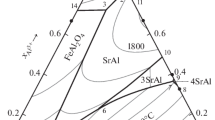
Abstract
The phase boundaries in the Fe–Mg–Al–O, Fe–Ca–Al–O, Fe–Sr–Al–O, and Fe–Ba–Al–O systems at 1600°C are determined using the theory of construction of solubility surfaces of components in a metal. The equilibrium constants of the formation of alkaline-metal aluminates from the components of a metallic melt are estimated. The compositions of the nonmetallic inclusions formed upon melt deoxidation and equilibrated with liquid iron are determined. A complex mechanism of deoxidation with the formation of oxide compounds containing aluminum and alkaline-earth metal is found to take place in all systems at 0.01 wt % aluminum dissolved in a metallic melt and an alkaline-earth metal concentration of 0.001 wt %. The deoxidation ability curves of the alkaline-earth metals are constructed for a fixed aluminum concentration [Al] = 0.01 wt %.





Similar content being viewed by others
REFERENCES
M. Breitzmann, H.-J. Engell, and D. Janke, “Refining of steel melts using alkaline earth metals,” Steel Res. Int. 59 (7), 289–294 (1988). https://doi.org/10.1002/srin.198801505
G. A. Irons and X.-P. Tong, “Treatment of steel with alkaline-earth elements,” ISIJ Int. 35 (7), 838–844 (1995). https://doi.org/10.2355/isijinternational.35.838
I. V. Ryabchikov, A. G. Panov, and A. E. Kornienko, “Characteristics of modifiers,” Steel Transl. 37, 516–521 (2007). https://doi.org/10.3103/S0967091207060113
A. A. Deryabin and E. Yu. Berestov, “On the mechanism of steel modification with alkaline-earth metals,” Elektrometallurgiya, No. 6, 35–38 (2008).
Yu. Ya. Skok, “Study of the deoxidation ability of the complex alloys containing alkaline-earth and rare-earth metals,” Protsessy Lit’ya, No. 3 (81), 8–12 (2010).
I. V. Bakin, N. A. Shaburova, I. V. Ryabchikov, V. G. Mizin, B. F. Belov, G. G. Mikhailov, and A. V. Senin, “Experimental study of refining and modification of steel with Si–Ca, Si–Sr, and Si–Ba alloys,” Steel Transl. 49 (8), 543–547 (2019). https://doi.org/10.3103/S0967091219080023
I. V. Ryabchikov, V. G. Mizin, R. G. Usmanov, V. A. Golubtsov, and V. G. Milyuts, “Criteria of quality evaluation of the steel deoxidizers and modifiers,” Stal’, No. 2, 24–27 (2015).
I. B. Provorova, E. V. Rozenberg, K. E. Baranovskii, V. I. Volosatikov, V. A. Rozum, A. N. Karas’, and M. S. Chernyavskaya, “A modifier for the outer-furnace treatment of the steel containing alkaline-earth metals,” Lit’e Metall. 83 (2), 14–18 (2016).
G. G. Mikhailov, O. V. Samoilova, L. A. Makrovets, and L. A. Smirnov, “Thermodynamic modeling of isotherms of oxygen solubility in liquid metal of the Fe–Mg–Al–O system,” Steel Transl. 49 (8), 522–527 (2019). https://doi.org/10.3103/S0967091219080114
G. G. Mikhailov, L. A. Makrovets, and D. A. Vydrin, “Barium as a deoxidizer and modifier of liquid steel,” Vestn. YuUrGU, Ser. Metall. 13 (1), 45–50 (2013).
G. G. Mikhailov, L. A. Makrovets, O. V. Samoilova, and L. A. Smirnov, “Phase equilibria in the liquid steel deoxidized with aluminum and calcium in the presence of magnesium,” Russian Metallurgy (Metally) 2020 (6), 640–648 (2020). https://doi.org/10.1134/S0036029520060130
G. G. Mikhailov, L. A. Makrovets, O. V. Samoilova, and I. V. Bakin, “Thermodynamic analysis of the deoxidation ability of strontium in liquid iron: phase stability diagram in Fe–Sr–O and Fe–Mg–Sr–O systems,” Chern. Metall. Byul. Nauchno-Tekh. Ekon. Inf. 75 (12), 1366–1373 (2019). https://doi.org/10.32339/0135-5910-2019-12-1366-1372
J.-H. Park and H. Todoroki, “Control of MgO·Al2O3 spinel inclusions in stainless steel,” ISIJ Int. 50 (10), 1333–1346 (2010). https://doi.org/10.2355/isijinternational.50.1333
H. Itoh, M. Hino, and S. Ban-Ya, “Thermodynamics on the formation of non-metallic inclusion of spinel (MgO·Al2O3) in liquid steel,” Tetsu-to-Hagané 84 (2), 85–90 (1998).
Steelmaking Data Sourcebook. Japan Society for the Promotion of Science. The 19th Committee on Steelmaking (Gordon & Breach, New York, 1988).
G. K. Sigworth and J. F. Elliott, “The thermodynamics of liquid dilute iron alloys,” Metal Sci. 8, 298–310 (1974).
G. G. Mikhailov and D. A. Zherebtsov, “On the interaction of calcium and oxygen in liquid iron,” Mater. Sci. Forum 843, 52–61 (2016). https://doi.org/10.4028/www.scientific.net/MSF.843.52
Yu. V. Balkovoi, R. A. Aleev, and V. K. Bakanov, Parameters of the First-Order Interaction in Iron-Based Melts (Chermetinformatsiya, Moscow, 1987).
S. W. Cho and H. Suito, “Assessment of calcium–oxygen equilibrium in liquid iron,” ISIJ Int. 34 (3), 265–269 (1994). https://doi.org/10.2355/isijinternational.34.265
H. Prox, M. Hino, and S. Ban-Ya, “Assessment of Al deoxidation equilibrium in liquid iron,” Tetsu-to-Hagané 83 (12), 773–778 (1997).
H.-Y. Zheng, S.-Q. Guo, M.-R. Qiao, et al., “Study on the modification of inclusions by Ca treatment in GCr18Mo bearing steel,” Adv. Manufact. 7 (4), 438–447 (2019). https://doi.org/10.1007/s40436-019-00266-1
O. V. Samoilova, L. A. Makrovets, and I. V. Bakin, “Phase diagram of a FeO–SrO–BaO system,” Vestn. YuUrGU, Ser. Metall. 20 (3), 5–11 (2020). https://doi.org/10.14529/met200301
O. V. Samoilova, L. A. Makrovets, and E. A. Trofimov, “Thermodynamic simulation of the phase diagram of the Cu2O–Na2O–K2O system,” Moscow University Chemistry Bulletin. 73 (3), 105–110 (2018). https://doi.org/10.3103/S0027131418030057
F. Stein and M. Palm, “Re-determination of transition temperatures in the Fe–Al system by differential thermal analysis,” Int. J. Mater. Res. 98 (7), 580–588 (2007).
Y. Du, J. R. Zhao, C. Zhang, et al., “Thermodynamic modeling of the Fe–Mg–Si system,” J. Mining Metall., Sect. B 43 (1), 39–56 (2007). https://doi.org/10.2298/JMMB0701039D
M. Berg, J. Lee, and D. Sichen, “Study on the equilibrium between liquid iron and calcium vapor,” Metall. Mater. Trans. B 48 (3), 1715–1720 (2017). https://doi.org/10.1007/s11663-017-0946-4
I. S. Kulikov, “Deoxidation of iron with alkaline-earth metals,” Russ. Metall. (Metally), No. 6, 9–15 (1985).
Yu. A. Ageev and S. A. Archugov, “Study of the solubility of alkaline-earth metals in liquid iron and iron-based alloys,” Zh. Fiz. Khim. LIX (4), 838–841 (1985).
B. Song, Q. Han, and C. Zhang, “Solubility of Ba in liquid iron and interaction effect of the third elements,” J. Univ. Sci. Technol. Beijing 7 (2), 82–85 (2000).
J. D. Seo and S. H. Kim, “Thermodynamic assessment of Mg deoxidation reaction of liquid iron and equilibria of [Mg]–[Al]–[O] and [Mg]–[S]–[O],” Steel Res. Int. 71 (4), 101–106 (2000). https://doi.org/10.1002/ srin.200005697
I. H. Jung, S. A. Decterov, and A. D. Pelton, “Computer applications of thermodynamic databases to inclusion engineering,” ISIJ Int. 44 (3), 527–536 (2004). https://doi.org/10.2355/isijinternational.44.527
K. Fujii, T. Nagasaka, and M. Hino, “Activities of the constituents in spinel solid solution and free energies of formation of MgO, MgO·Al2O3,” ISIJ Int. 40 (11), 1059–1066 (2004). https://doi.org/10.2355/isijinternational.40.1059
O. Kubaschewski and C. B. Alcock, Metallurgical Thermochemistry (Pergamon, New York, 1979).
E. Schürmann, U. Braun, and W. Pluschkell, “Investigation on the equilibria between Al–Ca–O-containing iron melts and CaO–Al2O3–FeOn slags,” Steel Res. Int. 69 (9), 355–358 (1998). https://doi.org/10.1002/srin.199805564
K. Taguchi, H. Ono-Nakazato, T. Usui, K. Marukawa, K. Katogi, and H. Kosaka, “Complex deoxidation equilibria of molten iron by aluminum and calcium,” ISIJ Int. 45 (11), 1572–1576 (2005). https://doi.org/10.2355/isijinternational.45.1572
I.-H. Jung, S. A. Decterov, and A. D. Pelton, “Thermodynamic model for deoxidation equilibria in steel,” Metall. Mater. Trans. B 35 (3), 493–507 (2004). https://doi.org/10.1007/s11663-004-0050-4
I. V. Bakin, G. G. Mikhailov, V. A. Golubtsov, I. V. Ryabchikov, and L. E. Dresvyankina, “Methods for improving the efficiency of steel modifying,” Mater. Sci. Forum 946, 215–222 (2019). https://doi.org/10.4028/www.scientific.net/MSF.946.215
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by E. Yablonskaya
Rights and permissions
About this article
Cite this article
Makrovets, L.A., Samoilova, O.V., Bakin, I.V. et al. Thermodynamic Analysis of the Deoxidation Ability of Alkaline-Earth Metals in the Presence of Aluminum. Russ. Metall. 2022, 575–582 (2022). https://doi.org/10.1134/S0036029522060180
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036029522060180