Abstract
With the improvement of medical and health care level in our society, the demand for antibacterial materials is increasing. In this work, we prepared the antibacterial materials by loading silver nanoparticles (AgNPs) on the dialdehyde cellulose (DAC) with in-situ synthesis method. DAC was prepared by pretreating cellulose fiber with sodium metaperiodate (NaIO4) to convert the hydroxyl group into aldehyde group, and then reacted with silver nitrate (AgNO3) to obtain AgNPs loaded on DAC. UV–Vis results show that the characteristic absorption peak of AgNPs at 428 nm appeared in the AgNPs-loaded-DAC. It was observed by SEM that the spherical AgNPs were distributed uniformly on the DAC surface without obvious flocculation. The color of DAC was not changed significantly, indicating that a small amount of AgNPs was loaded. In addition, sodium citrate (Na3C6H5O7) was added in the reaction of DAC and AgNO3 and its effect on the formation of AgNPs was studied. The results demonstrated that the color of DAC turned deeper and finally dark yellow with reaction time extended. When the reaction time was 60 h, the spherical AgNPs were gradually grown and transformed into triangular prism on the DAC surface. The antibacterial properties of AgNPs showed inhibition zones of 4.90 mm and 7.35 mm (60 h) against Gram-negative (E. coli) and Gram-positive (S. aureus), respectively, which increased by 40.00% and 14.85% compared with spherical AgNPs (2.5 h) obtained without Na3C6H5O7. The research of AgNPs-loaded cellulose-based materials promotes the development prospect of new nano-antibacterial materials.
Graphical abstract

Similar content being viewed by others
Introduction
With the development of social economy, human beings are facing the intrusion of bacteria and viruses while enjoying the development dividend. From the SARS in 2004 to the COVID-19 in 2020, the virus not only harms people’s health all over the world, but also restricts the development of the global economy (Liu et al. 2020). Therefore, people spend more efforts on improving their living environment. In order to effectively prevent the invasion of surrounding bacteria, people turn their attention to antibacterial materials.
In the long-term development of antibacterial drugs, it has been found that the combination of two or more antibacterial materials to form composites can not only obtain better antibacterial activity on the basis of reducing drug dosage and cytotoxicity, but also reduce the generation of bacterial drug resistance and increase the overall therapeutic effect of the materials (Roberto et al. 2019; Sun and Lü 2021). Until now, antibacterial substances are mainly composed of inorganic, organic and natural antibacterial materials. Due to the difference in physical and chemical properties of different types of antibacterial materials, the formation mechanism of composites is also different, which leads to some differences in antibacterial effects. For example, natural antibacterial materials (e.g., chitosan, insect protein and licorice etc.) were used to prepare antibacterial materials through physical modification, chemical modification, composite spinning and other methods (Ding 2010; Li and Luo 2012; Zhao et al. 2016). Using the antibacterial ability of metals (or their ions) such as silver (Ag), copper (Cu), zinc (Zn), etc., which are fixed on the surface or pores of porous materials such as zeolite and silica gel through physical adsorption or ion exchange methods. Then added to products to obtain antibacterial materials (Yu 2020). The surface functionalization of materials was carried out by using some organic polymers (e.g., quaternary ammonium salts and sodium alginate (SA) etc.) (Cheng et al. 2016; Kango et al. 2013; Sun et al. 2015a; Yadav and Maji 2018), it was found that materials after functionalization carry richer positive charges, which made them easier to combine with the bacterial cell membrane and easier to be further modified. This would provide greater possibilities for the preparation and application of multifunctional composite antibacterial materials (Bahrami et al. 2020; Li et al. 2019; Sun and Lü 2021).
At present, most of the antibacterial substrates we use are derived from petroleum, which is non-renewable and non-biodegradable. Cellulose resources (e.g., natural cellulose and bacterial cellulose) are considered to be environmentally friendly, green, and inexhaustible. Therefore, biodegradable and environmentally friendly cellulose-based antibacterial materials have become a research hotspot (e.g., reinforced composites, transparent packaging, high-performance filtration, biological medicine, etc.) (Chen and Xu 2017; Chen et al. 2018; Kang et al. 2020; Mishra et al. 2018). Alavi and Nokhodchi (2020) comprehensively summarized the antibacterial and wound healing properties of inorganic nanoparticles (e.g., AgNPs, ZnONPs, etc.). It was found that the active of killing eukaryotic and prokaryotic microorganisms mainly resulted from the release of ions from inorganic nanoparticles. They also illustrated the challenge of nanoparticles application in medicine and food packaging. Chook et al. (2012) first proposed the preparation of nano-silver sol by modified Torun reagent method, and then added the nano-silver sol to the cellulose-alkali (NaOH)/urea solution. The AgNPs smoothly entered the interior of the regenerated cellulose membrane, and obtained a AgNPs-loaded regenerated cellulose membrane with a thickness of about 0.5 mm, which imparted the membrane fairly stable antibacterial ability. In addition, Yu et al. (2021) developed a novel Pickering emulsion (CO-PE) with a rough surface, which clove essential oil (CO) was used as oil phase and carboxymethyl cellulose sodium (CMC-Na) modified cellulose nanocrystals (CNC) as stabilizer. It has strong antibacterial activity against Gram-negative (E. coli) and Gram-positive (S. aureus).
Silver nanoparticles (AgNPs) are widely applied in various fields because of their excellent antibacterial effect and no drug resistance. A new wound dressing based on chitosan-dialdehyde cellulose nanocrystal-silver nanoparticles (CS-DCNC-AgNPs) was proposed by (Dong and Li 2018; Kang et al. 2020). The antibacterial effect of material was improved by incorporation of AgNPs, and the mechanical strength and hydrophobicity of material were increased by DCNC cross-linked through hydrogen bonds. The research signified that CS-DCNC-AgNPs are a promising and safety antibacterial wound dressings. Kang et al. (2020) and Pang and Chen (2015) prepared composite antibacterial materials by adding AgNPs as antibacterial agent into microfibrillated cellulose (MFC) and evaluated its antibacterial performance, mechanical and barrier properties as the antibacterial packaging of winter jujube. The experimental results showed that the antibacterial performance increases with the increase of coating amount. When the coating amount reaches 5.8 g/m2, the diameter of the inhibition zone reached more than 20 mm. In addition, the elongation of coated paper was improved significantly with the increase of coating amount. Using periodate to extract oxidized cellulose nanofibers (CNFs) from bagasse to obtain dialdehyde-based nanocellulose (CDA) was studied by (Bansal et al. 2016; Kang et al. 2020). The aldehyde group of CDA reacted with the amino group of chitosan (CS) to form a Schiff-base based antimicrobial membranes. The obtained CDA/CS composite film shows excellent antibacterial properties to S. aureus and E. coli, demonstrating great application potential in the antibacterial packaging materials. Furthermore, He et al. (2020) developed a new natural formulation composed of carboxymethyl cellulose (CMC) and various contents of CNC immobilized AgNPs (CNC@AgNPs) for paper coating. The mechanical strength and antibacterial activities of the coated paper improved with the increasing content of CNC@AgNPs. After CMC was mixed with 7.00% CNC@AgNPs, the coated paper exhibited 1.26 times increase in tensile strength as well as the best antibacterial activities against E. coli and S. aureus compared with uncoated paper. When the CMC/CNC@AgNPs coated paper is applied to food quality preservation, the shelf-life of goods can be prolonged. Although the AgNPs-loaded materials demonstrate high antibacterial properties, there are few reports on the shape change of AgNPs. And there are fewer articles on whether the shape change of particle will affect its performance.
In this study, we successfully loaded AgNPs on the DAC surface by in situ synthesis. Firstly, dialdehyde cellulose (DAC) was prepared by selective oxidation of bleached softwood pulp fiber with sodium metaperiodate (NaIO4). Then DAC was used as the substrate and reductant to react with silver nitrate (AgNO3) to obtain AgNPs which were loaded on the surface of DAC. In the reaction process, the shape of the AgNPs was changed by adding sodium citrate (Na3C6H5O7) and the increase of reaction time. The structure of AgNPs-loaded DAC was characterized and their antibacterial properties were evaluated and compared.
Experimental
Materials
Sodium metaperiodate (NaIO4, analytical reagent grade (AR), 99%) and silver nitrate (AgNO3, AR, 99%) were purchased from Aladdin Chemical (Shanghai, China). Sodium citrate (Na3C6H5O7, AR, 99%) was obtained from MACKLIN Inc. (Shanghai, China). In addition, beef extract, peptone and AGAR medium were provided by Nanjing Clinic Biotechnology Co., Ltd. (China). Gram-negative (E. coli, Freeze-dried powder) and Gram-positive (S. aureus, Freeze-dried powder) were purchased from Beijing Aoboxing Biotechnology Co., Ltd. (China). Bleached softwood pulp fibers were provided by Hongta Renheng Papering Co., Ltd. (Guangdong, China). All other chemicals were used as received without further purification and deionized water (DI) was used throughout the experiments.
Preparation of dialdehyde cellulose (DAC)
20 g of bleached softwood pulp fiber with 68.08% moisture content was weighed to a 500 mL Erlenmeyer flask. 200 mL of NaIO4 with 0.20 mol/L concentration was then added. The mixture was stirred in the absence of light, at 50 ℃ in a water bath, for 3 h. After completion of the oxidation, the product was filtered by sintered glass funnel and then dialyzed against deionized water (DI) for 3 days to remove the contaminants (Jiang et al. 2020; Xu et al. 2017, 2020). The obtained dialdehyde cellulose was named DAC. The aldehyde group content was 3.6 mmol·g−1 at a reaction time of 2.5 h (the determination process was shown in supplementary information S1). Then, the DAC was used for the immobilization of silver nanoparticles (AgNPs) without drying.
In-situ synthesis of loaded with AgNPs on the DAC (AgNPs-loaded-DAC)
All glassware used in the experiment were cleaned well using chromic acid lotion, and rinsed with DI. In the synthesis process of antibacterial materials, silver nanoparticles (AgNPs) were in-situ reduced on the DAC surface with AgNO3 as silver precursor and without additional reductant. In addition to carrying AgNPs, the DAC were employed as reducing agent for in-situ reduction of silver ions (Ag+) (Jiang et al. 2020; Xu et al. 2017, 2020). Typically, the total volume of the reaction solution was fixed at 25 mL. 0.2 g of DAC and 24 mL DI were mixed in a 100 mL conical flask and stirred mildly at room temperature. Then 1 mL of AgNO3 solution was added, and the mixture was heated in a water bath at 90 ℃ for a certain of time to get loaded with AgNPs on the DAC, which was named AgNPs-loaded-DAC. Then the mixture was filtered into paper for the following characterization. The schematic illustration for process and mechanism of AgNPs-loaded-DAC was shown in Fig. 1. Specimens of different reaction time were obtained and named S1, S2, S3, S4, and S5 with 0.5 h, 1 h, 1.5 h, 2 h, and 2.5 h, respectively. The DAC without adding AgNO3 were used as the control and named as S0.
Furthermore, the shape changes of AgNPs loaded on the DAC was studied by adding Na3C6H5O7 and increasing reaction time. 14 mL of DI and 0.15 g of DAC were added to a 100 mL conical flask and stirred mildly. Immediately after, 1 mL of AgNO3 solution (0.05 M) and 10 mL of Na3C6H5O7 (75 mmol/L) were injected. The mixture reacted at room temperature for a certain of time to get AgNPs loaded on the DAC, which was named AgNPs-loaded-DAC. And the mixture was filtered into paper. Specimens of different reaction time (10 h, 20 h, 30 h, 40 h, 50 h and 60 h) were obtained.
Characterization
The shape of AgNPs was roughly monitored by visual inspection of the DAC by color changed. UV–Vis absorption spectra of the AgNPs-loaded-DAC were recorded on a Cary 5000 UV–Vis spectrophotometer with a scan range of 200–800 nm. The fiber sheets were directly used to measure the absorption spectra.
FT-IR spectra of bleaching softwood pulp fiber, DAC, and AgNPs-loaded-DAC were processed by using VERTEX 70 spectrometer in transmission mode. For the specimen preparation, a certain amount of KBr powder (Specpure grade) was used as the background. The specimens were cut into powder, and thoroughly mixed by grinding with an agate mortar and pestle to approximately 0.50% by weight in KBr, respectively. The spectra were obtained by operating at a nominal resolution of 4 cm−1 in the region between 400 and 4000 cm−1.
SEM observations were carried out on a S-4800 field emission scanning electron microscope operated at 3 kV. All specimens were sputtered with gold before observation. Elemental analysis of the particles was implemented by Energy-dispersive X-ray spectroscopy (EDS), which can provide a rapid qualitative and quantitative analysis of the elemental composition.
The AgNPs-loaded-DAC was measured by an X-ray diffractometer (XRD, D8-ADVANCE, Bruker, Germany). The samples were scanned at 40 kV and 30 mA in a 2θ range between 5° and 90° using Cu-Kα radiation (λ = 15.4 × 10–2 nm) at 1°/min.
The antibacterial activity of AgNPs-loaded-DAC against Gram-negative (E. coli, ATCC 8099) and Gram-positive (S. aureus, ATCC 25,923) was tested by agar diffusion method according to GB/T20944 (Behnam et al. 2018; Shemesh et al. 2015). On Luria–Bertani (LB) agar plates, a hundred microliter E. coli or S. aureus suspension was spread. Subsequently, circular AgNPs-loaded-DAC samples (16 mm in diameter) were placed on the LB agar plates and incubated overnight at 37 ℃. Their antibacterial activities were determined according to the inhibition zone width.
Results and discussion
Structure characterization of DAC and AgNPs-loaded-DAC
In the experiment, it was found that the color of DAC was changed through the surface observation. We recorded the AgNPs-loaded-DAC obtained by the reaction of DAC with AgNO3 solution at different times. The results are shown in Fig. 2a. Since the raw materials are white and the AgNPs-loaded-DAC show yellow, which is due to the yellow nature of Ag itself (Immanuel et al. 2017). It can be preliminarily determined that this color is presented by AgNPs, Ag+ has been reduced to Ag, and Ag is loaded on the DAC.
FT-IR was performed to characterize the change of the function groups of DAC and AgNPs-loaded-DAC. The results are shown in Fig. 2b. Compared to raw materials (Fig. 2b-a), the obvious characteristic absorption peak of carbonyl groups (C=O) was appeared at 1730 cm−1 for DAC (Fig. 2b-b) (Gong et al. 2013; Ruan et al. 2016; Sun et al. 2015b). The results show that raw cellulose fiber have been successfully oxidized to DAC by NaIO4. After the AgNPs were synthesized on the DAC (Fig. 2b-c), the adsorption band at 1730 cm−1 decreased significantly. This demonstrated that the aldehyde groups were oxidized in the process of in-situ synthesis of AgNPs. In addition, the characteristic absorption peak of cellulose fiber after NaIO4 oxidation did not change. Other main bonds can be assigned to: 3350 cm−1 (–OH stretching) (Peng et al. 2009), 2901 cm−1 (C–H stretching of –CH2 and C–H groups) (Jahan et al. 2011), 1643 cm−1 (water –OH bending vibration), 1431 cm−1 (–CH2 symmetric bending vibration), 1373 cm−1 (C–H bending vibration), 1165–1034 cm−1 (C–O stretching of skeletal) (Liu et al. 2011), 896 cm−1 (C–H deformation mode of the glycosidic linkage between the glucose units).
Besides, UV–Vis spectrum of AgNPs-loaded-DAC is shown in Fig. 2c (the insets showed loaded with AgNPs on the DAC). DAC (0 h) demonstrated no absorption peak at about 400 nm, indicating that there were no AgNPs on the DAC. After the reaction with AgNO3 for some time, the characteristic absorption peak of AgNPs appeared in all samples at 428 nm, which exhibited that silver ions (Ag+) in AgNO3 were reduced in-situ into AgNPs (Anderson et al. 2014; Hebeish et al. 2013). With the extension of reaction time (0–2.5 h), the characteristic absorption peak intensity of AgNPs increased, suggesting that prolonging reaction time would increase the loading capacity of AgNPs.
In order to characterize the morphology, particle size and loading amount of AgNPs on the DAC, the AgNPs-loaded-DAC was filtered into paper. The samples were observed by SEM, and the results are shown in Fig. 3. It can be seen from Fig. 3a that the surface of DAC was relatively smooth, while bright spherical nanoparticles could be observed on the surface of DAC loaded with AgNPs (Fig. 3c), and their distribution was relatively uniform without obvious flocculation. In order to further determine the particle size of AgNPs, the spherical particles in Fig. 3b were statistically analyzed by Nano Measurer Software. Most of the AgNPs demonstrated particle size between 20 and 200 nm, with an average particle size of 50 nm. The particles were detected by an EDS, and the results are shown in Fig. 3d. It could be determined that the spherical particles were AgNPs, and the weight content of AgNPs in the scanning area is 1.93 wt%.
Shape change of AgNPs on the DAC surface
In addition, with the increase of reaction time, the loading amount of AgNPs loaded on the DAC has a significant effect on the antibacterial performance. After the addition of Na3C6H5O7, the color of DAC as seen in Fig. 4a changed significantly. The raw material was white. With the extension of reaction time, the color of DAC changed from light yellow to dark yellow (Note: dark yellow after 60 h). Compared with Fig. 2a, the color of DAC was obviously deeper, indicating a higher loading amount of AgNPs.
The AgNPs were further characterized by using UV–Vis spectrophotometer. Usually, AgNPs exhibit unique and tunable optical properties due to their surface plasmon resonance (SPR) that are dependent on shape, size and size distribution of the nanoparticles. The optical properties of AgNPs measured using UV–Vis spectrophotometer can not only evidence their existence due to their SPR, but also indirectly characterize the shape of AgNPs to a certain extent. Different shape of AgNPs exhibit characteristic absorption peaks at different wavelength. It could be seen from Fig. 4b that the characteristic absorption peak of spherical AgNPs was at the wavelength of 428 nm before the reaction for 20 h, which proved that the shape of AgNPs on DAC is still spherical at this stage (Zhang et al. 2011b). With the extension of reaction time, the absorption peak at 428 nm gradually shifted to around 380 nm, and a new absorption peaks gradually appeared, which were located at around 600 nm. The appearance and enhancement of absorption peak at around 600 nm indicated the formation of triangular AgNPs, while the shift of characteristic peak indicated the shape of spherical AgNPs on the DAC started to change (Montazer et al. 2012; Silva and Unali 2011). It could be concluded that the spherical AgNPs were firstly formed in the reaction process. Then, with the addition of citrate and the extension of reaction time, these spherical AgNPs gradually growed and transformed into triangular prism shape, meanwhile, spherical AgNPs were still continuously generated. Therefore, both spherical and triangular AgNPs existed simultaneously on the DAC surface. During the synthesis, Na3C6H5O7 was used as both the capping agent to stabilize AgNPs and a structure-direction agent to change the nanoparticles shape from sphere to triangular (Chen et al. 2019; Rycenga et al. 2011).
In addition, Fig. 4c showed the spectral reflectance curve of each sample in the wavelength range of 360–750 nm, and the color change of sample could be clearly seen. With the extension of reaction time, the total color difference between the control (0 h) and the AgNP-loaded DAC (60 h) gradually increased. The yellow light of DAC decreased and the blue light increased. It was reported that the color of AgNPs would change significantly from yellow to blue when their shape was changed from sphere to triangular (Maryan et al. 2015; Prema et al. 2017). This was consistent with the results of UV–Vis spectra.
SEM is the most direct method to characterize the microscopic shape of AgNPs. The AgNPs-loaded-DAC obtained after 20 h and 60 h reaction were observed by SEM, and the results are shown in Fig. 5b, c. The surface of DAC was relatively smooth without any impurities attached (Fig. 5a), while the adhesion of AgNPs can be clearly seen on the surface of DAC after reaction for 20 h and 60 h, respectively (Fig. 5b, c). When the reaction time was within 20 h, most obtained AgNPs on the DAC surface were spherical with small size. When the reaction time was 60 h, it could be found that the obtained AgNPs had a larger size, and the large AgNPs were no longer spherical, but various heterogeneous shapes, such as trigonal, hexagonal and other irregular shapes (Zhang et al. 2011b). Na3C6H5O7 selectively attached to the silver (111) crystal facets with the increase of reaction time (Zhang et al. 2011a), which would induce the shape change of AgNPs from sphere to triangular. In generally, spherical AgNPs were firstly obtained on the DAC surface, and then with the increase of reaction time, more AgNPs were formed and their shape were gradually changed to triangular with the presence of Na3C6H5O7.
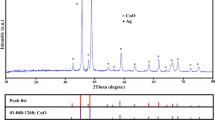
Furthermore, Fig. 6 shows the XRD patterns of the original cellulose fiber and AgNPs-loaded-DAC at different treatment times. It can be seen from Fig. 6 that the characteristic diffraction peak of cellulose I appeared in the XRD patterns of the sample before the reaction for 20 h, and there was no characteristic diffraction peak of AgNPs. When the reaction time was more than 20 h, the characteristic diffraction peaks of cellulose I and AgNPs appeared in the XRD spectrum of the samples. The characteristic diffraction peaks of AgNPs appear at 38.0°, 44.2°, 64.3°, 77.2° and 81.6°, corresponding to (111), (200), (220), (311) and (222) crystal planes (Ahmad et al. 2016; Silva and Unali 2011). SEM analysis shows that (1) the particle size of AgNPs formed by in-situ on the DAC surface is small and the content is low before the reaction for 20 h, which has not been detected, (2) the AgNPs loaded on the DAC are mainly amorphous at this time, and the AgNPs have not completely formed crystalline structure, so there is no characteristic diffraction peak in the XRD pattern (Wei et al. 2017). Therefore, a stable aggregation structure is formed when the reaction time is 60 h.
Antimicrobial activity of AgNPs-loaded-DAC
Gram-positive (S. aureus) bacteria and gram-negative (E. coli) bacteria were selected, and the antibacterial properties of the AgNPs-loaded-DAC obtained at different reaction time (0–60 h) were evaluated by the diameter of inhibition zones, and the results are shown in Fig. 7 and Table 1.
As can be seen from Fig. 7, the AgNPs-loaded-DAC obtained at different reaction time all showed obvious inhibition zones around the samples, indicating that the AgNPs-loaded-DAC were able to inhibit bacterial growth significantly. When Na3C6H5O7 was not added and the reaction time was 2.5 h, it can be concluded from Table 1 that the diameter of the inhibition zone of the samples against E. coli and S. aureus were 3.50 mm and 6.40 mm, respectively. With the increase of reaction time, more AgNPs were formed with the presence of Na3C6H5O7, and the diameter of inhibition zones increased gradually. When the reaction time was 60 h, the diameters of the inhibition zones of the samples against E. coli and S. aureus reached 4.90 mm and 7.35 mm, respectively. Compared with that of 2.5 h samples, the antibacterial properties of the sample (60 h) against E. coli and S. aureus increased by 40.00% and 14.85%. It was found that the shape transformation of the AgNPs from spherical to trigonal induced by Na3C6H5O7 did not affect their antibacterial properties. The increase of loading amount of AgNPs could improve the antibacterial properties. The AgNPs-loaded-DAC exhibited high antibacterial ability against both E. coli and S. aureus, indicating its broad-spectrum antibacterial properties.
Conclusions
In this study, bleached softwood pulp fibers were periodate oxidized to get DAC. Then, AgNPs were successfully synthesized and loaded on the DAC surface to prepare antibacterial materials. AgNPs were in-situ reduced on the DAC surface with AgNO3 as silver precursor and DAC as the reductant and stabilizer without using any additional reductant. It was found that the characteristic absorption peak of aldehyde group appeared in the infrared spectrum of DAC at 1730 cm−1, which indicated that hydroxyl groups of coniferous cellulose was selectively oxidized to aldehyde groups by NaIO4. In the UV–Vis absorption spectrum, the characteristic absorption peak of AgNPs at 428 nm appeared in the DAC. It was observed by SEM that the spherical AgNPs were distributed uniformly on the DAC surface without obvious flocculation, and the average size of AgNPs obtained by 2.5 h of reaction was about 50 nm. In addition, the shape change of AgNPs on the DAC surface was studied by adding Na3C6H5O7. With the increase of reaction time, more AgNPs were formed. When the reaction time was 60 h, the spherical AgNPs were gradually grown and transformed into triangular prism under the induction of Na3C6H5O7. The shape transformation of AgNPs from sphere to triangular did not show negative influence on their antibacterial performance. The AgNPs-loaded-DAC exhibited high antibacterial ability against E. coli and S. aureus, and showed inhibition zones of 4.90 mm and 7.35 mm (60 h), respectively, which increased by 40.00% and 14.85% compared with spherical AgNPs (2.5 h) due to the higher loading amount.
References
Ahmad I, Kamal T, Khan SB, Asiri AM (2016) An Efficient and easily retrievable dip catalyst based on silver-nanoparticles/chitosan-coated cellulose filter paper. Cellulose 23(6):1–12. https://doi.org/10.1007/s10570-016-1053-4
Alavi M, Nokhodchi A (2020) An overview on antimicrobial and wound healing properties of ZnO nanobiofilms, hydrogels, and bionanocomposites based on cellulose, chitosan, and alginate polymers. Carbohyd Polym 227:115349–115349. https://doi.org/10.1016/j.carbpol.2019.115349
Anderson R, Buscall R, Eldridge R, Mulvaney P, Scales P (2014) Concentrated synthesis of metal nanoparticles in water. RSC Adv 4(60):31914–31925. https://doi.org/10.1039/c4ra04223a
Bahrami A, Delshadi R, Jafari SM (2020) Active delivery of antimicrobial nanoparticles into microbial cells through surface functionalization strategies. Trends Food Sci Technol 99:217–228. https://doi.org/10.1016/j.tifs.2020.03.008
Bansal M, Chauhan GS, Kaushik A, Sharma A (2016) Extraction and functionalization of bagasse cellulose nanofibres to Schiff-based antimicrobial membranes. Int J Biol Macromol 91:887–894. https://doi.org/10.1016/j.ijbiomac.2016.06.045
Behnam A et al (2018) Direct covalent attachment of silver nanoparticles on radical-rich plasma polymer films for antibacterial applications. J Mater Chem B 6(37):5845–5853. https://doi.org/10.1039/C8TB01363B
Chen H, Xu Q-h (2017) Research progress of nanocellulose-based antibacterial materials. Paper Chem 29(2):4–7. https://doi.org/10.3969/j.issn.1007-2225.2017.02.002
Chen Q, Kang M, Zheng X, Zhou L, Wang P (2018) Application progress of nano-cellulose in paper-based functional materials. Chem Ind Forest Prod 38(4):5–12. https://doi.org/10.3969/j.issn.0253-2417.2018.04.001
Chen Z, Chang JW, Balasanthiran C, Milner ST, Rioux RM (2019) Anisotropic growth of silver nanoparticles is kinetically controlled by polyvinylpyrrolidone binding. J Am Chem Soc 141(10):4328–4337. https://doi.org/10.1021/jacs.8b11295
Cheng F, He J, Yan T, Liu C, Wei X, Li J, Huang Y (2016) Antibacterial and hemostatic composite gauze of N,O-carboxymethyl chitosan/oxidized regenerated cellulose. RSC Adv 6(97):94429–94436. https://doi.org/10.1039/C6RA15983D
Chook SW, Chia CH, Zakaria S, Ayob MK, Chee KL, Jamal R, Rahman RMFRA (2012) Antibacterial performance of Ag nanoparticles and AgGO nanocomposites prepared via rapid microwave-assisted synthesis method. Nanoscale Res Lett 7(1):541–548. https://doi.org/10.1186/1556-276X-7-541
Ding S (2010) Natural anti-bacterial agent and its application. J Tex Sci Tech. 000(005):50–53. https://doi.org/10.3969/j.issn.1009-3028.2010.05.017
Dong F, Li S (2018) Wound dressings based on Chitosan-Dialdehyde cellulose nanocrystals-silver nanoparticles: mechanical strength, antibacterial activity and cytotoxicity. Polym Basel 10(6):673–686. https://doi.org/10.3390/polym10060673
Gong R, Zhang J, Zhu J, Wang J, Lai Q, Bo J (2013) Loofah sponge activated by periodate oxidation as a carrier for covalent immobilization of lipase. Korean J Chem Eng 30(8):1620–1625. https://doi.org/10.1007/s11814-013-0102-z
He Y, Li H, Fei X, Peng L (2020) Carboxymethyl cellulose/cellulose nanocrystals immobilized silver nanoparticles as an effective coating to improve barrier and antibacterial properties of paper for food packaging applications. Carbohyd Polym 252:117156–117167. https://doi.org/10.1016/j.carbpol.2020.117156
Hebeish A, Hashem M, El-Hady M, Sharaf S (2013) Development of CMC hydrogels loaded with silver nanoparticles for medical applications. Carbohyd Polym 92(1):407–413. https://doi.org/10.1016/j.carbpol.2012.08.094
Immanuel G, Thangapandiyan S, Prema P (2017) CMC stabilized nano-silver synthesis, characterization and its antibacterial and synergistic effect with broad spectrum antibiotics. Carbohyd Polym 158:141–148. https://doi.org/10.1016/j.carbpol.2016.11.083
Jahan MS, Saeed A, He Z, Ni Y (2011) Jute as raw material for the preparation of microcrystalline cellulose. Cellulose 18(2):451–459. https://doi.org/10.1007/s10570-010-9481-z
Jiang Q, Lin Z, Gu B, Pang C, Wang X (2020) Green synthesis and immobilization of AgNPs by lumpy corn stalk as interlayer filling material for durable antibacterial. Ind Crops Prod 158:112987–112998. https://doi.org/10.1016/j.indcrop.2020.112987
Kang X, Yi L, Deng L, Zeng K, Ruan C (2020) Nanocellulose-based antibacterial composites and their applications in food packaging: a review. Food Sci Tech-Brazil 41(11):317–326. https://doi.org/10.7506/spkx1002-6630-20190509-087
Kango S, Kalia S, Celli A, Njuguna J, Habibi Y, Kumar R (2013) Surface modification of inorganic nanoparticles for development of organic-inorganic nanocomposites—a review. Prog Polym Sci 38(8):1232–1261. https://doi.org/10.1016/j.progpolymsci.2013.02.003
Li N, Luo H (2012) Application and development status of antimicrobial agent. Chem Ind Technol Dev 000(5):30–33. https://doi.org/10.3969/j.issn.1671-9905.2012.05.010
Li X, Bai H, Yang Y, Yoon J, Wang S, Zhang X (2019) Supramolecular antibacterial materials for combatting antibiotic resistance. Adv Mater 31(5):1805091–1805092. https://doi.org/10.1002/adma.201805092
Liu Z, Fatehi P, Sadeghi S, Ni Y (2011) Application of hemicelluloses precipitated via ethanol treatment of pre-hydrolysis liquor in high-yield pulp. Bioresour Technol 102(20):9613–9618. https://doi.org/10.1016/j.biortech.2011.07.049
Liu C, Jiang Z, Mao X, Wu H (2020) Research progress of common antibacterial materials. J xi’an Polytech Univ Nat Sci Ed 34(2):37–46
Maryan AS, Montazer M, Harifi T (2015) Synthesis of nano-silver on cellulosic denim fabric producing yellow colored garment with antibacterial properties. Carbohyd Polym 115(22):568–574. https://doi.org/10.1016/j.carbpol.2014.08.100
Mishra RK, Sabu A, Tiwari SK (2018) Materials chemistry and the futurist eco-friendly applications of nanocellulose: status and prospect. J Saudi Chem Soc 22(8):949–978. https://doi.org/10.1016/j.jscs.2018.02.005
Montazer M, Alimohammadi F, Shamei A, Rahimi MK (2012) In-situ synthesis of nano-silver on cotton using Tollens’ reagent. Carbohyd Polym 87(2):1706–1712. https://doi.org/10.1016/j.carbpol.2011.09.079
Pang X, Chen Y (2015) Study of antibacterial properties of nanometer ZnO. J Electron Packag 4:13–16. https://doi.org/10.3969/j.issn.1674-7100.2014.04.003
Peng F, Ren J-L, Xu F, Bian J, Peng P, Sun R-C (2009) Comparative study of hemicelluloses obtained by graded ethanol precipitation from sugarcane bagasse. J Agric Food Chem 57(14):6305–6317. https://doi.org/10.1021/jf900986b
Prema P, Thangapandiyan S, Immanuel G (2017) CMC stabilized nano-silver synthesis, characterization and its antibacterial and synergistic effect with broad spectrum antibiotics. Carbohyd Polym 158(9):141–148. https://doi.org/10.1016/j.carbpol.2016.11.083
Roberto Y-M, Muñoz-Bonilla A, Jesús-Tellez MAD, Maldonado-Textle H, Guerrero-Santos R (2019) Combinations of antimicrobial polymers with nanomaterials and bioactives to improve biocidal therapies. Polym Basel 11(11):1789–1800. https://doi.org/10.3390/polym11111789
Ruan C, Strømme M, Lindh J (2016) A green and simple approach for one-pot preparation of an efficient palladium adsorbent based on functionalized 2,3-dialdehyde cellulose. Cellulose 23(4):2627–2638. https://doi.org/10.1007/s10570-016-0976-0
Rycenga M et al (2011) Controlling the synthesis and assembly of silver nanostructures for plasmonic applications. Chem Rev 111(6):3669–3712. https://doi.org/10.1021/cr100275d
Shemesh R et al (2015) Antibacterial and antifungal LDPE films for active packaging. Polym Advan Technol 26(1):110–116. https://doi.org/10.1002/pat.3434
Silva AR, Unali G (2011) Controlled silver delivery by silver-cellulose nanocomposites prepared by a one-pot green synthesis assisted by microwaves. Nat Nanotechnol 22(31):315605–315618. https://doi.org/10.1088/0957-4484/22/31/315605
Sun Y, Lü Z (2021) Reserch progress of properties enhanced by quaternary ammonium salt polymers of nano-antibacterial materials. J Wuhan Univ Technol 43(1):12–20. https://doi.org/10.19843/j.cnki.CN42-1779/TQ.202010024
Sun B, Hou Q, Liu Z, Ni Y (2015) Sodium priodate oxidation of cellulose nanocrystal and its application as a paper wet strength additive. Cellulose 22(2):1135–1146. https://doi.org/10.1007/s10570-015-0575-5
Wei CS, Hua CC, Sarani Z, Min NH, Rahman J (2017) Effective immobilization of silver nanoparticles on a regenerated cellulose-chitosan composite membrane and its antibacterial activity. New J Chem 41(12):5061–5065. https://doi.org/10.1039/c7nj00319f
Xu Y, Li S, Chang P (2017) A facile method to produce silver nanoparticle-loaded regenerated cellulose membranes via the reduction of silver nitrate in a homogeneous system. Biore 12(4):9050–9062. https://doi.org/10.15376/biores.12.4.9050-9062
Xu Y, Shi Y, Lei F, Dai L (2020) A novel and green cellulose-based Schiff base-Cu (II) complex and its excellent antibacterial activity. Carbohyd Polym 230:115671–115695. https://doi.org/10.1016/j.carbpol.2019.115671
Yadav C, Maji PK (2018) Synergistic effect of cellulose nanofibres and bioextracts for fabricating high strength sodium alginate based composite biosponges with antibacterial properties. Carbohyd Polym 203:396–408. https://doi.org/10.1016/j.carbpol.2018.09.050
Yu L (2020) Research progress of nano-silver composite antibacterial materials. China Metal Bullet 1026(08):140–141. https://doi.org/10.3969/j.issn.1672-1667.2020.15.071
Yu H et al (2021) Cellulose nanocrystals based clove oil Pickering emulsion for enhanced antibacterial activity. Int J Biol Macromol 170:24–32. https://doi.org/10.1016/j.ijbiomac.2020.12.027
Zhang Q, Na L, Goebl J, Lu Z, Yin Y (2011a) A systematic study of the synthesis of silver nanoplates: is citrate a “magic” reagent. J Am Chem Soc 133(46):18931–18939. https://doi.org/10.1021/ja2080345
Zhang Q, Na L, Goebl J, Lu Z, Yin Y (2011b) A systematic study of the synthesis of silver nanoplates: is citrate a “magic” reagent. J Am Chem Soc 133(46):18931–18939. https://doi.org/10.1021/ja2080345
Zhao X, Zhu J, Li M, Liu Y (2016) Domestic application and development status of anti-bacterial agent. Int Mater Rev 30(07):68–73. https://doi.org/10.11896/j.issn.1005-023X.2016.07.012
Acknowledgments
The Project Supported by the National Natural Science Foundation of China (22178186) and the Foundation (No. 201730) of State Key Laboratory of Biobased Material and Green Papermaking, Qilu University of Technology, Shandong Academy of Sciences.
Funding
This study has no financial interest.
Author information
Authors and Affiliations
Contributions
All authors contributed as the main contributors of this work. All authors performed the literature search and analysis: all authors had drafted, revised the work and approved the final paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Du, P., Xu, Y., Shi, Y. et al. Preparation and shape change of silver nanoparticles (AgNPs) loaded on the dialdehyde cellulose by in-situ synthesis method. Cellulose 29, 6831–6843 (2022). https://doi.org/10.1007/s10570-022-04692-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-022-04692-6