Abstract
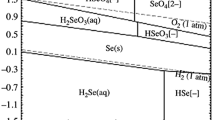
Selenium distillation residue (SDR), inevitably generated in the treatment of refining selenium by vacuum distillation of crude selenium, is a kind of solid residue with low utilization yet massive recycling value. It contains a diverse and complicated component of selenide, telluride, and high-level precious metals. In this study, oxidative acid leaching at atmospheric pressure is adopted to systematically treat SDR to separate selenium, tellurium, copper, and effectively enrich gold and silver. The leaching method in H2SO4–H2O2 mixed solution under an oxygen atmosphere was employed to investigate the effects of H2SO4 concentration, H2O2 concentration, oxygen flow rate, liquid–solid ratio, and leaching time on the recovery of Se, Te, and Cu. Under the optimal conditions, the recoveries of Se, Te, and Cu, respectively, achieved 87.96%, 87.02%, and 98.82% while Au, Ag, and Pb were enriched in the leaching residue, the contents were 0.42%, 16.5%, and 24.27%, respectively. Thermodynamic analysis indicated that insoluble Cu2Se could be oxidized to soluble H2SeO3 in the pH range of − 1.5 to 0, and the potential 0.83 V. XRD and EPMA results outline that CuSe, AgSe, CuTe, and AgTe exist in leaching residue resulting in incomplete leaching of Se, Te, and Cu. Besides, the existence of core–shell grain of AgCl@AgSe is another main reason for the incomplete leaching of selenium. Oxygen partial pressure can enhance mass transfer by diffusion and open the envelope phase. The leaching percentages of Se, Te, and Cu at 0.5 MPa are measured as 93.97%, 99.83%, and 97.42%, respectively.
Graphical Abstract











Similar content being viewed by others
References
Lina J, Valentina K, Nijolė D et al (2021) Ag–In–Se films on flexible architectural textiles as efficient material for optoelectronics applications: a preliminary study. Thin Solid Films 721:138566. https://doi.org/10.1016/j.tsf.2021.138566
Mishra PK, Babu PD, Ravikumar G et al (2015) Possible room temperature ferromagnetism in silicon doped tellurium semiconductor. J Alloys Compd 639:5–8. https://doi.org/10.1016/j.jallcom.2015.03.084
Hadar I, Song TB, Ke W et al (2019) Modern processing and insights on selenium solar cells: the world’s first photovoltaic device. Adv Energy Mater 9(16):1802766. https://doi.org/10.1002/aenm.201802766
Kang HK, Park SH, Jun DH et al (2011) Te doping in the GaAs tunnel junction for GaInP/GaAs tandem solar cells. Semicond Sci Technol 26135(7):75009–75005. https://doi.org/10.1088/0268-1242/26/7/075009
Zhang L, Xu Z (2018) A critical review of material flow, recycling technologies, challenges and future strategy for scattered metals from minerals to wastes. J Clean Prod 202(20):1001–1025. https://doi.org/10.1016/j.jclepro.2018.08.073
Spinks SC, Parnell J, Bellis D, Still J (2016) Remobilization and mineralization of selenium–tellurium in metamorphosed red beds: evidence from the Munster Basin, Ireland. Ore Geol Rev 72:114–127. https://doi.org/10.1016/j.oregeorev.2015.07.007
Moss RL, Tzimas E, Kara H, et al (2011) Critical metals in strategic energy technologies. assessing rare metals as supply-chain bottlenecks in low-carbon energy technologies. Publications Office of the European Union. https://doi.org/10.2790/35600
Shi GC, Liao YL, Su BW et al (2020) Kinetics of copper extraction from copper smelting slag by pressure oxidative leaching with sulfuric acid. Sep Purif Technol 241:10. https://doi.org/10.1016/j.seppur.2020.116699
Makuei FM, Senanayake G (2018) Extraction of tellurium from lead and copper bearing feed materials and interim metallurgical products—a short review. Miner Eng 115:79–87. https://doi.org/10.1016/j.mineng.2017.10.013
Ding Y, Zhang S, Liu B et al (2017) Integrated process for recycling copper anode slime from electronic waste smelting. Clean Prod 165(1):48–56. https://doi.org/10.1016/j.jclepro.2017.07.094
Khanlarian M, Rashchi F, Saba M (2019) A modified sulfation-roasting-leaching process for recovering Se, Cu, and Ag from copper anode slimes at a lower temperature. J Environ Manage 235:303–309. https://doi.org/10.1016/j.jenvman.2019.01.079
Lu DK, Chang YF, Yang HY et al (2015) Sequential removal of selenium and tellurium from copper anode slime with high nickel content. Trans Nonferr Metals Soc China 25(4):1307–1314. https://doi.org/10.1016/S1003-6326(15)63729-3
Li D, Guo XY, Xu ZP et al (2015) Leaching behavior of metals from copper anode slime using an alkali fusion-leaching process. Hydrometallurgy 157:9–12. https://doi.org/10.1016/j.hydromet.2015.07.008
Hyvärinen O, Lindroos L, Yllö E (1989) Recovering selenium from copper refinery slimes. JOM 41(7):42–43. https://doi.org/10.1007/BF03220271
Li D, Guo X, Xu Z et al (2016) Metal values separation from residue generated in alkali fusion-leaching of copper anode slime. Hydrometallurgy 165:290–294. https://doi.org/10.1016/j.hydromet.2016.01.021
Lee JC, Kurniawan K, Chung KW et al (2021) Metallurgical process for total recovery of all constituent metals from copper anode slimes: a review of established technologies and current progress. Met Mater Int 27(2):2160–2187. https://doi.org/10.1007/s12540-020-00716-7
Zhang Y, Hua Y, Gao X et al (2016) Recovery of zinc from a low-grade zinc oxide ore with high silicon by sulfuric acid curing and water leaching. Hydrometallurgy 166:16–21. https://doi.org/10.1016/j.hydromet.2016.08.010
Liu GQ, Wu YF, Tang AJ et al (2020) Recovery of scattered and precious metals from copper anode slime by hydrometallurgy: a review. Hydrometallurgy 197(8):105460. https://doi.org/10.1016/j.hydromet.2020.105460
Chen A, Peng Z, Hwang JY et al (2015) Recovery of silver and gold from copper anode slimes. JOM 67(2):493–502. https://doi.org/10.1007/s11837-014-1114-9
Han JW, Liang C, Liu W et al (2017) Pretreatment of tin anode slime using alkaline pressure oxidative leaching. Sep Purif Technol 174:389–395. https://doi.org/10.1016/j.seppur.2016.10.056
Liu WF, Yang TZ, Zhang DC et al (2014) Pretreatment of copper anode slime with alkaline pressure oxidative leaching. Int J Miner Process 128:48–54. https://doi.org/10.1016/j.minpro.2014.03.002
Kilic Y, Kartal G, Timur S (2013) An investigation of copper and selenium recovery from copper anode slimes. Int J Miner Process 124:75–82. https://doi.org/10.1016/j.minpro.2013.04.006
Hait J, Jana RK, Sanyal SK (2004) Mineralogical characteristics of copper electrorefining anode slime and its leached residues. Ind Eng Chem Res 43(9):2079–2087. https://doi.org/10.1021/ie0305465
Abdollahy M, Shafaei SZ (2004) Optimized leaching conditions for selenium from Sar-Cheshmeh copper anode slimes. Iran J Chem Chem Eng Int English Ed 23(2):101–108. https://doi.org/10.1016/j.ijpvp.2004.04.005
Seisko S, Aromaa J, Latostenmaa P, et al (2016) Effect of process variables on oxidative pressurized acid leaching of copper electrorefining anode slimes. E3s Web of Conferences. https://doi.org/10.1051/e3sconf/20160801006
Hait J, Jana RK, Kumar V et al (2002) Some studies on sulfuric acid leaching of anode slime with additives. Ind Eng Chem Res 41(25):6593–6599. https://doi.org/10.1021/ie020239j
Li XJ, Yang HY, Jin ZN et al (2017) Selenium leaching from copper anode slimes using a nitric acid-sulfuric acid mixture. Metallurgist 61:348–356. https://doi.org/10.1007/s11015-017-0500-2
Rao S, Liu Y, Wang D et al (2021) Pressure leaching of selenium and tellurium from scrap copper anode slimes in sulfuric acid-oxygen media. J Clean Prod. https://doi.org/10.1016/j.jclepro.2020.123989
Li XJ, Yang HY, Jin ZN et al (2017) Extraction of selenium from copper anode slimes in a sealed leaching system. Russ J Non-Ferr Metals 58(4):357–364. https://doi.org/10.3103/S1067821217040174
Hait J, Jana RK, Sanyal SK (2009) Processing of copper electrorefining anode slime: a review. Miner Process Extract Metall 118(4):240–252. https://doi.org/10.1179/174328509X431463
Xiao L, Wang YL, Yu Y et al (2019) Enhanced selective recovery of selenium from anode slime using MnO2 in dilute H2SO4 solution as oxidant. J Clean Prod 209:494–504. https://doi.org/10.1016/j.jclepro.2018.10.144
Dong ZL, Jiang T, Xu B et al (2020) Comprehensive recoveries of selenium, copper, gold, silver and lead from a copper anode slime with a clean and economical hydrometallurgical process. Chem Eng J. https://doi.org/10.1016/j.cej.2020.124762
Cao L, Liao YL, Shi GC et al (2019) Leaching behavior of zinc and copper from zinc refinery residue and filtration performance of pulp under the hydrothermal process. Int J Miner Metall Mater 26(1):21–32. https://doi.org/10.1007/s12613-019-1706-z
Gharabaghi M, Irannajad M, Azadmehr AR (2013) Leaching kinetics of nickel extraction from hazardous waste by sulphuric acid and optimization dissolution conditions. Chem Eng Res Des 91(2):325–331. https://doi.org/10.1016/j.cherd.2012.11.016
Yang HY, Li XJ, Tong LL et al (2018) Leaching kinetics of selenium from copper anode slimes by nitric acid-sulfuric acid mixture. Chinese J Nonferr Metals 28(1):186–192. https://doi.org/10.1016/S1003-6326(18)64652-7
Yin CS, Ji L, Chen XY et al (2020) Efficient leaching of scheelite in sulfuric acid and hydrogen peroxide solution. Hydrometallurgy 192:11. https://doi.org/10.1016/j.hydromet.2020.105292
Acknowledgements
This research was supported by the National Natural Science Foundation of China (U1902221), the Basic Research Plan of Yunnan Province (2019FA020).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
The contributing editor for this article was Zhi Sun.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhen, T., Luo, H., Liu, L. et al. Selective Recovery of Valuable Metals (Se, Te, Cu) from the Selenium Distillation Residue by Sulfuric Acid Oxidative Leaching. J. Sustain. Metall. 8, 1191–1203 (2022). https://doi.org/10.1007/s40831-022-00547-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-022-00547-3