Abstract
Summary
This study includes 1005 men from the Gothenburg part of the Osteoporotic Fracture in Men Study (MrOS). Included are 66 men with anemia (hemoglobin < 130 g/L). The follow-up time was up to 16 years, and the main results are that anemia is associated with all fractures and non-vertebral osteoporotic fractures.
Introduction
Anemia and osteoporotic fractures are conditions that are associated with increased morbidity and mortality. Clinical studies have suggested that anemia can be used as a predictor of future osteoporotic fractures.
Method
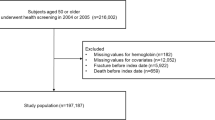
Men from the Osteoporotic Fractures in Men Study (MrOS) Sweden, Gothenburg, with available hemoglobin (Hb) values (n = 1005, median age 75.3 years (SD 3.2)), were included in the current analyses. Of these, 66 suffered from anemia, defined as Hb < 130 g/L. Median follow-up time for fracture was 10.1 years and the longest follow-up time was 16.1 years.
Results
Men with anemia had, at baseline, experienced more falls and had a higher prevalence of diabetes, cancer, prostate cancer, hypertension, and stroke. Anemia was not statistically significantly associated with bone mineral density (BMD). Men with anemia had higher serum levels of fibroblast growth factor 23 (iFGF23) (p < 0.001) and phosphate (p = 0.001) and lower serum levels of testosterone (p < 0.001) and estradiol (p < 0.001). Moreover, men with anemia had an increased risk of any fracture (hazard ratio (HR) 1.97, 95% CI 1.28–3.02) and non-vertebral osteoporotic fracture (HR 2.15, 95% CI 1.18–3.93), after adjustment for age and total hip BMD, in 10 years. The risk for any fracture was increased in 10 and 16 years independently of falls, comorbidities, inflammation, and sex hormones. The age-adjusted risk of hip fracture was increased in men with anemia (HR 2.32, 95% CI 1.06–5.12), in 10 years, although this was no longer statistically significant after further adjustment for total hip BMD.
Conclusions
Anemia is associated with an increased risk for any fracture and non-vertebral osteoporotic fracture in elderly men with a long follow-up time. The cause is probably multifactorial and our results support that anemia can be used as a predictor for future fracture.
Similar content being viewed by others
Introduction
Osteoporosis is an important public health issue because of potential devastating results of osteoporotic fractures. Major osteoporotic fractures are typically defined as fractures of the hip, vertebral body, distal forearm, or humerus [1, 2]. They are associated with increased morbidity and mortality for the individual and are a major economic burden for the society [3]. The risk of osteoporosis is highly dependent on age [4]. Anemia is another public health issue that gets increasingly common in older age [5]. Anemia is defined by the World Health Organization (WHO) as hemoglobin (Hb) < 130 g/L in men and < 120 g/L in women [6]. Blood and bone cells live in close proximity in the bone marrow and all three bone cells, osteoblasts [7], osteoclasts [8, 9], and osteocytes [10, 11], have been implicated to play a role in the hematopoietic stem cell niche and in the differentiation of blood cells. A recent meta-analysis reported that hematopoietic disorders with increased marrow cell proliferation are associated with significant deterioration of bone health, independent of the lineage of the affected blood cells [12]. The association between blood and bone health is also seen in non-proliferative hematopoietic diseases such as Diamond-Blackfan anemia and Fanconi anemia [13]. In recent years, accumulating evidence suggests that anemia is a risk factor for osteoporotic fractures, with most evidence available for non-vertebral fractures in men [14,15,16,17,18]. The primary aim of the present study was to investigate if anemia could predict fracture in a well-defined population-based cohort of ambulatory elderly men, with a long follow-up time.
Methods
Study population
The MrOS (Osteoporotic Fractures in Men) study is a prospective, international, multicenter, observational study. The primary objective of the study was to evaluate risk factors for fracture and osteoporosis in elderly men. The overall study design has previously been described [19]. Gothenburg was one of three Swedish sites and recruited 1010 subjects. Men in the age group of 70–80 years living in Gothenburg were randomly identified from national population registries and invited to participate by letter. At the first visit (April 2002–December 2004), subjects answered standardized questionnaires and underwent blood tests and bone mineral density (BMD) measurements. Men with available Hb values were included in the present study (n = 1005). The MrOS study was approved by the ethics committee at the University of Gothenburg, Sweden (M 014–01 and LU 611/2012).
Assessment of covariates
Body weight and height were measured using standard equipment and body mass index (BMI) calculated as weight in kilograms divided by height (in meters) squared (kg/m2). Hand grip strength was measured using a Jamar® dynamometer. Areal BMD (aBMD; g/cm2) was assessed using DXA with the Hologic QDR 4500/A-Delphi (Hologic, Waltham, MA). aBMD will hereinafter be referred to as BMD. The results for total hip BMD and lumbar spine BMD were standardized with a method that previously has been described [19]. The coefficients of variation (CVs) for the BMD measurements were 0.5–3%. Information regarding general health (hypertension, myocardial infarction, stroke, chronic bronchitis, cancer), falls in the previous year, and smoking was gathered from a standardized questionnaire. Diabetes mellitus was defined as previously diagnosed diabetes, fasting plasma glucose concentration > 7.0 mM/L, or the use of insulin or other hypoglycemic medication. Information regarding prevalent prostate cancer was gathered from the National Prostate Cancer Register of Sweden.
Blood sampling and analytical methods
Blood samples were collected at the baseline visit, at around 8.00 a.m., following an overnight fast and abstinence from smoking. Hb was analyzed immediately in an automated cell counter (CellDyn 4000; Abbott Diagnostics, Abbott Park, IL, USA), at Sahlgrenska University Hospital, Gothenburg, Sweden. Plasma and serum samples were frozen within 1 h and stored at − 80 °C until required for analysis. Methods regarding plasma/serum concentrations of intact fibroblast growth factor 23 (iFGF23), glucose, osteocalcin, N-terminal propeptide of type 1 collagen (P1NP), testosterone, estradiol, erythropoietin (EPO), C-reactive protein (CRP), intact parathyroid hormone (iPTH), 25(OH)D, and cystatin C, as well as calculation using cystatin C-based formula for estimated glomerular filtration rate (eGFR), have previously been described [20,21,22,23]. The serum levels of alkaline phosphatase (ALP), albumin-adjusted calcium, and phosphate were analyzed according to the routine laboratory technique used at Sahlgrenska University Hospital. Hypogonadism was defined as testosterone levels < 8 nmol/L.
Assessment of fractures
The follow-up time was recorded from the date of baseline visit to the time of fracture, to the end of the study, June 1, 2018, or until death — whichever came first. All Swedish citizens have a unique personal registration number. This enables access to information in X-ray archives concerning the time and the site of fracture. The follow-up time for each fracture type was recorded; namely, any fracture, vertebral fractures, hip fractures, and non-vertebral osteoporotic fractures. Non-vertebral osteoporotic fractures were defined as fractures of the hip, pelvis, proximal humerus, and forearm. Deaths were documented from the National Cause of Death Register that include virtually all deaths in Sweden.
Statistical analyses
All parametric values are presented as mean with standard deviation (SD), and values that were not normally distributed are presented as median with interquartile range (IQR). Skewed continuous variables were analyzed in the log scale. Differences in means were tested through permutation t-test for continuous variables and with the Fisher exact test for dichotomous variables. Since 25(OH)D varies according to season, a Z-score was calculated and an expected value of 25(OH)D was attained for each participant according to season [24] and was used in statistical analysis at 25(OH)D values. Hb was analyzed as a dichotomous variable, i.e., Hb < 130 g/L (anemia) or Hb ≥ 130 g/L (not anemia). Using fractures (any, non-vertebral osteoporotic, hip and vertebral) as an event, we calculated the probability of being fracture free during the follow-up period, depending on whether the subject had anemia or not, and plotted them according to the Kaplan–Meier method using a log‐rank test for statistical comparison of the equality in the fractures between the anemic and non-anemic groups. Follow-up time was derived separately for any fracture, vertebral fracture, non-vertebral fracture, and hip fracture outcomes. Patients were followed until fracture, death, or emigration. Poisson regression was used to determine if anemia was associated with risk of fractures after adjusting for other covariates. As Poisson regression uses updated age at each time, it takes partly care of issue of competing risk of mortality. Linear regression analysis was performed to determine if anemia was associated with iFGF23 after adjustment for other covariates. Double-sided tests were used throughout and a p value of < 0.05 was regarded as statistically significant. The software used was SAS for Windows, version 9.3 (SAS Institute, Inc. Cary, NC, USA); Stata version 15.1 (StataCorp LLC, College Station, TX, 77,845, USA); and a database and statistics program package developed at the Department of Community Medicine and Public Health, Gothenburg University.
Results
The current cohort consisted of 1005 men with a mean age of 75.3 years (SD 3.2) and a mean BMI of 26.2 kg/m2 (SD 3.5). Hb values were normally distributed. The mean value of Hb was 147 g/L (SD 12). The cohort included 66 men with anemia, of whom 14 had a Hb < 120 g/L. Baseline demographic and laboratory values for the whole study group and the subjects with or without anemia are presented in Table 1. Men with anemia had experienced more falls in the previous year before baseline and had lower hand strength and slower walking speed. Men with anemia had a higher prevalence of hypertension, stroke, diabetes, cancer, and prostate cancer. The 10-year survival rate was 31% in the anemic group and 49% in the non-anemic group (p = 0.009). We were neither able to establish a statistically significant difference in total hip BMD between the anemic and the non-anemic group (0.94 vs. 0.96, p = 0.159), nor in the difference between bone remodeling markers in anemic and non-anemic subjects. Subjects with anemia had higher EPO, iFGF23, CRP, and phosphate and lower eGFR, while we were not able to show statistical difference regarding PTH, D vitamin, and calcium levels. The association between anemia and iFGF23 was independent of age, EPO, and eGFR (β = 6.56, standard error = 2.84, p = 0.021).
Risk of fractures and anemia
During a median follow-up time of 10.1 years (SD 4.6, maximal follow-up time 16.1 years), 346 subjects suffered from any fracture: 27 of 66 anemic subjects (41%) and 319 of 938 (34%) of non-anemic subjects. Analysis according to fracture type showed that 162 subjects suffered from a non-vertebral fracture (14 of 66 (21%) anemic and 152 of 938 (16%) non-anemic), 110 from a hip fracture (10 of 66 (15%) anemic and 100 of 938 (11%) non-anemic), and 140 from a vertebral fracture (8 of 66 (12%) anemic and 132 of 938 (14%) non-anemic). Figure 1 depicts a Kaplan–Meier curve and shows the probability of being fracture free in the follow-up period for any fracture, non-vertebral fracture, hip fracture, and vertebral fracture, in the anemic and non-anemic groups. Men with anemia were less likely to be free from any (p = 0.003), non-vertebral (p = 0.022), and hip fracture (p = 0.020) in the follow-up period. For vertebral fractures, the probability of being fracture free was the same for the anemic and non-anemic subjects (p = 0.624). We calculated the risk for specific fractures by anemia status with up to 10 and up to 16 years’ follow-up time and adjusted for age and total hip BMD (see Table 2). The HR for any fracture when anemic was 1.97 (95% CI 1.28–3.02) in 10 years and 1.69 (95% CI 1.13–2.52) in 16 years, compared with non-anemic subjects, adjusted for age and total hip BMD. Anemia predicted for non-vertebral fractures (HR in 10 years 2.35, 95% CI 1.29–4.27) but not for vertebral fractures (HR in 10 years 1.59, 95% CI 0.77–3.29) after adjustment for age and total hip BMD. Anemic subjects had an increased age-adjusted risk of hip fracture after both 10 and 16 years of follow-up, albeit not significant after further adjustment for total hip BMD.
Further adjustment was done to assess the risk for all fractures, adjusting for various diseases and conditions (see Table 3). This analysis shows that anemia is independently associated with increased risk for all fractures at 10 and 16 years after adjusting for age, year of birth, smoking, hip BMD, falls, stroke, cancer, diabetes, kidney function, testosterone and estrogen levels, CRP, and EPO.
Discussion
In this prospective observational study of 1005 ambulatory elderly men, we found that anemia increased the risk of any fracture independently of age, falls, total hip BMD, and various comorbidities and conditions, even with a long follow-up time of up to 16 years. The increased fracture risk was driven by non-vertebral fractures, where the 10-year risk was increased by 115%, independently of age and total hip BMD. The age-adjusted hip fracture risk was increased by 132%, 10 years from baseline, but, probably due to few fracture events and few anemic men, the risk increase was not statistically significant after adjusted for total hip BMD.
Our results are in agreement with previous studies. A population-based study from Tromsö, Norway, found that anemia was associated with an increased risk of non-vertebral fractures but not for vertebral fractures, in men but not in women, after adjustment for BMD [15]. Similar information was reported from MrOS USA, which only studied men [17]. The National Health Screening Program in Korea reported an association between anemia and both vertebral and non-vertebral fractures, although the risk increase was higher for non-vertebral fractures [16]. The Korean cohort included 72,131 subjects, while our cohort, as well as the MrOS USA and the Tromsö cohorts, was smaller, thus making it harder to show a statistically significant risk difference. Furthermore, no adjustments were made for BMD in the Korean cohort [15,16,17]. Conflicting results are published on the association between Hb values or anemia and fracture in women [14,15,16].
In agreement with other studies, we showed that anemia is associated with increased frailty, morbidity, and mortality [5, 25]; however, the risk for fractures was independent of baseline falls and various diseases and conditions. Men with anemia had a higher prevalence of diabetes, as previously described [26]. Diabetes is known to be associated with increased risk of osteoporotic fractures [27], as well as being associated with hypogonadism [28]. The prevalence of prostate cancer was markedly increased in the anemic population compared to the non-anemic population, as well as the prevalence of hypogonadism. Hypogonadism is associated with prostate cancer, partly due to its treatment with androgen deprivation therapy (ADT), as well as the prostate cancer in itself being associated with lower testosterone values [29]. Both low testosterone and low estradiol in men are known to be associated with decreased BMD and increased risk for fractures [19, 30], as well as being associated with anemia, in our, as well as previous studies [23, 31]. Chronic kidney disease (CKD) and inflammation are known causes of secondary anemia and have both been associated with increased risk of fracture [32, 33], which is in agreement with our results. The mechanism by which anemia increases fracture risk is probably multifactorial and it is likely that anemia, to some extent, serves as a proxy for the underlying disease burden. Another mechanisms through which anemia might be associated with fracture risk is via higher phosphate and iFGF23, both related to anemia in our study. Both are associated with decreased renal function [34]. Hyperphosphatemia has been related to anemia [35] and increased fracture risk [36]. FGF23 is produced by osteocytes/blasts and is a known effector of bone mineralization, but our group has previously shown that Hb as a continuous variable correlates with iFGF23 [37]. We further demonstrated that anemia was associated with iFGF23, independently of renal function, EPO, and age. Contradicting results are published on the association between FGF23 and fracture risk [38, 39]. Another possible mechanism through which anemia affects bone is via elevated levels of EPO in the blood. Our group has previously shown that increased levels of plasma EPO are associated with increased risk of fracture in elderly men, although most noticeable for vertebral fractures [21]. EPO production is stimulated via hypoxia and bone homeostasis is effected by oxygen tension. Preclinical studies have shown that hypoxia has a negative effect on bone both by inhibiting osteoblast formation and stimulating osteoclast formation [40]. Clinical studies have shown that short-term exposure of high altitude, which is associated with hypoxia, can lead to decreased BMD [41, 42], and hypoxia during sleep in elderly men leads to increased risk for falls and fractures [43]. Thus, it is possible that anemia increased fracture risk partly because of relative hypoxia. In the bone marrow, EPO controls the proliferation and differentiation of erythrocytes [44]. Several preclinical studies have shown that EPO has a detrimental effect on bone mass in adult rodents, but other studies in growing mice and using traumatic models have reported a stimulation of bone formation by EPO [45]. Our group has recently shown that EPO is associated with increased BMD in elderly men [21]. It has been suggested that EPO is a cross-link between the hematopoietic stem cell niche and bone cells. Osteoblasts can produce EPO, which results in the expansion of the hematopoietic stem cell niche and is associated with selective expansion of erythroid cells [46].
In our present study on anemia, we were not able to establish a statistically significant association with lower BMD. The reason for this can be either a real lack of association or a lack of power in our study owing to the small number of men with anemia. The latter would be supported by the loss of statistical significance after adding BMD in multivariate Poisson regression for hip fractures. Our group has recently shown that Hb analyzed as a continuous variable is associated with total hip BMD after adjustment for age and BMI. When adjusted for estradiol or osteocalcin, the association was no longer significant [47]. Hb has been associated with lower bone mass in two Italian cohort studies [48, 49]. In these studies, bone mass was measured with peripheral quantitative computed tomography (pQCT) or ultrasound-derived T- and Z-score, respectively. In another study where BMD has been measured with DXA, no association between anemia and cross-sectional BMD was seen. In that study, an association between fast annual change in BMD and anemia was seen [50]. It is therefore possible that anemia is associated with bone mass in a way that was not captured by a single DXA or anemia is associated with bone fragility not captured by DXA. In a recent meta-analysis by Steer et al., an association between bone marrow cellularity evaluated with a trephine biopsy, and bone density change was seen, whereas no association was seen with Hb measurement and bone density change [12]. Peripheral blood cell count may not be the best way to evaluate hematopoiesis, rather a trephine biopsy would be preferable; however, this was not collected in the MrOS cohort.
An important strength of our study is the long follow-up time for fractures and a relatively large cohort. The fracture data are reliable owing to the unique personal registration number of all Swedish citizens, making it possible to access information in X-ray archives about the time and the site of fracture. Fasting and standardized sampling of data were used for all blood samples. We acknowledge several limitations in our study. First, it is a cross-sectional study, with only single measurements of blood variables and BMD. A significant limitation is that the anemic group only contains 66 subjects. Thus, the total amount of events was few in the anemic group which makes statistical analysis more vulnerable and true associations can be missed. Since this is an observational study, causality cannot be determined. Although multiple covariates were tested, we cannot rule out the possibility of residual confounding.
In conclusion, our findings indicate that anemia, after controlling for multiple factors, is predictive for any fracture and non-vertebral osteoporotic fractures in elderly men. The underlying mechanism is probably multifactorial but measuring Hb might be useful for long-term risk prediction for fractures.
References
Cummings SR, Melton LJ (2002) Epidemiology and outcomes of osteoporotic fractures. Lancet (London, England) 359(9319):1761–1767
Pisani P, Renna MD, Conversano F, Casciaro E, Di Paola M, Quarta E et al (2016) Major osteoporotic fragility fractures: risk factor updates and societal impact. World J Orthop 7(3):171–181
Borgström F, Karlsson L, Ortsäter G, Norton N, Halbout P, Cooper C et al (2020) Fragility fractures in Europe: burden, management and opportunities. Arch Osteoporos 15(1):59
Siris ES, Brenneman SK, Barrett-Connor E, Miller PD, Sajjan S, Berger ML et al (2006) The effect of age and bone mineral density on the absolute, excess, and relative risk of fracture in postmenopausal women aged 50–99: results from the National Osteoporosis Risk Assessment (NORA). Osteoporos Int 17(4):565–574
Culleton BF, Manns BJ, Zhang J, Tonelli M, Klarenbach S, Hemmelgarn BR (2006) Impact of anemia on hospitalization and mortality in older adults. Blood 107(10):3841–3846
McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B (2009) Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr 12(4):444–454
Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC et al (2003) Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425(6960):841–846
Kollet O, Dar A, Shivtiel S, Kalinkovich A, Lapid K, Sztainberg Y et al (2006) Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med 12(6):657–664
Mansour A, Abou-Ezzi G, Sitnicka E, W. Jacobsen SE, Wakkach A, Blin-Wakkach C (2012) Osteoclasts promote the formation of hematopoietic stem cell niches in the bone marrow. J Exp Med 209(3):537–49
Fulzele K, Krause DS, Panaroni C, Saini V, Barry KJ, Liu X et al (2013) Myelopoiesis is regulated by osteocytes through Gsalpha-dependent signaling. Blood 121(6):930–939
Sato M, Asada N, Kawano Y, Wakahashi K, Minagawa K, Kawano H et al (2013) Osteocytes regulate primary lymphoid organs and fat metabolism. Cell Metab 18(5):749–758
Steer K, Stavnichuk M, Morris M, Komarova SV (2017) Bone health in patients with hematopoietic disorders of bone marrow origin: systematic review and meta-analysis. J Bone Miner Res 32(4):731–742
Valderrabano RJ, Wu JY (2019) Bone and blood interactions in human health and disease. Bone 119:65–70
Chen Z, Thomson CA, Aickin M, Nicholas JS, Van Wyck D, Lewis CE et al (2010) The relationship between incidence of fractures and anemia in older multiethnic women. J Am Geriatr Soc 58(12):2337–2344
Jorgensen L, Skjelbakken T, Lochen ML, Ahmed L, Bjornerem A, Joakimsen R et al (2010) Anemia and the risk of non-vertebral fractures: the Tromso Study. Osteoporos Int 21(10):1761–1768
Lee EA, Shin DW, Yoo JH, Ko HY, Jeong SM (2019) Anemia and risk of fractures in older Korean adults: a nationwide population-based study. J Bone Miner Res 34(6):1049–1057
Valderrabano RJ, Lee J, Lui LY, Hoffman AR, Cummings SR, Orwoll ES et al (2017) Older men with anemia have increased fracture risk independent of bone mineral density. J Clin Endocrinol Metab 102(7):2199–2206
Kim KM, Lui L-Y, Cauley JA, Ensrud KE, Orwoll ES, Schousboe JT et al (2020) Red cell sistribution width is a risk factor for hip fracture in elderly men without anemia. J Bone Miner Res 35(5):869–874
Mellstrom D, Johnell O, Ljunggren O, Eriksson AL, Lorentzon M, Mallmin H et al (2006) Free testosterone is an independent predictor of BMD and prevalent fractures in elderly men: MrOS Sweden. J Bone Miner Res 21(4):529–535
Johansson H, Oden A, Lerner UH, Jutberger H, Lorentzon M, Barrett-Connor E et al (2012) High serum adiponectin predicts incident fractures in elderly men: osteoporotic fractures in men (MrOS) Sweden. J Bone Miner Res 27(6):1390–1396
Kristjansdottir HL, Lewerin C, Lerner UH, Herlitz H, Johansson P, Johansson H et al (2020) High plasma erythropoietin predicts incident fractures in elderly men with normal renal function: the MrOS Sweden Cohort. J Bone Miner Res 35:298–305
Lewerin C, Ljungman S, Nilsson-Ehle H (2007) Glomerular filtration rate as measured by serum cystatin C is an important determinant of plasma homocysteine and serum methylmalonic acid in the elderly. J Intern Med 261(1):65–73
Lewerin C, Nilsson-Ehle H, Jacobsson S, Johansson H, Sundh V, Karlsson MK et al (2014) Serum estradiol associates with blood hemoglobin in elderly men: the MrOS Sweden study. J Clin Endocrinol Metab 99(7):2549–2556
Haghsheno M-A, Mellström D, Behre C-J, Damber J-E, Johansson H, Karlsson M et al (2013) Low 25-OH vitamin D is associated with benign prostatic hyperplasia. J Urol 190(2):608–614
Guralnik J, Ershler W, Artz A, Lazo-Langner A, Walston J, Pahor M et al (2022) Unexplained anemia of aging: etiology, health consequences, and diagnostic criteria. J Am Geriatr Soc 70(3):891–899
Taderegew MM, Gebremariam T, Tareke AA, Woldeamanuel GG (2020) Anemia and its associated factors among type 2 diabetes mellitus patients attending Debre Berhan Referral Hospital, North-East Ethiopia: a cross-sectional study. J Blood Med 11:47–58
Janghorbani M, Van Dam RM, Willett WC, Hu FB (2007) Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol 166(5):495–505
Bebb R, Millar A, Brock G (2018) Sexual dysfunction and hypogonadism in men with diabetes. Can J Diabetes 42(Suppl 1):S228–S233
Gravina GL, Di Sante S, Limoncin E, Mollaioli D, Ciocca G, Carosa E et al (2015) Challenges to treat hypogonadism in prostate cancer patients: implications for endocrinologists, urologists and radiotherapists. Transl Androl Urol 4(2):139–147
Mellstrom D, Vandenput L, Mallmin H, Holmberg AH, Lorentzon M, Oden A et al (2008) Older men with low serum estradiol and high serum SHBG have an increased risk of fractures. J Bone Miner Res 23(10):1552–1560
Ferrucci L, Maggio M, Bandinelli S, Basaria S, Lauretani F, Ble A et al (2006) Low testosterone levels and the risk of anemia in older men and women. Arch Intern Med 166(13):1380–1388
Eriksson AL, Movérare-Skrtic S, Ljunggren Ö, Karlsson M, Mellström D, Ohlsson C (2014) High-sensitivity CRP is an independent risk factor for all fractures and vertebral fractures in elderly men: the MrOS Sweden study. J Bone Miner Res 29(2):418–423
Bover J, Ureña-Torres P, Laiz Alonso AM, Torregrosa JV, Rodríguez-García M, Castro-Alonso C et al (2019) Osteoporosis, bone mineral density and CKD-MBD (II): therapeutic implications. Nefrologia 39(3):227–242
David V, Francis C, Babitt JL (2017) Ironing out the cross talk between FGF23 and inflammation. Am J Physiol-Renal Physiol 312(1):F1–F8
Tran L, Batech M, Rhee CM, Streja E, Kalantar-Zadeh K, Jacobsen SJ et al (2016) Serum phosphorus and association with anemia among a large diverse population with and without chronic kidney disease. Nephrol Dial Transplant 31(4):636–645
Campos-Obando N, Koek WNH, Hooker ER, van der Eerden BC, Pols HA, Hofman A et al (2017) Serum phosphate is associated with fracture risk: the Rotterdam study and MrOS. J Bone Miner Res 32(6):1182–1193
Lewerin C, Ljunggren O, Nilsson-Ehle H, Karlsson MK, Herlitz H, Lorentzon M et al (2017) Low serum iron is associated with high serum intact FGF23 in elderly men: the Swedish MrOS study. Bone 98:1–8
Isakova T, Cai X, Lee J, Katz R, Cauley JA, Fried LF et al (2016) Associations of FGF23 with change in bone mineral density and fracture risk in older individuals. J Bone Miner Res 31(4):742–748
Mirza MA, Karlsson MK, Mellström D, Orwoll E, Ohlsson C, Ljunggren Ö et al (2011) Serum fibroblast growth factor-23 (FGF-23) and fracture risk in elderly men. J Bone Miner Res 26(4):857–864
Arnett TR (2010) Acidosis, hypoxia and bone. Arch Biochem Biophys 503(1):103–109
Basu M, Malhotra AS, Pal K, Chatterjee T, Ghosh D, Haldar K et al (2013) Determination of bone mass using multisite quantitative ultrasound and biochemical markers of bone turnover during residency at extreme altitude: a longitudinal study. High Alt Med Biol 14(2):150–154
Tanaka H, Minowa K, Satoh T, Koike T (1992) Bone atrophy at high altitude. J Bone Miner Metab 10(1):31–36
Cauley JA, Blackwell TL, Redline S, Ensrud KE, Ancoli-Israel S, Fink HA et al (2014) Hypoxia during sleep and the risk of falls and fractures in older men: the Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc 62(10):1853–1859
Lacombe C, Mayeux P (1998) Biology of erythropoietin. Haematologica 83(8):724–732
Hiram-Bab S, Neumann D, Gabet Y (2017) Erythropoietin in bone - controversies and consensus. Cytokine 89:155–159
Rankin Erinn B, Wu C, Khatri R, Wilson Tremika LS, Andersen R, Araldi E et al (2012) The HIF signaling pathway in osteoblasts directly modulates erythropoiesis through the production of EPO. Cell 149(1):63–74
Kristjansdottir HL, Mellström D, Johansson P, Karlsson M, Vandenput L, Lorentzon M et al (2020) High platelet count is associated with low bone mineral density: the MrOS Sweden cohort. Osteoporos Int 32(5):865–871
Cesari M, Pahor M, Lauretani F, Penninx BW, Bartali B, Russo R et al (2005) Bone density and hemoglobin levels in older persons: results from the InCHIANTI study. Osteoporos Int 16(6):691–699
Laudisio A, Marzetti E, Pagano F, Bernabei R, Zuccala G (2009) Haemoglobin levels are associated with bone mineral density in the elderly: a population-based study. Clin Rheumatol 28(2):145–151
Valderrabano RJ, Lui LY, Lee J, Cummings SR, Orwoll ES, Hoffman AR et al (2017) Bone density loss is associated with blood cell counts. J Bone Miner Res 32(2):212–220
Acknowledgements
We acknowledge Valter Sundh for help with statistical analysis.
Funding
Open access funding provided by University of Gothenburg. The study was financed by grants from the Swedish state under agreement between the Swedish government and the county councils, the ALF agreement (ALFGBG 872941 (HLK), ALFGBG 73940 (DM), ALFGBG 718341 (UHL), ALFGBG-720331 (CO)), Göteborgs läkaresällskap (GLS-880451 HLK), SU fonder (SU-890891 HLK), Swedish Research Council (2016–01001 CO), Lundberg Foundation (2017–0081 CO), Torsten Söderberg Foundation (M65/15, MT3/20 CO), Novo Nordisk Foundation (NNF180C0033898 CO), and Knut and Alice Wallenberg Foundation (KAW2015.0317, 2020.0230 CO).
Author information
Authors and Affiliations
Contributions
All authors have made substantial contributions to the conception and design of the study, acquisition of data, and analysis and interpretation of data and have played a role in drafting the article or revising it critically. All authors approve this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Hallgerdur Lind Kristjansdottir, Dan Mellström, Peter Johansson, Magnus Karlsson, Liesbeth Vandenput, Hans Herlitz, Ulf H. Lerner, and Catharina Lewerin have no disclosures. Mattias Lorentzon has received lecture or consulting fees from Amgen, Lilly, Meda, UCB Pharma, Renapharma, Radius Health, and Consilient Health, all outside the submitted work. Claes Ohlsson has two patent applications within the field of probiotics and osteoporosis.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kristjansdottir, H.L., Mellström, D., Johansson, P. et al. Anemia is associated with increased risk of non-vertebral osteoporotic fractures in elderly men: the MrOS Sweden cohort. Arch Osteoporos 17, 85 (2022). https://doi.org/10.1007/s11657-022-01130-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-022-01130-9