Clin Exp Rheumatol. 2016;34(5 Suppl 101):S96-s99.
Hetland ML, Lindegaard HM, Hansen A, et al. Do changes in prescription practice in patients with rheumatoid arthritis treated with biological agents affect treatment response and adherence to therapy? Results from the nationwide Danish DANBIO Registry. Ann Rheum Dis. 2008;67(7):1023–6.
Pappas DA, Litman HJ, Lesperance T, et al. Persistence on biologic DMARD monotherapy after achieving rheumatoid arthritis disease control on combination therapy: retrospective analysis of corrona registry data. Rheumatol Int. 2021;41(2):381–90.
Neovius M, Arkema EV, Olsson H, et al. Drug survival on TNF inhibitors in patients with rheumatoid arthritis comparison of adalimumab, etanercept and infliximab. Ann Rheum Dis. 2015;74(2):354–60.
Bergman M, Zhou L, Patel P, Sawant R, Clewell J, Tundia N. Healthcare costs of not achieving remission in patients with rheumatoid arthritis in the United States: a retrospective cohort study. Adv Ther. 2021;38(5):2558–70.
Kim SY, Servi A, Polinski JM, et al. Validation of rheumatoid arthritis diagnoses in health care utilization data. Arthritis Res Ther. 2011;13(1):R32.
Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749–59.
Sun JW, Rogers JR, Her Q, et al. Adaptation and validation of the combined comorbidity score for ICD-10-CM. Med Care. 2017;55(12):1046–51.
Kim DH, Glynn RJ, Avorn J, et al. Validation of a claims-based frailty index against physical performance and adverse health outcomes in the health and retirement study. J Gerontol A Biol Sci Med Sci. 2019;74(8):1271–6.
Zhang Y, Li R, Tsai C-L. Regularization parameter selections via generalized information criterion. J Am Stat Assoc. 2010;105(489):312–23.
Chand S. On tuning parameter selection of lasso-type methods—a monte carlo study. In: Paper presented at: Proceedings of 2012 9th International Bhurban Conference on Applied Sciences & Technology (IBCAST); 2012.
Desai RJ, Wang SV, Vaduganathan M, Evers T, Schneeweiss S. Comparison of machine learning methods with traditional models for use of administrative claims with electronic medical records to predict heart failure outcomes. JAMA Netw Open. 2020;3(1):e1918962–e1918962.
Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36.
Dore RK, Antonova J, Burudpakdee C, Wang X, Ozbay B, Genovese MC. SAT0133 treatment patterns, persistence, and durability in rheumatoid arthritis patients with inadequate response to biologic disease-modifying anti-rheumatic drugs (BDMARDS). Ann Rheum Dis. 2019;78(Suppl 2):1135–6.
Chastek B, Chen CI, Proudfoot C, Shinde S, Kuznik A, Wei W. Treatment persistence and healthcare costs among patients with rheumatoid arthritis changing biologics in the USA. Adv Ther. 2017;34(11):2422–35.
Meijboom RW, Gardarsdottir H, Becker ML, et al. Switching TNFα inhibitors: patterns and determinants. Pharmacol Res Perspect. 2021;9(4): e00843.
Li K-J, Chang C-L, Hsin C-Y, Tang C-H. Switching and discontinuation pattern of biologic disease-modifying antirheumatic drugs and tofacitinib for patients with rheumatoid arthritis in Taiwan. Front Pharmacol. 2021;2021:12.
Fletcher A, Lassere M, March L, et al. Patterns of biologic and targeted-synthetic disease-modifying antirheumatic drug use in rheumatoid arthritis in Australia. Rheumatology. 2022;2022:5.
Saevarsdottir S, Wedrén S, Seddighzadeh M, et al. Patients with early rheumatoid arthritis who smoke are less likely to respond to treatment with methotrexate and tumor necrosis factor inhibitors: observations from the Epidemiological Investigation of Rheumatoid Arthritis and the Swedish Rheumatology Register cohorts. Arthritis Rheum. 2011;63(1):26–36.
Schäfer M, Meißner Y, Kekow J, et al. Obesity reduces the real-world effectiveness of cytokine-targeted but not cell-targeted disease-modifying agents in rheumatoid arthritis. Rheumatology. 2019;59(8):1916–26.
Højgaard P, Glintborg B, Kristensen LE, Gudbjornsson B, Love TJ, Dreyer L. The influence of obesity on response to tumour necrosis factor-α inhibitors in psoriatic arthritis: results from the DANBIO and ICEBIO registries. Rheumatology. 2016;55(12):2191–9.
Micheroli R, Hebeisen M, Wildi LM, et al. Impact of obesity on the response to tumor necrosis factor inhibitors in axial spondyloarthritis. Arthritis Res Ther. 2017;19(1):164–164.
Suh YS, Cheon YH, Kim HO, et al. Medication nonadherence in Korean patients with rheumatoid arthritis: the importance of belief about medication and illness perception. Korean J Intern Med. 2018;33(1):203–10.
Wabe N, Lee A, Wechalekar M, McWilliams L, Proudman S, Wiese M. Factors associated with medication adherence in a longitudinal study of rheumatoid arthritis patients. Int J Clin Pract. 2019;73(7): e13375.
Rodrigues JR, Faria DS, Neves JS, et al. Positive affect as a predictor of adherence in patients with Rheumatoid Arthritis. Acta Reumatol Portuguesa. 2019;44(2):132–7.
Pasma A, van’t Spijker A, Hazes JM, Busschbach JJ, Luime JJ. Factors associated with adherence to pharmaceutical treatment for rheumatoid arthritis patients: a systematic review. Semin Arthritis Rheum. 2013;43(1):18–28.
Morgan C, McBeth J, Cordingley L, et al. The influence of behavioural and psychological factors on medication adherence over time in rheumatoid arthritis patients: a study in the biologics era. Rheumatol (Oxf). 2015;54(10):1780–91.
Kumar K, Raza K, Nightingale P, et al. Determinants of adherence to disease modifying anti-rheumatic drugs in White British and South Asian patients with rheumatoid arthritis: a cross sectional study. BMC Musculoskelet Disord. 2015;16:396.
Laganà B, Zullo A, Scribano ML, et al. Sex differences in response to TNF-inhibiting drugs in patients with spondyloarthropathies or inflammatory bowel diseases. Front Pharmacol. 2019;10:47.
Jawaheer D, Olsen J, Hetland ML. Sex differences in response to anti-tumor necrosis factor therapy in early and established rheumatoid arthritis—results from the DANBIO registry. J Rheumatol. 2012;39(1):46–53.
Rusman T, Ten Wolde S, Euser SM, van der Ploeg T, van Hall O, van der Horst-Bruinsma IE. Gender differences in retention rate of tumor necrosis factor alpha inhibitor treatment in ankylosing spondylitis: a retrospective cohort study in daily practice. Int J Rheum Dis. 2018;21(4):836–42.
Hider SL, Tanveer W, Brownfield A, Mattey DL, Packham JC. Depression in RA patients treated with anti-TNF is common and under-recognized in the rheumatology clinic. Rheumatol (Oxf). 2009;48(9):1152–4.
Lwin MN, Serhal L, Holroyd C, Edwards CJ. Rheumatoid arthritis: the impact of mental health on disease: a narrative review. Rheumatol Therapy. 2020;7(3):457–71.
Calip GS, Adimadhyam S, Xing S, Rincon JC, Lee WJ, Anguiano RH. Medication adherence and persistence over time with self-administered TNF-alpha inhibitors among young adult, middle-aged, and older patients with rheumatologic conditions. Semin Arthritis Rheum. 2017;47(2):157–64.
Kristensen LE, Saxne T, Nilsson J-Å, Geborek P. Impact of concomitant DMARD therapy on adherence to treatment with etanercept and infliximab in rheumatoid arthritis. Results from a six-year observational study in southern Sweden. Arthritis Res Therapy. 2006;8(6):R174.
Engel-Nitz NM, Ogale S, Kulakodlu M. Use of anti-tumor necrosis factor therapy: a retrospective study of monotherapy and adherence to combination therapy with non-biologic disease-modifying anti-rheumatic drugs. Rheumatol Ther. 2015;2(2):127–39.
Blum MA, Koo D, Doshi JA. Measurement and rates of persistence with and adherence to biologics for rheumatoid arthritis: a systematic review. Clin Ther. 2011;33(7):901–13.
Aaltonen KJ, Virkki LM, Malmivaara A, Konttinen YT, Nordström DC, Blom M. Systematic review and meta-analysis of the efficacy and safety of existing TNF blocking agents in treatment of rheumatoid arthritis. PLoS ONE. 2012;7(1): e30275.
Klareskog L, van der Heijde D, de Jager JP, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. The Lancet. 2004;363(9410):675–81.
Emery P, Breedveld FC, Hall S, et al. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. The Lancet. 2008;372(9636):375–82.
Smolen JS, Emery P, Fleischmann R, et al. Adjustment of therapy in rheumatoid arthritis on the basis of achievement of stable low disease activity with adalimumab plus methotrexate or methotrexate alone: the randomised controlled OPTIMA trial. The Lancet. 2014;383(9914):321–32.
Dessein PH, Joffe BI, Stanwix AE. High sensitivity C-reactive protein as a disease activity marker in rheumatoid arthritis. J Rheumatol. 2004;31(6):1095–7.
Graf J, Scherzer R, Grunfeld C, Imboden J. Levels of C-reactive protein associated with high and very high cardiovascular risk are prevalent in patients with rheumatoid arthritis. PLoS ONE. 2009;4(7): e6242.
Pope JE, Choy EH. C-reactive protein and implications in rheumatoid arthritis and associated comorbidities. Semin Arthritis Rheum. 2021;51(1):219–29.
Cylwik B, Chrostek L, Gindzienska-Sieskiewicz E, Sierakowski S, Szmitkowski M. Relationship between serum acute-phase proteins and high disease activity in patients with rheumatoid arthritis. Adv Med Sci. 2010;55(1):80–5.
Yildirim K, Karatay S, Melikoglu MA, Gureser G, Ugur M, Senel K. Associations between acute phase reactant levels and disease activity score (DAS28) in patients with rheumatoid arthritis. Ann Clin Lab Sci. 2004;34(4):423–6.
England BR, Thiele GM, Mikuls TR. Anticitrullinated protein antibodies: origin and role in the pathogenesis of rheumatoid arthritis. Curr Opin Rheumatol. 2017;29(1):57–64.
McInnes IB, Schett G. Pathogenetic insights from the treatment of rheumatoid arthritis. The Lancet. 2017;389(10086):2328–37.
Courvoisier DS, Chatzidionysiou K, Mongin D, et al. The impact of seropositivity on the effectiveness of biologic anti-rheumatic agents: results from a collaboration of 16 registries. Rheumatology. 2020;60(2):820–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This project was supported by AbbVie (grant number: 2018A018586).
Role of the study sponsor
The sponsor provided the data access to the authors at Brigham and Women’s Hospital via the third-party agreement. The authors at Brigham and Women’s Hospital analyzed the data, interpreted the results, determined the final wording of the manuscript, and had the final decision to submit the manuscript for publication. The sponsor was given the opportunity to make nonbinding comments on a draft of the manuscript. Publication of this article was not contingent upon approval by the sponsor.
Conflicts of interest/Competing interests
Jin and Landon declare that they have no conflict of interest. Desai reports receiving research grants from Bayer, Vertex, and Novartis for unrelated research. Liede and Krueger are full-time AbbVie employees and therefore own AbbVie stock. Kim has received research grants to the Brigham and Women’s Hospital from Pfizer, AbbVie, Roche and Bristol-Myers Squibb.
Availability of data and material
The data that support the findings of this study are not publicly available.
Code availability
The codes that support the findings of this study are available from the corresponding author, SCK, upon reasonable request.
Authors' contributions
All authors meet the journal’s criteria for authorship. All authors have contributed to the work and approve the presented findings.
Ethics approval
The study protocol was reviewed and approved by the Institutional Review Board (IRB) of the Brigham and Women’s Hospital (IRB protocol number: 2019P003604).
Consent to participate
Patient-informed consent was not required, since the data were deidentified.
Consent for publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jin, Y., Landon, J., Krueger, W. et al. Predictors of Treatment Change Among Patients with Rheumatoid Arthritis Treated with TNF Inhibitors as First-Line Biologic Agent in the USA: A Cohort Study from Longitudinal Electronic Health Records. BioDrugs 36, 521–535 (2022). https://doi.org/10.1007/s40259-022-00542-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-022-00542-w