Abstract
Objective
Hepatocellular carcinoma (HCC) is a malignant tumor associated with high morbidity and mortality rates. In many non-prostate solid tumors such as HCC, prostate-specific membrane antigens (PSMA) are overexpressed in tumor-associated endothelial cells. Therefore, the aim of this study was to evaluate the performance of [68Ga]Ga-PSMA-617 PET imaging on HCC with different animal models, including cell line-derived xenografts (CDX) and patient-derived xenografts (PDX), and to explore its mechanisms of function.
Methods
[68Ga]Ga-PSMA-617 was prepared. The expression level of PSMA in two human hepatocellular cancer cells (HepG2 and HuH-7) was evaluated, and the cellular uptakes of [68Ga]Ga-PSMA-617 were assayed. HepG2 and HuH-7 subcutaneous xenograft models, HepG2 orthotopic xenograft models, and four different groups of PDX models were prepared. Preclinical pharmacokinetics and performance of [68Ga]Ga-PSMA-617 were evaluated in different types of HCC xenografts models using small animal PET and biodistribution studies.
Results
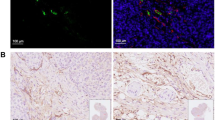
Low PSMA expression level of HepG2 and HuH-7 cells was observed, and the cellular uptake and blocking study confirmed the non-specificity of the PSMA-targeted probe binding to HepG2 and HuH-7 cells. In the subcutaneous xenograft models, the tumor uptakes at 0.5 h were 0.76 ± 0.12%ID/g (HepG2 tumors) and 0.78 ± 0.08%ID/g (HuH-7 tumors), respectively, which were significantly higher than those of the blocking groups (0.23 ± 0.04%ID/g and 0.20 ± 0.04%ID/g, respectively). In the orthotopic xenograft models, PET images clearly displayed the tumor locations based on the preferential accumulation of [68Ga]Ga-PSMA-617 in tumor tissue versus normal liver tissue, suggesting the possibility of using [68Ga]Ga-PSMA-617 PET imaging to detect primary HCC lesions in deep tissue. In the four different groups of HCC PDX models, PET imaging with [68Ga]Ga-PSMA-617 provided clear tumor uptakes with prominent tumor-to-background contrast, further demonstrating its potential for the clinical imaging of PSMA-positive HCC lesions. The staining of tumor tissue sections with CD31- and PSMA-specific antibodies visualized the tumor-associated blood vessels and PSMA expression on endothelial cells in subcutaneous, orthotopic tissues, and PDX tissues, confirming the imaging with [68Ga]Ga-PSMA-617 might be mediated by targeting tumor associated endothelium.
Conclusion
In this study, in vivo PET on different types of HCC xenograft models illustrated high uptake within tumors, which confirmed that [68Ga]Ga-PSMA-617 PET may be a promising imaging modality for HCC by targeting tumor associated endothelium.








Similar content being viewed by others
References
Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–14. https://doi.org/10.1016/S0140-6736(18)30010-2.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. https://doi.org/10.3322/caac.21492.
Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11(4):317–70. https://doi.org/10.1007/s12072-017-9799-9.
European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. https://doi.org/10.1016/j.jhep.2018.03.019.
Lin CY, Liao CW, Chu LY, Yen KY, Jeng LB, Hsu CN, et al. Predictive value of 18F-FDG PET/CT for vascular invasion in patients with hepatocellular carcinoma before liver transplantation. Clin Nucl Med. 2017;42:e18–e187. https://doi.org/10.1097/RLU.0000000000001545.
Castilla-Lièvre MA, Franco D, Gervais P, Kuhnast B, Agostini H, Marthey L, Désarnaud S, Helal BO. Diagnostic value of combining 11C-choline and 18F-FDG PET/CT in hepatocellular carcinoma. Eur J Nucl Med Mol Imaging. 2016;43(5):852–9. https://doi.org/10.1007/s00259-015-3241-0.
Chotipanich C, Kunawudhi A, Promteangtrong C, Tungsuppawattanakit P, Sricharunrat T, Wongsa P. Diagnosis of hepatocellular carcinoma using C11 CHOLINE PET/CT: comparison with F18 FDG, contrast enhanced MRI and MDCT. Asian Pac J Cancer Prev. 2016;17:3569–73.
Filippi L, Schillaci O, Bagni O. Recent advances in PET probes for hepatocellular carcinoma characterization. Exp Rev Med Devices. 2019;16(5):341–50. https://doi.org/10.1080/17434440.2019.1608817.
Zhou F, Shang W, Yu X, Tian J. Glypican-3: a promising biomarker for hepatocellular carcinoma diagnosis and treatment. Med Res Rev. 2018;38(2):741–67. https://doi.org/10.1002/med.21455.
Xu H, Tang Y, Zhao Y, Wang F, Gao X, Deng D, et al. SPECT imaging of hepatocellular carcinoma detection by the GPC3 receptor. Mol Pharm. 2021;18(5):2082–90. https://doi.org/10.1021/acs.molpharmaceut.1c00060.
Sham JG, Kievit FM, Grierson JR, Chiarelli PA, Miyaoka RS, Zhang M, et al. Glypican-3-targeting F(ab')2 for 89Zr PET of hepatocellular carcinoma. J Nucl Med. 2014;55(12):2032–7. https://doi.org/10.2967/jnumed.114.145102.
Labadie KP, Ludwig AD, Lehnert AL, Hamlin DK, Kenoyer AL, Sullivan KM, et al. Glypican-3 targeted delivery of 89Zr and 90Y as a theranostic radionuclide platform for hepatocellular carcinoma. Sci Rep. 2021;11(1):3731. https://doi.org/10.1038/s41598-021-82172-w.
An S, Zhang D, Zhang Y, Wang C, Shi L, Wei W, Huang G, Liu J. GPC3-targeted immunoPET imaging of hepatocellular carcinomas. Eur J Nucl Med Mol Imaging. 2022. https://doi.org/10.1007/s00259-022-05723-x.
Bacich DJ, Pinto JT, Tong WP, Heston WD. Cloning, expression, genomic localization, and enzymatic activities of the mouse homolog of prostate-specific membrane antigen/NAALADase/folate hydrolase. Mamm Genome. 2001;12(2):117–23. https://doi.org/10.1007/s003350010240.
Ross JS, Sheehan CE, Fisher HA, Kaufman RP Jr, Kaur P, Gray K, et al. Correlation of primary tumor prostate-specific membrane antigen expression with disease recurrence in prostate cancer. Clin Cancer Res. 2003;9:6357–62.
Elsasser-Beile U, Buhler P, Wolf P. Targeted therapies for prostate cancer against the prostate specific membrane antigen. Curr Drug Targets. 2009;10:118–25. https://doi.org/10.2174/138945009787354601.
Bühler P, Wolf P, Elsässer-Beile U. Targeting the prostate-specific membrane antigen for prostate cancer therapy. Immunotherapy. 2009;1:471–81. https://doi.org/10.2217/imt.09.17.
Slovin SF. Targeting novel antigens for prostate cancer treatment: Focus on prostate-specific membrane antigen. Expert Opin Ther Targets. 2005;9:561–70. https://doi.org/10.1517/14728222.9.3.561.
Chang SS, Reuter VE, Heston WD, Bander NH, Grauer LS, Gaudin PB. Five different anti-prostate- specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res. 1999;59:3192–8.
Wernicke AG, Varma S, Greenwood EA, Christos PJ, Chao KS, Liu H, et al. Prostate-specific membrane antigen expression in tumor-associated vasculature of breast cancers. APMIS. 2014;122(6):482–9. https://doi.org/10.1111/apm.12195.
Baccala A, Sercia L, Li J, Heston W, Zhou M. Expression of prostate-specific membrane antigen in tumor-associated neovasculature of renal neoplasms. Urology. 2007;70(2):385–90. https://doi.org/10.1016/j.urology.2007.03.025.
Haffner MC, Kronberger IE, Ross JS, Sheehan CE, Zitt M, Mühlmann G, et al. Prostate-specific membrane antigen expression in the neovasculature of gastric and colorectal cancers. Hum Pathol. 2009;40(12):1754–61. https://doi.org/10.1016/j.humpath.2009.06.003.
Wang HL, Wang SS, Song WH, Pan Y, Yu HP, Si TG, et al. Expression of prostate-specific membrane antigen in lung cancer cells and tumor neovasculature endothelial cells and its clinical significance. PLoS ONE. 2015;10(5):e0125924. https://doi.org/10.1371/journal.pone.0125924.
Kasoha M, Unger C, Solomayer EF, Bohle RM, Zaharia C, Khreich F, et al. Prostate-specific membrane antigen (PSMA) expression in breast cancer and its metastases. Clin Exp Metastasis. 2017;34(8):479–90. https://doi.org/10.1007/s10585-018-9878-x.
Nimmagadda S, Pullambhatla M, Chen Y, Parsana P, Lisok A, Chatterjee S, et al. Low-level endogenous PSMA expression in nonprostatic tumor xenografts is sufficient for in vivo tumor targeting and imaging. J Nucl Med. 2018;59(3):486–93. https://doi.org/10.2967/jnumed.117.191221.
Denmeade SR, Mhaka AM, Rosen DM, Brennen WN, Dalrymple S, Dach I, et al. Engineering a prostate-specific membrane antigen-activated tumor endothelial cell prodrug for cancer therapy. Sci Transl Med. 2012;4(140):140ra86. https://doi.org/10.1126/scitranslmed.3003886.
Patel D, Loh H, Le K, Stevanovic A, Mansberg R. Incidental detection of hepatocellular carcinoma on 68Ga-labeled prostate-specific membrane antigen PET/CT. Clin Nucl Med. 2017;42(11):881–4. https://doi.org/10.1097/RLU.0000000000001832.
Taneja S, Taneja R, Kashyap V, Jha A, Jena A. 68Ga-PSMA uptake in hepatocellular carcinoma. Clin Nucl Med. 2017;42(1):e69–70. https://doi.org/10.1097/RLU.0000000000001355.
Sasikumar A, Joy A, Nanabala R, Pillai MR, Thomas B, Vikraman KR. (68)Ga-PSMA PET/CT imaging in primary hepatocellular carcinoma. Eur J Nucl Med Mol Imaging. 2016;43(4):795–6. https://doi.org/10.1007/s00259-015-3297-x.
Kuyumcu S, Has-Simsek D, Iliaz R, Sanli Y, Buyukkaya F, Akyuz F, et al. Evidence of prostate-specific membrane antigen expression in hepatocellular carcinoma using 68Ga-PSMA PET/CT. Clin Nucl Med. 2019;44(9):702–6. https://doi.org/10.1097/RLU.0000000000002701.
Kesler M, Levine C, Hershkovitz D, Mishani E, Menachem Y, Lerman H, et al. 68Ga-PSMA is a novel PET-CT tracer for imaging of hepatocellular carcinoma: a prospective pilot study. J Nucl Med. 2019;60(2):185–91. https://doi.org/10.2967/jnumed.118.214833.
Schmittgen TD, Zakrajsek BA, Hill RE, Liu Q, Reeves JJ, Axford PD, et al. Expression pattern of mouse homolog of prostate-specific membrane antigen (FOLH1) in the transgenic adenocarcinoma of the mouse prostate model. Prostate. 2003;55(4):308–16. https://doi.org/10.1002/pros.10241.
Bass LA, Wang M, Welch MJ, Anderson CJ. In vivo transchelation of copper-64 from TETA-octreotide to superoxide dismutase in rat liver. Bioconjug Chem. 2000;11(4):527–32. https://doi.org/10.1021/bc990167l.
Rylova SN, Stoykow C, Del Pozzo L, Abiraj K, Tamma ML, Kiefer Y, et al. The somatostatin receptor 2 antagonist 64Cu-NODAGA-JR11 outperforms 64Cu-DOTA-TATE in a mouse xenograft model. PLoS ONE. 2018;13(4):e0195802. https://doi.org/10.1371/journal.pone.0195802.
Hu LY, Bauer N, Knight LM, Li Z, Liu S, Anderson CJ, et al. Characterization and evaluation of (64) Cu-labeled A20FMDV2 conjugates for imaging the integrin αvβ 6. Mol Imaging Biol. 2014;16(4):567–77. https://doi.org/10.1007/s11307-013-0717-9.
Hu B, Li H, Guo W, Sun YF, Zhang X, Tang WG, et al. Establishment of a hepatocellular carcinoma patient-derived xenograft platform and its application in biomarker identification. Int J Cancer. 2020;146(6):1606–17. https://doi.org/10.1002/ijc.32564.
Chun YS, Pawlik TM, Vauthey JN. 8th Edition of the AJCC cancer staging manual: pancreas and hepatobiliary cancers. Ann Surg Oncol. 2018;25(4):845–7. https://doi.org/10.1245/s10434-017-6025-x.
Acknowledgements
We would also like to acknowledge the service provided by Beijing Novel Medical Equipment Ltd. for image acquisition.
Funding
This work was funded by the National Natural Science Foundation of China (No. 82030052).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All experimental schemes were performed under the guidance and approved by the Institutional Animal Care and Use Committee of Tongji Medical College of Huazhong University of Science and Technology. Extensive efforts were made to ensure minimal suffering of the animals used during the study.
Competing interests
Weibo Cai is a scientific advisor, stockholder, and grantee of Focus-X Therapeutics, Inc. All other authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Preclinical Imaging
Supplementary information
ESM 1
(DOCX 6.71 mb)
Rights and permissions
About this article
Cite this article
Lu, Q., Long, Y., Fan, K. et al. PET imaging of hepatocellular carcinoma by targeting tumor-associated endothelium using [68Ga]Ga-PSMA-617. Eur J Nucl Med Mol Imaging 49, 4000–4013 (2022). https://doi.org/10.1007/s00259-022-05884-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-022-05884-9