Abstract
A multi-faceted energy intensive technology that can be used for water disinfection and synthesis of electrolysed water (EW) is the need of the hour to achieve a sustainable post COVID 19 water management strategy. Direct sunlight driven processes are legislatively green technologies and hold the key in environmental sustenance. The development of a laboratory proto type reactor powered by a photovoltaic module for the treatment open source river water is described in this paper. This paper reports on the efficacy of the developed proto type technology for multipurpose application namely: (1) the production of Electrolysed water (EW) in a cost efficient manner using direct sunlight and (2) the removal of organic impurity from water using direct sunlight without the use of any photo catalyst or membrane. The prototype reactor utilizes chemical spray pyrolysis deposited highly photo-conducting indium sulphide thin films grown on fluorine doped tin oxide (F:SnO2) substrate (coated using chemical spray pyrolysis technique in-house) as the photo electrode. Dissolved organic matter arising in river water has distinctive fluorescence properties, and this research has utilized it to identify dissolved organic substances in both random samples and treated water. The work proves that photovoltaic module powered electrolytic reactors consisting of In2S3 electrodes can be used for treatment of river water. A diaphragm free, energy intensive route for the production of electrolysed water with the use of non-hazardous NaCl as the electrolyte has been demonstrated here. We conclude that In2S3 electrodes can be used for non-photo catalytic reduction of humic-derived impurities in river water. These results are also encouraging on the prospects of treating Nitrates present in the river water. The likes of techniques as described in this report that do not use photo catalyst or membranes may pave way for real time photovoltaic module powered floating reactors that can decontaminate water bodies on a large scale. The technique used by us demonstrates that a chlorine free route can be optimized for the synthesis of EW eliminating the production of large amounts of wastewater with high levels of biological oxygen demand (BOD).
Similar content being viewed by others
INTRODUCTION
Water quality is a major economic and environmental issue in both developed and developing countries. Water quality refers to the chemical, physical, biological and radiological characteristics of water [1]. The most common standards used to assess water quality relate to the health of the ecosystem, safety of human contact and drinking water [2]. Any change in the physical and chemical characteristics will result in the alteration in water quality and affect the flora, fauna and human population associated with it. Water has been responsible for big epidemics in the world like typhus and cholera. W.H.O. recognized the disinfection as one of the most important barriers for protection of public health [2]. Several important technologies on water disinfection have been reported by using membrane technology, UV irradiation, reverse osmosis, photo-catalysis, electrochemical disinfection etc. [3–7]. Electrolytic disinfection is regarded as a disruptive technology because of its benefits [8–10]. The cost of the electrode system in this technology has kept electrolytic disinfection unaffordable.
Electrolyzed water has been used industrially as one of the useful sanitizers in food, aquaculture, agriculture, medical, and energy industry [11]. Electrolysis of water results in the formation of a variety of chemical species such as hypochlorous acid, chlorine gas, hypochlorite ion and hydrochloric acid at the anode’s side. This mixture of acid, gas, ion and liquid released at the anode side is called Electrolysed water (EW). EW is of three forms—in acidic form, a neutral pH form, and in an alkaline form. Acidic EW exhibits an acid pH, a high oxidation reduction potential, and high free chlorine concentrations which makes it effective as an antimicrobial agent [12, 13]. It is well known that if the EW generator has a membrane separating the electrodes, highly acidic (pH < 3.0) water will be produced at the anode, and alkaline water (pH ≥ 11.0) will be produced at the cathode [14]. It is questionable if it is possible to generate EW with an effective sanitizing capability through an energy intensive route without the use of membranes. A multi-faceted energy intensive technology that can be used for water disinfection and synthesis of EW is the need of the hour to achieve a sustainable post COVID 19 water management strategy. Direct sunlight driven processes are legislatively green technologies and hold the key in environmental sustenance.
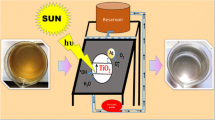
This paper reports on the efficacy of a proto type technology for multipurpose application namely: (1) the production of EW in a cost efficient manner using direct sunlight and (2) the removal of organic impurity from water using direct sunlight without the use of any photo catalyst or a membrane. In this work highly photo conducting In2S3 thin films grown using chemical spray pyrolysis technique is used as the photo electrode and a Cu electrode serves the counter electrode [15–18]. The efficacy of the reactor in treating river water in removing identified contaminants without use of any membrane is verified.
EXPERIMENTAL
The containers used to collect and store water samples were made of polyethylene which are resistant to breakage and cost effective. Storage in bottles made of polyethylene did not significantly alter the silica, sodium, total alkalinity, chloride, and boron content, or the specific conductance, pH, or hardness of the water during a storage period of about 5 months. All of the new bottles were thoroughly cleansed, filled with water, and allowed to soak for 10 days. The soaking removes much of the water-soluble material from the container surface. A sample consisted of 2 L which was sufficient for the normal routine chemical analysis. Seven study sites were selected along the flow terrain of the river. Water samples (2 L) were collected from the seven sites. During collection, the bottle was kept under the surface of the water, so as to eliminate the impurities floating on the surface. Care was taken to avoid bubbling during sampling.
To develop electrodes for the electrolysis unit spray pyrolysis method was used. A solution containing the mixture of indium chloride (InCl3) and thiourea (CS(NH2)2) was used as the precursor solution for spraying. Fluorinedoped SnO2 coated glass slides, with dimensions of 37 × 12 × 1.4 mm3, were used as the substrates for film deposition. The In/S ratio in the solution was fixed by using the molar concentration of InCl3 and CS(NH2)2 in the ratio 1.2 : 8. Total volume of the solution sprayed was 20 mL and the rate of spray was 4 mL/min. Detailed reports on the preparation and characterization of In2S3 and their photosensitivity have been reported by us previously [15, 16].
An in-house fabricated electrolysis unit was used for water treatment. The unit has previously been used for water and nutrient recovery from urine [19]. The unit consisted of a 350 mL cylindrical glass jar which served as the water storage tank. Indium sulphide thin film spray coated over the fluorine doped SnO2 substrate with a surface area of 12.56 cm2 served as the photo-anode. A Cu electrode (98% pure) of the same area was used as the cathode. These two electrodes were separated by a distance of 0.5 cm and powered by a 12 V, 0.5 A c : Si solar panel. The current rating of 0.04 A/cm2 was maintained during the electrolysis process. Electrolyte solution made from 0.02 M NaCl was mixed with the test water sample during the treatment process. The proto-type unit was maintained at 28 ± 2°C during the electrolysis. The unit was loaded with the test solution and placed under direct sunlight. The treated water was extracted over time and analysed.
Fluorescence Spectroscopy is commonly used in the study of dissolved organic matter in natural water. It is a rapid, reagent less technique that requires little sample preparation. [20–22]. Fluorescence Spectrum was generated using a JASCO International Ltd. Spectrophotometer (Model no. FP8000). The emission and excitation slit widths were set at 5 nm. The scan rate for emission was 2000 nm/min. The sensitivity of the instrument was selected to High (Maximum gain) for data collection. Fluorescence Spectrum was recorded for the samples after solar treatment for 5 and 10 h respectively.
RESULTS AND DISCUSSION
Table 1 presents the results of the water quality test taken for the random samples collected from the study sites. One of the critical observations was the presence of E. coli form bacteria in the water all along the basins. Only for samples taken from the upstream point where the water collected was from within the forest sanctuary area E. coli bacteria was absent. We have quantified the pH, Turbidity, fluoride, chloride and Nitrate content in the samples along the basin. The presence of Nitrates in the random test sample is indicative of the effect of agricultural activities on the river sides. The origin of nitrates in the river water may be assigned to activities like fertilizers, livestock facilities, septic systems, natural deposits and household waste flowing into the river.
Figure 1 shows the water fluorescence under excitation of 300 nm, for water samples collected at seven different basins of the river. The spectrum shows a relatively sharp emission with peak emission intensity at a wavelength of ~330 nm. For all samples there was broad emission ranging from 350 to 550 nm. Gaussian peak fit program was used to fit the emission spectrum of the samples. The results of the peak fit for raw water samples are shown in Table 2. The fit data had a residue value greater than 0.998. We observe consistent excitation/emission pairs from this exercise. Specifically, the λex/λem pair of 300 nm/334 nm is present for all samples. The λex/λem pair of 300 nm/398–400 nm, λex/λem pair of 300 nm/451–458 nm, λex/λem pair of 300 nm/521–532 nm are common for all samples. The sample from the urban location containing a community water pumping station showed distinct λex/λem pairs of 300 nm/388 nm, 300 nm/415 nm, 300 nm/438 nm and 300 nm/470 nm.
The most common fluorophores in natural surface waters are humic-like derived from the breakdown of plant material (peaks C and A) [20]. These humic-like derived fluorophores exhibit fluorescence at excitation/emission wavelengths λex 304–347 nm – λem 405–461 nm (Peak C) and λex 217–261 nm, λem 395–449 nm (Peak A). In addition to humic-like material tryptophan-like and tyrosine-like material as “free” molecules or bound in amino acids and proteins (commonly referred to as peaks T and B respectively), also exhibit fluorescence at distinctive wavelengths in natural waters [21]. Peaks T and B are related to microbial activity and may be transported into a system (allochthonous) or be created by microbial activity within a system (autochthonous) [22]. Tryptophan-like fluorescence (peak T1) occurs in this study at λex/λem 275–296/330–378 nm while tyrosine-like fluorescence (peak B) was not commonly seen and is not addressed in this work. Peak T also has a shorter wavelength excitation/emission pair (named T2) with excitation between λex 216–247 nm and emission between λem 329–378 nm. Tryptophan-like fluorescence may be exhibited by natural waters where tryptophan is present as “free” molecules or else bound in proteins, peptides or humic structures.
Our fluorescence analysis is based on the study of the effect of excitation at 300 nm because for this excitation wavelength we observed a consistent emission at 334 nm which could be used as a reference excitation emission pair. For a certified drinking water sample only the λex/λem – 300 nm/334 nm pair was observed. Under this excitation wavelength the other emissions recorded in the samples could be classified into 4 bands: (1) 388–400 nm, (2) 425–438 nm, (3) 451–470 nm and (4) 521–535 nm. The first two bands could be associated to the peak C and A which are reported to be humic derivatives [23]. The other two bands are not reported for natural surface waters for the excitation of 300 nm in literature. These two fluorescence may correspond to flora specific to the river terrain or to other impurities in the water.
Figure 2 compares the spectrum for water samples collected from Aaratupuzha (urban locality downstream) and subjected to 5 and 10 h of treatment using the solar powered electrolysis unit with the electrodes made with the stoichiometric ratio In/S = 1.2/8. The figure also includes the fluorescence spectra for a branded mineral drinking water sample as reference. This sample yielded only a sharp emission at ~330 nm. The comparison between the spectra of other samples hence proved that the water samples collected from us was contaminated. It is evident from the figure that the relative intensities of the fluorescence from the humic derivatives decreases progressively as the treatment time is increased. The EW was removed through the duct at the top of the unit and was taken for water quality analysis. The water at the bottom of the unit after treatment contained sediments which was removed using the lower duct. It is evident that the fluorescence signals from the treated water samples decrease with processing time. This is due to the removal of the humic derivative in the sedimentation. Table 3 summarizes the results of the water quality test taken for the EW which show that quality improvement has been achieved and demonstrates feasibility of water purification through our prototype.
The technique used by us demonstrates that a chlorine free route can be optimized for the synthesis of EW eliminating the production of large amounts of wastewater with high levels of biological oxygen demand (BOD). The EW was removed through the duct at the top of the unit and was taken for water quality analysis. The technique used by us demonstrates that a chlorine free route can be optimized for the synthesis of EW eliminating the production of large amounts of wastewater with high levels of biological oxygen demand (BOD). As seen in Table 2 the EW obtained after 10 h of treatment has low pH. EW may find several applications in the agriculture and food industry due to its disinfectant action. The fluorescence spectra of the EW did not contain the emissions associated with the humic derivatives identified in the random test sample. The pH of the EW was found to progress from 4.6 to 3.9 at the end of 11 h of electrolysis. Acidic EW can be used as a surface disinfectant for the removal of bacterial contamination. E. coli bacteria has been totally removed as a result of the process. The treated water shows an increase in electrical conductance with treatment time. This may be ascribed to the formation of hypochlorous acid (HOCl) and hydrogen chloride (HCl) as a byproduct of the electrolysis. The BOD removal efficiency after electrolytic treatment was calculated out using the equation:
where C0 and C are the BOD after and before electrolysis. The removal percentage of BOD decreases progressively with time indication fatigue in the working electrodes.
CONCLUSIONS
Dissolved organic matter arising in river water has distinctive fluorescence properties, and this research has utilized it to identify dissolved organic substances in both random samples and treated water. These findings have great significance for integrated river basin management and improving water quality utilizing fluorescence as a water quality management tool which can be implemented economically. The work proves that photovoltaic module powered electrolytic reactors consisting of In2S3 electrodes can be used for treatment of river water. A diaphragm free, energy intensive route for the production of electrolysed water with the use of non-hazardous NaCl has been demonstrated here. Solar detoxification as demonstrated by the work shows that decrease in fluorescence in the treated water samples is a signature of the reduction in the humic-derived fluorescence materials. We conclude that In2S3 electrodes can be used for non-photo catalytic reduction of humic-derived impurities in river water. These results are also encouraging on the prospects of treating Nitrates present in the river water. The likes of techniques as described in this report that do not use photocatalyst may pave way for real time photovoltaic module powered floating reactors that can decontaminate water bodies on a large scale. The technique used by us demonstrates that a chlorine free route can be optimized for the synthesis of EW eliminating the production of large amounts of wastewater with high levels of biological oxygen demand (BOD).
REFERENCES
Guidelines for Drinking-Water Quality, Geneva: World Health Organization, 2011, 4th ed.
Guidelines for Drinking-Water Quality, Geneva: World Health Organization, 2004, 3rd ed., vol. 1.
Madaeni, S.S., The application of membrane technology for water disinfection, Water Res., 1999, vol. 33, no. 2, pp. 301–308.
Alzahrani, S. and Mohammad, A.W., Challenges and trends in membrane technology implementation for produced water treatment: A review, J. Water Process Eng., 2014, vol. 4, pp. 107–133.
Chatterley, C. and Linden, K., Demonstration and evaluation of germicidal UV-LEDs for point-of-use water disinfection, J. Water Health, 2010, vol. 8, no. 3, pp. 479–486.
Gogate, P.R., Application of cavitational reactors for water disinfection: Current status and path forward, J. Environ. Manage., 2007, vol. 85, no. 4, pp. 801–815.
Water Treatment and Disinfection By-Products, Plewa, M.J. and Richardson, S.D., Eds., J. Environ. Sci., 2017, vol. 58, pp. 1–350.
Bergmann, M.E.H., Iourtchouk, T., Schöps, K., and Bouzek, K., New UV irradiation and direct electrolysis-promising methods for water disinfection, Chem. Eng. J., 2002, vol. 85, pp. 111–117.
Bergmann, M.E.H., in Electrochemistry for the Environment, New York: Springer, 2009, pp. 163–204.
Drogui, P., Elmaleh, S., Rumeau, M., Bernard, C., and Rambaud, A., Hydrogen peroxide production by water electrolysis: Application to disinfection, J. Appl. Electrochem., 2001, vol. 31, no. 8, pp. 877–882.
Setareh, G. and Shiroodi Mahmoudreza Ovissipour, in Postharvest Disinfection of Fruits and Vegetables, New York: Academic, 2018, pp. 67–89.
O’Bryan, C.A., Pendleton, S.J., Ricke, S.C., and Crandall, P.G., in Postharvest Disinfection of Fruits and Vegetables, New York: Academic, 2018, pp. 195–212.
Sapers, G.M., in The Produce Contamination Problem Causes and Solutions Food Science and Technology, New York: Elsevier, 2009, 2nd ed., pp. 393–424.
Bergmann, M.E.H. and Rollin, J., Product and by-product formation in laboratory studies on disinfection electrolysis of water using boron-doped diamond anodes, Catal. Today, 2007, vol. 124, nos. 3–4, pp. 198–203.
Jayakrishnan, R., Sebastian, T., John, T.T., Sudha Kartha, C., and Vijayakumar, K.P., Photoconductivity in sprayed β-In2S3 thin films under sub-band-gap excitation of 1.96 eV, J. Appl. Phys., 2007, vol. 102, 043109.
Jayakrishnan, R., John, T.T., Sudha Kartha, C., Vijayakumar, K.P., Jain Deepthi, Sharath Chandra, L.S., and Ganesan, V., Do the grain boundaries of In2S3 thin films have a role in subband gap photosensitivity to 632.8 nm?, J. Appl. Phys., 2008, vol. 103, 053106.
Jayakrishnan, R., Sebastian, T., Vijayakumar, K.P., and Sudha Kartha, C., Effect of defect bands in β-In2S3 thin films, J. Appl. Phys., 2012, vol. 111, 093714.
Jayakrishnan, R., John, T.T., Sudha Kartha, C., Vijayakumar, K.P., Abe, T., and Kashiwaba, Y., Defect analysis of sprayed β-In2S3 thin films using photoluminescence studies, Semicond. Sci. Technol., 2005, vol. 20, pp. 1162–1168.
Jayakrishnan, R., Sreerev, T.R., and Varma, A., Water and nutrient recovery from urine: A lead up trail using nano-structured In2S3 photo electrodes, Chin. Phys. B, 2021, vol. 30, no. 5, 056103.
Coble, P.G., Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy, Mar. Chem., 1996, no. 4, pp. 325–346.
Hudson, N., Baker, A., Ward, D., Reynolds, D.M., Brunsdon, C., Carliell-Marquet, C., and Browning, S., Can fluorescence spectrometry be used as a surrogate for the Biochemical Oxygen Demand (BOD) test in water quality assessment? An example from South West England, Sci. Total Environ., 2008, vol. 391, pp. 149–158.
Parlanti, E., Wörz, K., Geoffry, L., and Lamotte, M., Dissolved organic matter fluorescence spectroscopy as a tool to estimate biological activity in a coastal zone submitted to anthropogenic inputs, Org. Geochem., 2000, vol. 31, pp. 1765–1781.
Sierra, M.M.D., Giovanela, M., Parlanti, E., and Soriano-Sierra, E.J., Fluorescence fingerprint of fulvic and humic acids from varied origins as viewed by single-scan and excitation/emission matrix techniques, Chemosphere, 2005, pp. 715–733.
Funding
R.J. would also like to thank DoECC, Government of Kerala for financial support vide DoECC/E3/R&D/1751/2017.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declares that he has no conflicts of interest.
About this article
Cite this article
Jayakrishnan, R. Direct Sunlight Driven In2S3 Thin Film Based Water Treatment Proto-Type. J. Water Chem. Technol. 44, 73–78 (2022). https://doi.org/10.3103/S1063455X22020060
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1063455X22020060