Abstract
High activity of metal complex catalytic systems comprising nitrogenous bases and copper sulfates, acetates, and basic carbonate in oxidation of hydrogen sulfide and light mercaptans with atmospheric oxygen was established. Their activities are close to that observed for the chlorine-containing analogs, while no corrosion effect is exerted. An increase in the initial concentration of H2S has negligible effect on its conversion rate. Mercaptans do not affect the H2S oxidation rate, whereas an increase in the hydrogen sulfide concentration decelerates mercaptan oxidation. This is a diffusion-controlled reaction; hence, intensification of mixing of the catalyst with the hydrocarbons affords a 30–40% increase in the demercaptanization efficiency. The efficiencies displayed in demercaptanization processes by the catalytic systems and by the conventional scavengers neutralizing light sulfur impurities were compared.

Similar content being viewed by others
Sulfur impurities contained in fossil hydrocarbons pose a risk of corrosion rupture to pipelines, reduce the service life of equipment, and contribute to environmental deterioration [1, 2]. Especially corrosive are hydrogen sulfide and methyl and ethyl mercaptans, and their high volatility causes environmental hazards. Accordingly, the appropriate standards accepted in the Russian Federation set the limit on the content of hydrogen sulfide, as well as on those of methyl and ethyl mercaptans in the stage of crude oil preprocessing in the fields before transportation, at 20 and 40 ppm, respectively [3]. More stringent requirements are imposed on the hydrogen sulfide content in fuel oil. Specifically, it is limited by 2 ppm [4] and, according to the appropriate technical regulations of the Russian Federation, by 10 ppm in view of high operating temperatures (70–100°C). These temperatures are responsible for significantly increased H2S concentration in the vapor above the liquid, reaching 80–400 ppm in some cases, which level is close to the lethal hydrogen sulfide concentration (713 mg/kg). At the 9.2 ppm H2S concentration in fuel oil its content in the vapor phase may reach 2070 ppm [2], which is thrice the lethal concentration.
At present, the method of oxidative demercaptanization [5, 6] is extensively used in crude oil production and refining. It is based on extraction of hydrogen sulfide and light mercaptans with aqueous alkaline solutions into sulfides and mercaptides. Alkali is regenerated by oxidation of sulfides and mercaptides with oxygen in the presence of cobalt phthalocyanines to di- and polysulfides and elemental sulfur:
Due to low cost, ready availability of extractant, and low consumption of catalyst this method is efficient, though only at low contents of hydrogen sulfide and mercaptans. The degree of removal of mercaptans in the liquid flow regime at high H2S concentrations is low, and large amounts of highly toxic waste are formed, which entails additional costs for their detoxification and disposal.
Sulfhydryl impurities are also removed with the use of various neutralizing reagents (scavengers) [4, 7], which are most effective in removing hydrogen sulfide and light methyl mercaptan but exhibit sharply decreased efficiency with respect to heavier mercaptans (from C2 upwards). Moreover, many components of these agents are highly toxic, which concerns, e.g., such a commonly used scavenger as formaldehyde. It is recognized as a carcinogenic substance [GN (Hygienic Standard) 1.1.725-98], classified as a category 1 highly toxic compound, which is much more hazardous than hydrogen sulfide (MPC of formaldehyde in the air of the working area is 0.5 mg/m3, which is 20 times lower than that of hydrogen sulfide). Another significant drawback suffered by the demercaptanization methods based on treatment with chemical agents is the commonly observed reformation of sulfhydryl impurities under the action of oxygen and water which are always present in tanks containing treated petroleum products [8, 9].
Formaldehyde and alkyl triazine, when consumed at a rate of 2.3 and 1.7 kg per ton, respectively (Table 1, items 4 and 5), afford reducing the hydrogen sulfide content in fuel oil to levels close to those set by the appropriate EU standards already within 1 h. These rates correspond to the scavenger/hydrogen sulfide weight ratio of 23 for the formaldehyde-based scavenger and of 17 for oil-soluble scavenger alkyl triazine.
(Columns 1–3) Mass ratio of fuel oil M-100 to diesel fuel DT Euro-3 is 9 : 1. (Columns 4, 5) Secondary component of the fuel oil from the visbreaking unit. T = 90°C.
Unfortunately, the authors of the above-cited works did not present the time dependence of the residual content of hydrogen sulfide at lower concentrations of the scavenger and at its minimum concentration at the indicated content of H2S. Basing on the stoichiometry of the reaction of hydrogen sulfide with formaldehyde, it can be expected that the residual amount of unreacted formaldehyde will be 16.0 mol, which corresponds to its content of 480 ppm [7]. As mentioned above, high concentrations of formaldehyde in fuel oil are extremely undesirable because of its high toxicity and ability to be oxidized to corrosion-active formic acid [9].
Scavengers based on alkyl triazines surpass in efficiency other scavengers under consideration (Table 1, examples 1, 5). However, even in these cases, a fairly large amount of the active component remains unreacted, and its decomposition during transportation and storage (T = 70–80°C) may lead to the formation of toxic amines [8, 9].
Reagent Koltek PS1615 proved to be the least effective hydrogen sulfide scavenger. For example, at its concentration of 1500 ppm the residual hydrogen sulfide content of <2 ppm is achieved within 3 h. A decrease in the Koltek PS1615 concentration to 700 ppm causes significant deceleration of hydrogen sulfide scavenging. The efficiency of ethyl mercaptan scavenging by agent nos. 1, 2, and 3 is extremely low (Table 1) [7].
An alternative method of treatment of petroleum products to remove hydrogen sulfide and mercaptans is based on their oxidation with atmospheric oxygen to less corrosive and less volatile di- and polysulfides, whose content in natural hydrocarbons is not standardized. The reaction is carried out directly in hydrocarbon media in the presence of metal complex catalysts. This approach does not require the use of a scavenger, excludes the alkaline extraction stage, and, consequently, avoids formation of sulfur-alkaline effluents [10, 11]; it is applicable to various fossil hydrocarbons (crude oil, gas condensate, natural and associated petroleum gas), as well as to their processing products [12, 13].
Most of the known relevant studies are dedicated to oxidation of thiols and performed with the use of model systems [14]. At the same time, the influence of hydrogen sulfide on the proceeding of demercaptanization in the presence of such catalytic systems was not evaluated. Transition metal derivatives such as Cu(I) and Cu(II) chlorides used in those studies can be sources of corrosive hydrogen chloride [15].
With the view of reducing the risk of corrosion, we investigated herein catalysts comprising copper sulfates, acetates, and basic carbonate and also evaluated the influence of hydrogen sulfide on the character of demercaptanization.
EXPERIMENTAL
The following chemicals were used: copper sulfate (CuSO4·5H2O, 99+%, Sigma-Aldrich); copper(II) acetate monohydrate ((CH3COO)2Cu·H2O, 98+%, Sigma-Aldrich); copper(II) carbonate basic (Cu2CO3(OH)2, ≥95%, Sigma-Aldrich); anhydrous copper(II) chloride (CuCl2, 99+%, Sigma-Aldrich); monoethanolamine (MEA, extrapure, C2H7NO, Laverna); ethylenediamine (C2H8N2, ≥99%, Sigma-Aldrich); 1-octanethiol (C8H18S, 98.5+%, Sigma-Aldrich); heptane, HPLC grade (CH3(CH2)5CH3, ≥99%, Sigma-Aldrich); toluene, HPLC grade (C6H5–CH3, 99.9%, Sigma-Aldrich); coconut oil fatty acids diethanolamide (surfactant) ((CH3(CH2)nC( = O)N(CH2CH2OH)2, where n = 14–16, 95+%, trade name Awaxan F, OOO Kompaniya Veresk); hydrogen sulfide from the standard H2S+N2 gas mixture (hydrogen sulfide content 24.9 vol %, OOO Monitoring); heavy fuel oil M-100 from gas condensate (Astrakhan GPP); diesel fuel Euro-3 (L); methanol ([CH3OH, technical grade, GOST (State Standard) 2222-95, TOO Metinghhim]; hexahydro-1,3,5-tris(hydroxyethyl)-s-triazine (C9H21N3O3, 78.0%, Siwei Development Group Ltd.); and distilled water.
The instruments used in this study were as follows:
—potentiometer (METEX M-4660A multimeter, voltage measurement accuracy ±(0.05% + 3dgts);
—IONIKS 111.050, EVL-1M3.1 electrodes;
—Varian 3800 gas chromatograph equipped with a 50 m × 0.32 mm × 0.52 μm capillary column HP-5 (Agilent Technologies), 1170 injector, 250°C, flow split ratio 1:40, constant flow (flow rate 3 mL/min), 50°C (8 min), 270°C (10°C/min), pulsed flame photometric detector (S mode), Galaxie 1.9 program; andr
—Crystal-5000 gas chromatograph equipped with a 25 m×0.32 mm Optima 5 column (Macherey-Nagel), with precolumn backpurging, flame photometric detector, Chromatec Analytic software.
Catalytic systems based on copper salts were obtained by reacting the prescribed amounts of copper sulfate, copper(II) acetate monohydrate, copper carbonate basic, and copper(II) chloride with monoethanolamine (MEA) or a MEA/water solution at 40°C under continuous stirring in an air atmosphere. The above-listed chemicals were not subjected to further purification. The compatibility with hydrocarbons was improved by using a nonionic surfactant, coconut oil fatty acids diethanolamide or 1,2-diaminoethane.
The following catalytic systems were obtained:
—K-1: 12 wt % CuCl2; 20 wt % H2O; 68 wt % MEA,
—K-2: 10 wt % CuSO4·5H2O; 8 wt % (CH3COO)2Cu·H2O; 20 wt % H2O; 62 wt % MEA. The surfactant was introduced at a rate of 0.75 g per 100 g of the catalyst.
—Catalytic system K-3 with a lower content of metal salts (10 wt %) was obtained by diluting K-2 containing the prescribed amount of MEA/water solution with the monoethanolamine concentration of 80 wt %, which allowed maintaining the water concentration in the final product (20 wt %).
Catalytic system K-4 comprising basic copper carbonate was composed of 6.2 wt % copper salt, 20 wt % H2O, 71.8 wt % MEA, and 2.0% 1,2-diaminoethane taken for improving the stability of the solution.
All the obtained catalytic systems are solutions stable at room temperature.
The activity of the catalysts in oxidation of H2S and ethyl mercaptans (C2H5SH) was examined using model mixtures of fuel oil, produced from gas condensate, with diesel fuel introduced for decreasing viscosity of the reaction medium. The mixtures were prepared by adding solutions with prescribed concentrations of hydrogen sulfide in diesel fuel (preliminarily deoxygenated) to the initial fuel oil at the weight ratio of 9 : 1 and 9.5 : 0.5. Hydrogen sulfide from the standard H2S+N2 gas mixture with the hydrogen sulfide content of 24.9 vol % was used. Oxidation of the model mixture was carried out at 90°C (typical initial temperature of fuel oil transportation in the storage tank) and 110°C in hermetically sealed glass reactors with a volume of 0.7–0.8 L, equipped with water jackets (up to 90°C) or with flexible heating elements (above 90°C). The volumes of the vapor phase above the solution and of the liquid phase in the reaction zone were within the 0.2–1.0 ratio. The specified amount of the catalyst was introduced as an emulsion in the diesel fuel. Samples of 6–8 mL volumes were periodically pipetted under controlled temperature conditions and dissolved in the same volume of toluene. The content of hydrogen sulfide and mercaptan sulfur in the obtained solutions was determined by the potentiometric technique [16]. EVL-1M3.1 silver/silver chloride electrode was used as a reference electrode, and IONIKS 111.050 sulfide ion-selective electrode, as a measuring electrode. The electrode pair was calibrated in the 50–700 ppm range using toluene solutions of octanethiol.
For selective analysis of hydrogen sulfide and ethyl mercaptan in the liquid phase, these impurities were separated from the fuel oil by passing a helium stream, which was followed by freezing out in a trap at a liquid nitrogen temperature of –196°C; the prescribed volume of heptane was used as a scavenger.
The content of H2S and thiols in the liquid and vapor phases was determined by GLC on a Varian 3800 chromatograph equipped with a 50 m × 0.32 mm HP-5 capillary column (Agilent Technologies) and a flame photometric (S-mode) detector, using helium as a carrier gas. A Crystal-5000 chromatograph equipped with a 25 m × 0.32 mm Optima 5 column (Macherey-Nagel) and a flame photometric detector, with precolumn backpurging, was employed for direct chromatographic analysis of the mixtures of fuel oil solutions in toluene. The Galaxie 1.9 (Varian 3800) and Chromatec Analytic (Crystal-5000) programs were used to control the chromatograph and to collect and process the experimental data.
RESULTS AND DISCUSSION
Table 2 summarizes the data on the influence of the composition of the chlorine-containing and chlorine-free catalysts and of the initial concentration of hydrogen sulfide on demercaptanization of gas condensate fuel oil. The time taken by complete conversion of hydrogen sulfide with the participation of the CuCl2-based catalytic system K-1 does not exceed 4 h and is independent on the initial concentration of H2S. The latter finding indicates the lack of noticeable inhibition by the substrate [17], which could be expected to result from the interaction of hydrogen sulfide with Cu(I) and Cu(II), giving the corresponding sulfides [18], and from withdrawal of the catalyst from the reaction sphere. About the same situation is seen for K-2 system based on copper sulfate and copper acetate, for which the conversion of hydrogen sulfide reached 90% after 4 h at its initial concentration of 128 ppm (Table 2). An increase in the content of hydrogen sulfide causes reduction of the ethyl mercaptan consumption rate. For example, after 4 h at an initial concentration [H2S]0 = 42 ppm the residual content of ethyl mercaptan is negligible, and at [H2S]0 = 128 ppm it is 21 ppm (Table 2). Almost complete exhaustion of mercaptan under these conditions is observed only after 8 h.
The rate of demercaptanization depends on the ratio of the volumes of the vapor phase above the reaction solution (Vg) and of the reaction solution (Vl), since atmospheric oxygen is involved in the reaction. A decrease in the Vg/Vl ratio from 0.4 to 0.2 further decelerates oxidation of ethyl mercaptan, whose residual content after 8 h is 12 ppm (Table 2). With Vg/Vl increasing to >1.0 demercaptanization is accelerated, but oxidation of the fuel oil components may lead to formation of corrosive water-soluble acids and, thereby, to deterioration of the properties of the final product [19]. Therefore, the Vg/Vl = 0.4 ratio seems to be optimal for demercaptanization of fuel oil containing sulfur impurities in the considered concentrations. The results obtained convincingly show that the catalysts with the total content of copper sulfates and acetates or basic carbonate afford nearly 100% conversion of ethyl mercaptan and hydrogen sulfide at the initial concentration of the latter of 128 ppm within less than 8 h. Such treatment times are quite acceptable for commercial implementation of the process of interest.
From the cost reduction perspective, it seemed essential to explore the suitability of the catalysts with lower contents of transition metal salts for the treatment of petroleum products. A decrease in the content of the copper salts leads to deceleration of demercaptanization, most prominent at a high initial concentration of hydrogen sulfide (Table 2). For example, complete conversion of hydrogen sulfide is achieved within >8 h, and that of ethyl mercaptan, within 20 h. This is also acceptable for commercial application, since the process can also be conducted in temporary tanks for storage of commercial products.
The choice of the temperature regime (T = 90°C) in this study was dictated by the process conditions for the production and transportation of fuel oil. At T ≤ 60°C the viscosity of the medium sharply increases, leading to diffusion inhibition of the reactions under study; accordingly, even in temporary tanks the fuel oil is stored at temperatures no lower than 75–80°C. Temperature rising to 110°C in the presence of a catalyst soluble in hydrocarbons sharply accelerates mercaptan oxidation [19], but leads to an increase in acidity of the aqueous extract to pH = 4.0–4.5 and to darkening of the fuel oil due to oxidation of its hydrocarbon components [19].
Another expected adverse effect associated with high temperatures in oxidizing environments (air oxygen, oxidation catalysts) is corrosion of equipment. We showed previously [20] that the catalyst being developed is not corrosive at room temperature [20]. Experiments on exposure of unalloyed carbon steel samples in the reaction medium in the presence of K-2 and K-3 catalysts at 90°C showed that the model samples do not suffer corrosion within 200 h (Fig. 1). This evidences suitability of these metal complex catalysts for commercial application. At the same time, in the presence of copper dichloride-based catalyst K-1, a noticeable corrosion effect is observed under the same conditions (Fig. 1, sample S-1).
The catalytic activity of the systems studied is close to that of their analogs based on Cu(I) chloride [12] within comparable hydrogen sulfide and C2H5SH concentration ranges. This allows avoiding the use of corrosive chlorine-containing components in the catalyst composition.
The method by which the catalyst is introduced into the reaction volume in the case of commercial implementation of the process is essential for demercaptanization efficiency (optimal catalyst consumption rate and time required for achievement of the desired degree of conversion under the specified process conditions) (Figs. 2 and 3). Use of diffuser-confuser type turbulent mixers in the stage of the catalyst introduction into the raw material stream [21] leads to enhancement of the demercaptanization efficiency due to nearly uniform distribution of the catalyst with high surface area of contact of the phases. Also, mass transfer between the liquid and vapor phases is significantly intensified. Under laboratory conditions, a rotor-pulsation apparatus (RPA) was used it the catalyst introduction stage.
Time dependence of the rate of hydrogen sulfide conversion in the presence of K-2 catalyst introduced by different methods and at varied concentration on the kinetics of hydrogen sulfide conversion. Catalytic system K-2; gas phase air; Vg/Vl = 0.4; [H2S]0=128 ppm; 90°C. (1) [K-2] = 75 ppm, stirring in RPA; (2) [K-2] = 118 ppm, stirring with magnetic stirrer; (3) [K-2] = 78 ppm, stirring with magnetic stirrer; and (4) [K-2] = 40 ppm, stirring in RPA.
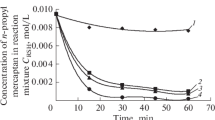
Time dependence of the rate of ethyl mercaptan conversion in the presence of K-2 catalyst introduced by different methods and at varied concentration. Catalytic system K-2, 90°C; gas phase air; Vg/Vl = 0.4; [H2S]0 = 72 ppm. (1) [K-2] = 75 ppm, stirring in RPA and (2) [K-2] = 118 ppm, stirring with magnetic stirrer.
Owing to the resultant circulating flow of liquid through the system of channels, a high degree of dispersion of the catalyst introduced is achieved in the rotor-pulsation unit having the design considered [22]. The reaction mixture was periodically stirred.
The time dependences of the current concentration of hydrogen sulfide at the catalyst contents of 75 ppm (using RPA) and 118 ppm (using magnetic stirrer) are practically identical (Fig. 2, curves 1 and 2). This indicates that the use of RPA in the catalyst introduction stage causes intensification of mass transfer, which allows reducing catalyst concentration without compromising the efficiency of treatment of the raw material. In the process run at the catalyst concentration of 78 ppm under stirring with magnetic stirrer the hydrogen sulfide scavenging noticeably decelerates (Fig. 2, curve 3) in comparison with the case of using RPA (Fig. 2, curve 1). The method of catalyst introduction affects the oxidation of ethyl mercaptan in a similar way (Fig. 3, curves 1 and 2). A decrease in the catalyst concentration to 40 ppm, despite homogenization of the reaction medium in the RPA, leads to decline and total loss of activity of the catalyst (Fig. 2, curve 4). Apparently, this is the threshold concentration for the considered type of the raw material.
In petroleum products treated with the use of scavengers, sulfhydryl impurities can again accumulate if the compounds formed by mercaptan and hydrogen sulfide with the trapping reagent will decompose under the action of oxygen and water which are always present in tanks containing petroleum products [8, 9]. The catalytic demercaptanization method compares favorably with the methods based on treatment with chemical agents in that no reformation of sulfhydryl impurities occurred over time; rather, a slow decrease in the ethyl mercaptan concentration was observed (Table 3).
Thus, based on the results of the studies performed, sequential introduction of air and the catalyst into the stream of the transported raw hydrocarbons or their processing products containing sulfhydryl impurities was proposed for commercial implementation of alkali-free oxidative demercaptanization [23]. It is advisable that environment-unfriendly chlorine-containing catalysts be replaced without loss of efficiency by catalysts comprising nitrogenous bases and copper sulfates, acetates, or carbonates. In this case, hydrogen sulfide and mercaptans are oxidized to sulfanes and di- and polysulfides directly in the pipeline and temporary storage tanks [23]. The unreacted air, after passing the separation stage, is released to the flare or, if in a small amount, dissipates in atmosphere.
CONCLUSIONS
Our study revealed effectiveness of the developed catalytic compositions in oxidation of sulfhydryl derivatives in hydrocarbons. The TON at nearly complete conversion of hydrogen sulfide and ethyl mercaptan in the fuel oil medium can reach ≥80 mol –SH/mol Cu, while the properties of the product are fully compliant with appropriate GOST [24]. The technology being developed can find application in crude oil preprocessing in the fields, as well as in utilization of associated petroleum gas and demercaptanization of fuel oil.
Change history
27 August 2022
An Erratum to this paper has been published: https://doi.org/10.1134/S0965544122070179
REFERENCES
Skuratov, Yu.I., Duka, G.G., and Miziti, A., Vvedenie v ekologicheskuyu khimiyu (Introduction to Ecological Chemistry), Moscow: Vysshaya Shkola, 1994.
Garcia, J., Crude Oil Quality Group Conf., 2005, p. 16. http://www.coqa-inc.org/20050929nalco.pdf
GOST (State Standard) R 51858-2002: Crude Petroleum: General Specifications, 2002.
Sitdikova, A.V., Sadretdinov, I.F., Alyab’ev, A.S., Kovin, A.S., and Kladov, V.S., Online Neftegaz. Delo, 2012, no. 2, pp. 479–489.
Sittig, M., Combining Oxygen and Hydrocarbons for Profit, Houston, TX: Gulf, 1962.
Borisenkova, S.A., Vil’danov, A.F., and Mazgarov, A.M., Mendeleev Chem. J., 1995, vol. 39, no. 5, pp. 92–98.
Dorogochinskaya, V.A., Tonkonogov, B.P., and Romanova, O.V., Trudy Ross. Gos. Univ. Nefti i Gaza im. I.M. Gubkina, 2013, no. 1(270), pp. 58–68.
Popadin, N.V., Nurakhmedova, A.F., Prokhorov, E.M., and Tarakanov, G.V., Vestn. Astrakhan. Gos. Tekh. Univ., 2014, no. 2(58), pp. 31–41.
Mazgarov, A.M., Vil’danov, A.F., Korobkov, F.A., Komleva, T.I., Khrushcheva, I.K., and Nabiev, A.I., Ekspoz. Neft’ Gaz, 2015, no. 5(44), pp. 73–76.
Tarkhanova, I.G. and Smirnov, V.V., RF Patent 2404225, Byull. Izobret., 2010, no. 32, publ. November 20, 2010.
Isichenko, I.V., Gavrilov, Yu.A., Pletneva, I.V., and Silkina, E.N., Patent EA018297, 2013, EAPO Bull., 2013, no. 6, publ. June 6, 2013.
Pletneva, I.V., Gavrilov, Yu.A., Silkina, E.N., and Isichenko, I.V., Vestn. Mosk. Inst. Tonk. Khim. Tekhnol., 2014, vol. 9, no. 2, pp. 99–103.
Gavrilov, Yu.A., Pletneva, I.V., and Silkina, E.N., Russ. Chem. Bull., 2013, vol. 62, pp. 1590–1594. https://doi.org/10.1007/s11172-013-0229-4
Gantman, M.G., Doctoral (Chem.) Dissertation, Moscow: Mosk. Gos. Univ., 2008.
GOST (State Standard) 9.908-85: Unified System of Corrosion and Ageing Protection: Metals and Alloys: Methods for Determination of Corrosion and Corrosion Resistance Indices, Moscow: Izd. Standartov, 1999.
GOST (State Standard) R 52030-2003: Petroleum Products: Potentiometric Method for Determination of Mercaptan Sulfur.
Varfolomeev, S.D., Khimicheskaya enzimologiya (Chemical Enzymology), Moscow: Akademiya, 2005.
Handbuch der präparativen anorganischen Chemie, Brauer, G., Ed., Stuttgart: Ferdinand Enke, 3rd ed., 1978, vol. 4.
Gavrilov, Yu.A., Pletneva, I.V., and Silkina, E.N., Khim. Tekhnol., 2015, no. 5, pp. 282–287.
Gavrilov, Yu.A., Pletneva, I.V., and Silkina, E.N., Tonk. Khim. Tekhnol., 2015, vol. 10, no. 4, pp. 56–63.
Zakharov, V.P., Berlin, A.A., Monakov, Yu.B., and Deberdeev, R.Ya., Fiziko-khimicheskie osnovy protekaniya bystrykh zhidkofaznykh protsessov (Physicochemical Fundamentals of Rapid Liquid Phase Processes), Moscow: Nauka, 2008.
Balabudkin, M.A., Rotorno-pul’satsionnye apparaty v khimiko-farmatsevticheskoi promyshlennosti (Rotor-Pulsation Apparatuses in Chemicopharmaceutical Industry), Moscow: Meditsina, 1983.
Pletneva, I.V., Gavrilov, Yu.A., Silkina, E.N., Isichenko, I.V., and Kulakov, A.V., Development of the process of demercaptanization of natural hydrocarbons, IV Rossiiskaya konferentsiya “Aktual’nye problemy neftekhimii” (IV Russian Conf. “Topical Problems of Petrochemistry”), Zvenigorod, 2012.
GOST (State Standard) 10585-99: Oil Fuel: Fuel Oil: Specifications, 1999.
Funding
This study was supported within the framework of the State Assignment to the Semenov Federal Research Center of Chemical Physics, Russian Academy of Sciences (project no. 0082-2019-0004).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest requiring disclosure in this article.
Additional information
Translated from Neftekhimiya, 2022, Vol. 62, No. 4, pp. 510–518 https://doi.org/10.31857/S0028242122040062.
The original online version of this article was revised due to a retrospective Open Access order.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pletneva, I.V., Gavrilov, Y.A., Moiseeva, N.I. et al. Noncorrosive Metal Complex Catalysts for Oxidation of Hydrogen Sulfide and Mercaptans in Petroleum Products. Pet. Chem. 62, 628–635 (2022). https://doi.org/10.1134/S0965544122050061
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965544122050061