Abstract
In the present study, the waste part of the banana tree was used as a precursor, and copper chloride salt was used as an impregnating agent for the preparation of adsorbent to remove both cyanide and phenol from synthetic wastewater. Initially, thermogravimetric analysis was used to determine the rate of carbonization of the material with temperature, and thus, the optimum temperature (370 °C) and time of carbonization (35 min) were assessed. Different samples of adsorbents were prepared next by varying the weight ratio of pseudo-stem of waste banana tree to copper salt from 1:1 to 30:1. All the samples were then tested for removal of both the pollutants, and the ratio (20:1) corresponding to maximum removal of both the pollutants was considered as optimum. Therefore, further studies were conducted with the adsorbent prepared at optimum ratio, temperature and time and such adsorbent was termed as copper impregnated activated banana tree (CIABT). One variable at a time approach was followed to find out the most effective condition based on the maximum removal of pollutants. Maximum removal of 95.99 ± 1.03% and 97.33 ± 0.04% was achieved for cyanide (initial concentration: 100 ppm) and phenol (initial concentration: 450 ppm), respectively, at an optimum contact time of 150 min, the particle size of 90 μ, the adsorbent dosage of 10 g/L, pH 8.0 using CIABT at 25 °C. Hybrid artificial neural network–particle swarm optimization were employed for modelling-optimization of removal of both the pollutants while achieving 91.4–99.99% and 86.43–99.99% removal of cyanide and phenol, respectively, from simulated wastewater.
Similar content being viewed by others
Introduction
Coke-oven plant is an integral part of the steel industry where destructive distillation of bituminous coal is done for the production of coke. The huge volume of water is utilized for quenching and cooling of red hot coke and for recovery of by-products from by-product slot-type coke ovens (Rai et al. 2020). During such processes, the major portion of water is being contaminated with a number of pollutants, and hence, a large volume of wastewater, commonly termed as coke-oven wastewater, is being generated. Among the various pollutants, cyanide and phenolic compounds are major contributors to the total chemical oxygen demand (COD) in coke-oven wastewater (Mishra et al. 2018). Cyanide and phenol are extremely toxic and harmful to humans (Mohsen et al. 2015). Owing to several detrimental effects of cyanide and phenol on environment and ecosystem, coke-oven wastewater must be treated properly before being discharged to the environment. As primary treatment, different chemical reaction methods have been used to treat high concentrations of cyanide and phenol for coke-oven wastewater (Kumar and Pal 2012). However, all these processes have several limitations, such as high chemical cost and generation of secondary pollutants. Therefore, alternative environmentally friendly, low-cost treatment methods are in search. Adsorption is one of the most favoured, technically simple and efficacious methods for the treatment of cyanide and phenol from wastewater (Pramanik et al. 2019). Various low-cost adsorbents, derived from waste materials such as coconut shells, apricot stone, banana peel, coffee husk, sawdust, plywood dust and corncob were used for the removal of said pollutants from wastewater (Kuyucak and Akcil 2013; Depci et al. 2014; Abbas et al. 2014; Gebresemati et al. 2017; Singh et al. 2018; Pramanik et al. 2019; Agarwal et al. 2019). Since these adsorbents were prepared from waste materials, they are economical. Moreover, the valorization of such waste materials solves the solid waste disposal problem adeptly. Impregnation of metal ions onto adsorbents derived from such waste materials is another practice to increase the adsorptive capability of the native adsorbent. Metal impregnated activated carbon (MIAC) has shown its great potential to adsorb cyanide and phenol from wastewater in a number of studies (Agarwal et al. 2013a, 2019; Sing and Balomajumder 2015; Pramanik et al. 2019). However, the amount of metal used as an impregnating agent with precursor material was found high in most of the research works, which led to an increase in the preparation cost of the adsorbents. The goal of this research is to create a suitable adsorbent from waste material by reducing the amount of impregnating metal ions while maintaining the same efficacy.
India is one of the largest banana-producing countries in the world (Chowdhury et al. 2018). After harvesting bananas, the farmers usually cut the banana trees. These trees are generally being lie down over the agricultural field as post-harvesting residue (Chowdhury et al. 2018). This lignocellulosic residue, considered as agro-wastes, has the problem of proper disposal. Thus, fruitful utilization of such waste material will not only lessen the problem of solid waste disposal but also contribute to the Indian economy by valorizing the waste material. Since it is a “pseudo-stem”, mechanical pre-treatment cost for size reduction is much less than that required for hardwood biomass. All these favourable points attribute altogether to the potential use of pseudo-stem of the banana tree as the prospective precursor for carbonization. As per the knowledge of the current research group concerned, only a few research studies were carried out on the prospective use of pseudo-stem of the banana tree (Fernandes et al. 2012; Abdullah et al. 2014; Chowdhury et al. 2018). However, the study on removal of both cyanide and phenol using metal impregnated adsorbent prepared from pseudo-stem of banana tree dust as a precursor is very terse. Therefore, in the present research work, the potential of agro-waste-derived adsorbent prepared from pseudo-stem of waste banana tree, impregnated with copper chloride salt, on the removal of both cyanide and phenol has been investigated. Thermogravimetric analysis followed by an experimental variation of impregnation ratio of waste material to copper chloride salt was done to assess the optimum condition of carbonization vis-à-vis the metal impregnation process. To make the impregnation process economic, sustainable and eco-friendly, minimum carbonization temperature and time and maximum impregnation ratio of waste material to copper chloride salt were chosen for preparation of adsorbent from its precursor. An artificial neural network (ANN) model was created for the current study since 1st principle-based model for the adsorption process is not available (Lahiri and Ghanta 2010; Ochoa-Estopier et al. 2013). After the model is ready, particle swarm optimization is used to determine the best-operating conditions and the optimum responses (Prata et al. 2009; Karri and Sahu 2018). The application of such soft techniques for finding out optimum conditions is novel of its kind.
Materials and methods
All of the experiments were carried out three times in the laboratory, and the findings were presented as Mean \(\pm {\text{SD}}\) values. Chemicals used for all experimentations were of analytical grade (Merck, India).
Preparation of agro-waste-derived adsorbent
The pseudo-stem of the banana tree, an agricultural waste, was collected from an agricultural field and sundried for 3–4 days. The stem was cut into small pieces having sizes 3ʺ–4ʺ. These were then crushed in a jaw crusher followed by grinding in a ball mill to get very fine particles of banana stem and after screen analysis, 85 µ particle was taken for preparation of adsorbent.
Thermogravimetric analysis (TGA) (Model: Shimadzu: TG-DTG60/60H) was done to obtain the optimum carbonization condition of the prepared material. The thermal degradation of the materials during pyrolysis in the N2 atmosphere at a flow rate of 200 mL/min and heating rate of 40 °C/min was observed. After judicial scrutiny of the data, optimum temperature and time of carbonization were selected. Waste pseudo-banana stem dust was then mixed with copper chloride dihydrate salt having a weight ratio varying from 1:1 to 30:1. All these mixtures were kept separately in heat-resistant silica crucibles and then carbonized in a muffle furnace at the specific condition as obtained from TGA. Samples thus prepared were stored in plastic zip packets for further experimentations on the removal of cyanide and phenol individually. The optimum weight ratio was selected by comparing the efficacies of all these copper impregnated samples in the removal of these pollutants. The stock solutions of cyanide and phenol were made by diluting the standard cyanide solution (1000 ppm, Merck KGaA, 64271 Darmstadt) with distilled water and dissolving 1.0 g of phenol (EMPARTARR ACS Phenol) in 1000 mL of distilled water, respectively. 100 mL solution of phenol (450 ppm) and Cyanide (100 ppm) were mixed separately with 1.0 g of each carbonized sample taken in 250-mL Erlenmeyer flask subsequently placed at BOD Incubator Shaker at 250 rpm for 150 min. The samples were filtered with Whatman filter paper (Ashless, Diameter 125 mm). For analysing the residual cyanide concentration, the ion electric method was used. 50 mL of residual cyanide solution sample was taken first. The sample was then mixed thoroughly with 1 mL of 10 M of NaOH buffer solution to prevent any possibility of toxic HCN fumes. Finally, 20 mL of the resultant solution was taken, and the cyanide concentration was measured by Orion Ion Meter (Sn-B36531). For analysing the residual phenol concentration, the spectrophotometric method was followed (Kang et al. 2000). Initially, 50 mL of residual phenol solution was taken. Then 1.0 mL of hydroxylamine hydrochloride solution, 1.0 mL of sodium nitroprusside solution and 3 mL of buffer solution (pH 12) were well mixed with it. The sample was then left for 15 min. Finally, a Visible Spectrophotometer (UV-2300, TECHCOM) at the wavelength of 700 nm was used to analyse the resultant solution. Based on the removal efficacy of each carbonized sample, the weight ratio of banana stem dust and copper chloride salt was chosen for the preparation of the final adsorbent. The adsorbent thus prepared was named copper impregnated activated banana tree (CIABT) and used for further studies.
To observe the influence of copper impregnation, waste pseudo-banana stem dust was also carbonized without copper chloride salt at the temperature and time as obtained from TGA analysis and the prepared material was termed as a carbonized banana tree (CBT).
Characterization of adsorbent
Scanning electron microscope (Hitachie-S-3000N, Japan) and energy-dispersive X-ray spectroscopy were used to analyse CIABT before and after adsorption of both cyanide and phenol. While the former was used to find out the morphological characteristics of CIABT, the latter one was used to get elemental analysis of the same. CHNS analysis of CIABT was done using CHNS analyser (Model: VARIO MACRO CUBE, Elementar, Germany). The amount of CIABT sample taken for analysis was 16.44 g. X-ray fluorescence (XRF) analysis is useful for getting elemental analysis of the solid sample. About 4.0 g of CIABT sample was taken in compressed powder form for XRF analysis (Model: AXIOS, Panalytical, Netherlands). Fourier transform infrared spectroscopy (FTIR) studies of CIABT, before and after adsorption of pollutants, were carried out using PerkinElmer FTIR spectrometer (Malaysia) for analysing their functional groups. Wave numbers were varied in the range of 400–4000 cm−1.
Removal of cyanide and phenol using carbonized banana tree (CBT) and copper impregnated activated banana tree (CIABT)
Carbonized banana tree (CBT) and copper impregnated activated banana tree (CIABT) were used separately as adsorbents for cyanide and phenol removal, taking initial concentrations (IC) of 100 ppm and 450 ppm, respectively. 1.0 g of both the adsorbents was taken to treat 100 mL cyanide and phenol solutions separately. The flasks were then shaken for 150 min at 250 rpm in a BOD Incubator Shaker. The residual concentrations of phenol and cyanide in both samples were determined as per the procedure described above.
Removal of cyanide and phenol using CIABT
Effect of operating variables on the adsorption of cyanide and phenol using CIABT following one variable at a time (OVAT) approach
By altering each variable individually in a judicial manner, the effect of adsorption time, weight of adsorbent, particle size and initial pH of the solution on the elimination of both pollutants was investigated. Adsorption time for both cyanide (100 ppm) and phenol (450 ppm) removal was varied from 15 to 150 min. The weight and particle size of CIABT were varied from 0.1 to 1.0 g and 45 to 120 µ, respectively. Initially, the pH of synthetic solutions was also varied between 7 and 9. The volume of the aqueous solution of cyanide and phenol was maintained at 100 mL for each set of experiments. After determining the most suitable condition corresponding to maximum removal of both the pollutants using such one variable at a time (OVAT) analysis, time variation studies on removal of both the pollutants were performed at this condition. Separately, a fixed amount of adsorbent was contacted with phenol and cyanide solutions. IC of cyanide and phenol were changed between 50 and 150 ppm and 300 and 550 ppm, respectively. The solutions were then placed in the BOD shaker at 120 rpm for 150 min at 25 °C. After a definite time interval, samples were collected and subsequently filtered with Whatman filter paper. Residual cyanide and phenol concentrations were analysed separately.
Equilibrium study for the adsorption of cyanide and phenol using CIABT
From the stock solutions, various concentrations of cyanide (50–150 ppm) and phenol (300–550 ppm) were prepared. Each solution of cyanide and phenol was taken in a 250-mL Erlenmeyer flask containing CIABT (1.0 g/100 mL) and shaken at 120 rpm for 24 h at 25 °C. The solution was then filtered and analysed to find out the equilibrium concentration of cyanide and phenol.
Regeneration and reusability study
For the reusability study, desorption method was performed at various pH (8–10) conditions. After adsorption with cyanide and phenol, the spent CIABT samples were collected and dried for 24 h at room temperature. Then, 1.0 g of dried spent CIABT was added to 100 mL solutions at different pH ranging from 8 to 10 taken in 250-mL Erlenmeyer flasks. The flasks were then placed in a BOD shaker and stirred for 2.5 h. After filtering the solutions, the adsorbents were dried and reused for cyanide and phenol adsorption.
Since the experimentations were carried out in a laboratory, all safety measures were followed as per standard operating procedure (SOP). During the experiments, the research teams used gloves, lab coats and masks and followed other safety protocols. All hazardous chemicals were handled with caution, and cyanide waste was disposed of in compliance with University Environmental Health and Safety (EHS) hazardous waste policies. The cyanide waste adsorbent was disposed of in a covered container with a label.
Theoretical analysis
Application of artificial intelligence (AI)-based neural network (ANN) modelling technique
Detailed experimentations were carried out where CIABT was used to remove cyanide and phenol from simulated wastewater. It is very difficult to build the phenomenological model for such adsorption processes. Since a lot of experimental data was generated in this work, a data-driven ANN model is tried in the present work. In this type of scenario, ANN has proven to be an excellent tool for modelling nonlinear systems. In the last 20 years, ANN models were developed in the diverse field to model complex systems, as evident from a large number of research papers (Lahiri and Ghanta 2010; Ochoa-Estopier et al. 2013).
In the present research, ANN is applied to predict the individual removal of cyanide and phenol considering input parameters, such as, (1) initial concentration (IC) of cyanide and phenol, (2) contact time. (3) Particle size of the adsorbent, (4) pH of the media and (5) adsorbent dose. The removal percentage of cyanide and phenol individually is taken as the target output.
As shown in Fig. 1, an ANN model typically comprises of three (03) layers: input layer, hidden layer and output layer. In the present research work to develop ANN model, five (05) nodes for input layers and one (01) node for output layers have been considered and are shown in Fig. 1. Each node in the hidden layer has an activation function that is provided by the user. ANN training began with 80% of the input–output data set (selected at random) for weight updation. Validation was done on 10% of the data, and examination of training is stopped when validation error starts increasing. Rest 10% data was used for testing. The goodness of ANN model was judged by the test error (on unseen data). The mean square error (MSE) and \(R^{2}\) on test data are used to evaluate the performance of the ANN model. To create an ANN model, the user must provide the following information: (a) the training algorithm, (b) the activation functions in the hidden layer and output layer nodes and (c) the number of hidden layer nodes (Upendar et al. 2020). Optimum nodes in the hidden layer, best ANN algorithm, optimum activation function in the hidden layer and output layer were determined during ANN model building. The effectiveness of the ANN model was assessed using the coefficient of \(R^{2}\) and MSE which are defined as follows:
Coefficient of determination
Mean square error (MSE)
where N is the number of experimental data, Yi,pred is the ith data point of predicted output, Yi,exp is the ith data point of experimental output,\(\overline{Y}_{{{\text{pred}}}}\) is the mean value of ANN-predicted output and \(\overline{{Y_{\exp } }}\) is the mean value of experimental output (Lahiri and Ghanta 2010; Sarkar et al. 2021). Although a large number of ANN model was evaluated in this study, the execution time in Intel i-5 processor was less than one hour.
Development of particle swarm optimization (PSO)
Following the development of a reliable and accurate ANN model, the optimization technique was used to optimize the input parameters, such as contact time, initial concentration, solution pH, particle size and adsorbent dose, in order to maximize the removal of cyanide and phenol. Since ANN equations are complex and contain a variety of weights and exponential factors, applying a standard optimization procedure to an ANN model is difficult. Because of its simplicity and convenience of implementation, particle swarm optimization (PSO) was used in this investigation. Kennedy and Eberhart invented particle swarm optimization (PSO) in 1995 as a progressive computation technique. A swarm of bird-like particles make up PSO. Every particle occupies a specific location in search space. The strength of each particle shows the quality of its position (Sarkar et al. 2021). For better achievement of results and performance, ANN evolutionary algorithm was combined with PSO optimization process (Karri and Sahu 2018).
Particle swarm optimization (PSO) is the same as the genetic algorithm in which the system is starting with a population of random solutions. However, in comparison to the genetic algorithm; each potential solution allows a randomized velocity and that potential solution called particle are “flown” through cyberspace. Advantages of PSO are (1) it is totally comprehensive to escalate design variable; (2) it can be smoothly implemented; (3) it is derivative-free; (4) it has very less algorithm parameter and (5) it is very efficient global search algorithm (Prata et al. 2009).
Results and discussion
Selection and preparation of copper impregnated activated banana tree (CIABT)
Initially, thermogravimetric analysis (TGA) has been done to assess the thermal degradation behaviour of dried pseudo-stem of banana tree dust. The thermal degradation of the material during pyrolysis in N2 atmosphere at a flow rate of 200 mL/min and heating rate of 40 °C/min has been observed in the form of residual mass percentage \([ = (({\text{residual}}\,{\text{mass}}*100)/{\text{initial}}\,{\text{mass}})]\) vs temperature and time individually, as shown in Fig. 2a, b, respectively. It is seen that on increasing the heating temperature, the mass loss increases and the decomposition rate is faster at 300 °C. The amount of mass loss is found maximum at around 370 °C after 35 min. Therefore, the carbonization temperature and time of banana tree dust have been fixed at 370 °C and 35 min, respectively.
The weight ratio of the waste pseudo-stem of banana tree dust to copper chloride salt has been varied from 1:1 to 30:1 during carbonization at the preset condition in the muffle furnace. The sample prepared at individual condition has been tested for both the pollutants. The removal of cyanide and phenol has been observed to vary from 99.98 \(\pm\) 0.012% to 87.48 \(\pm\) 0.717% and from 99.27 \(\pm\) 2.62% to 95.72 \(\pm\) 0.73%, respectively, when the ratio of weight ratio of banana tree dust to copper chloride has been varied from 1:1 to 30:1. Results are shown in Table 1.
Removal is found to decrease with an increase in the weight ratio of waste banana tree to copper chloride salt. This may be due to the less impregnation of copper onto adsorbent at a higher weight ratio and, thereby, less interaction with anionic pollutants. Removal of both cyanide and phenol has been seen to decrease minutely when the weight ratio has been increased from 25:1 to 30:1. Naturally, in the current study, the ideal condition for preparing adsorbent has been chosen as weight ratio: 20:1, temperature: 370 °C, time: 35 min.
Characterization of adsorbent
Figure 3a–c shows SEM images of CIABT before and after cyanide and phenol adsorption, respectively. No such commendable difference has been seen during SEM analysis of CIABT, before and after adsorption. The images of EDAX analysis for native CIABT and CIABT after adsorption of cyanide and phenol are shown in Fig. 4a–c, respectively. The presence of copper peak confirms the impregnation of copper onto banana tree dust during carbonization in the presence of copper chloride salt.
CHNS analysis for untreated CIABT has been performed where compositions of carbon (C), hydrogen (H), nitrogen (N) and sulphur (S) have been observed as 54.8%, 3.22%, 0.3% and 0.19%, respectively. Higher content of carbon provides an additional benefit in the adsorption of pollutants (Sadhu et al. 2013).
Elemental composition of native CIABT has been obtained by XRF analysis. It reveals that CIABT contains major percentage of O (23.97%), Cu (19.28%), K (26.45%), Cl (11.9%), Ca (6.62%), Mg (5.33%) and minor percentage of Al (0.042%), Si (1.94%), P (2.84%), S (0.96%), Fe (0.45%) and Ba (0.23%).
Figure 5 shows FTIR graphs for CIABT before and after cyanide and phenol adsorption. Very sharp and intense peaks have been observed at wave number of 1627.49 cm−1 and 3294 cm−1 for cyanide and phenol treated CIABT as compared to untreated CIABT. The wave number 1627.49 cm−1 corresponding to the C–N stretch and wave number 3294 cm−1 corresponding to the N–H (strong) stretch represents the functional group of CN− and primary amines, respectively. The presence of C–N and N–H stretch on treated one confirms the adsorption of cyanide onto CIABT. Again, the sharp, intense peak for phenol treated CIABT at wave number 3294 cm−1 corresponds to the O–H stretch, which reflects the presence of a functional group of phenol. Thus, the adsorption of phenol on CIABT is also confirmed.
Removal of cyanide and phenol using copper impregnated activated banana tree (CIABT) and carbonized banana tree (CBT)
A comparative study has been made to evaluate the removal efficacies of CIABT and carbonized banana trees (CBT). Results are shown in Fig. 6. The percentage removal of cyanide using CIABT and carbonized banana tree (CBT) has been found as 99.99% and 69.10%, respectively, while for phenol, the percentage removal has been observed as 98.76 and 21.18%, respectively. Higher removal with copper impregnated adsorbent may be due to the electrostatic interaction between impregnated copper and anionic pollutants, which favours the binding of pollutants onto the surface of the adsorbent. Pramanik et al. (2019) have shown a similar type of observation where the removal efficacy of pollutants is increased with the copper impregnated adsorbent (Pramanik et al. 2019).
Removal of cyanide and phenol following OVAT approach
Effect of contact time
The trend of removal of cyanide and phenol has been observed when contact time varies from 15 to 150 min (Fig. 7a). Removal of cyanide and phenol increases from 59 \(\pm\) 0.38% to 95 \(\pm\) 1.03% and from 66.22 \(\pm\) 1.94% to 97.33 \(\pm\) 0.04%, respectively, when contacting time increases from 15 to 150 min. The rate of increase in the removal of phenol decreases after 30 min while that for cyanide decreases after 120 min. However, since maximum removal of cyanide and phenol is obtained at 150 min, the next set of experiments have been performed for 150 min. Agarwal et al. (2013a, b) and Pramanik et al. (2019) showed the same trend on the removal of both the pollutants.
Effect of particle size
The particle size of the adsorbent has been varied from 45 to 120 µ to evaluate the effect of particle size on the removal of both the pollutants. Removal of cyanide and phenol decreases from 92.19 \(\pm\) 1.4% to 87.66%\(\pm\) 3.76% and from 98.5 \(\pm\) 1.47% to 90.04%\(\pm\) 1.47%, respectively, when particle size increases from 45 to 120 µ (Fig. 7b). Since adsorption is a surface phenomenon, higher surface area of smaller adsorbent results in higher removal. Therefore, the selection of the smallest particle (45 µ) is expected. However, since the grinding cost for preparing 45 µ is higher than other larger particles, an optimum size of 90 µ has been selected for both the removal of cyanide (90.25 \(\pm\) 3.46%) and phenol (97.30 \(\pm\) 0.514%). Kulkarni et al. 2013 reported the same where the removal of phenol decreased with the increase in particle size of the adsorbent. Agarwal et al. 2019 have shown a similar observation for the removal of cyanide.
Effect of pH
To observe the effect of pH on removal of cyanide and phenol, the pH of the solution has been varied from 7 to 9. The removals of cyanide and phenol decrease from 93.95 \(\pm\) 0.63% to 88.75 \(\pm\) 2.47%% and from 99 \(\pm\) 1.40% to 98.34 \(\pm\) 2.35%, respectively, when the pH of the solution increases from 7 to 9 (Fig. 7c). Lower removal at higher pH may be due to the electrostatic repulsion between negatively charged adsorbent surface and anionic pollutants. Therefore, further studies have been carried out at pH 8, which is almost close to the pH of real coke-oven wastewater. Sing et al. (2015) and Dwivedi et al. (2016) have shown a similar observation for the removal of cyanide. Agarwal et al. (2013a, b) have also shown a similar observation where the removal of cyanide and phenol decreases with increases of pH (> 9).
Effect of adsorbent dose
Removals of cyanide and phenol increase from 41 \(\pm\) 11.54% to 95.7 \(\pm\) 1.27% and 83.32 \(\pm\) 1.63% to 95.85 \(\pm\) 1.67%, respectively, when adsorbent dose increases from 2 to 20 g/L (Fig. 7d). The higher the adsorbent dose, the higher will be surface area. No such remarkable variation on the removal of both the pollutants beyond the adsorbent dose of 10 g/L has been observed. Therefore, an adsorbent dose of CIABT for the removal of cyanide and phenol has been chosen 10 g/L for further studies. Similar kinds of observations have been made by Agarwal et al. (2019) and Dwivedi et al. (2016) for the removal of cyanide. Higher removal at higher adsorbent dose for both cyanide and phenol has also been shown by Agarwal et al. (2013a, b).
Time variation study at optimum condition as obtained from OVAT analysis
Once the most effective condition has been assessed, the variations of removal of cyanide and phenol with time have been studied (Fig. 8a, b), respectively. The removal of cyanide decreases from 95.31 \(\pm\) 0.52% to 84.38 \(\pm\) 3.73% when the IC of cyanide increases from 50 to 150 ppm at 150 min (Fig. 8a). Similarly, the removal of phenol decreases from 95.62 \(\pm\) 0.93% to 86.01 \(\pm\) 0.004% when the IC of phenol increases from 300 to 550 ppm (Fig. 8b). Lower removal at higher concentrations may be due to the inadequacy of the amount of adsorbent. Pramanik et al. (2019) have made similar observation.
To assess whether any copper leaching occurred during adsorption, the collected solution was also tested for copper using Atomic absorption spectroscopy (AAS) (Model: iCE 3500, Thermo Fisher Scientific). A very less amount of copper was observed in the solution. Hence, it can be stated that the leaching of copper from CIABT is less.
Equilibrium study
Equilibrium data are fitted to two parameters isotherm models viz., Langmuir \(\left( {q_{{\text{e}}} = \frac{{q^{0} C_{{\text{e}}} k_{{\text{L}}} }}{{1 + k_{{\text{L}}} C_{{\text{e}}} }}} \right)\) and Freundlich \(\left( {\mathop {q_{{\text{e}}} = k}\nolimits_{{\text{F}}} \mathop c\nolimits_{{\text{e}}} \mathop n\limits^{1} } \right)\), where \(q^{0}\) is the adsorption equilibrium constant as used in the Langmuir isotherm (L/mg adsorbate), \(C_{{\text{e}}}\) is the equilibrium concentration for both cyanide and phenol after adsorption (ppm), \(k_{{\text{L}}}\) is the adsorption equilibrium constant as used in Langmuir isotherm (L/mg adsorbate), \(n\) is the Freundlich intensity parameter, \(k_{{\text{F}}}\) is the adsorption equilibrium constant as used in Freundlich adsorption isotherm model ((mg adsorbate/g CIABT)/ mg adsorbate/L))1/n. Table 2 shows the parameter values for both isotherm models. Based on the \(R^{2}\), it can be concluded that the Freundlich model explains best the equilibrium data for cyanide removal, while the Langmuir model is the best for phenol removal.
Regeneration and reusability study
To assess the adsorption capability of regenerated spent CIABT, it has been reutilized for the removal of cyanide (Initial concentration: 100 ppm) and phenol (Initial concentration: 450 ppm) in the next cycle of operation. About 80% of cyanide and 67% of phenol removal indicate the good adsorption efficacy of regenerated CIABT. After two months, similar removal of both the pollutants has been observed using the same CIABT (1 g) sample, which confirms the stability of the present adsorbent.
ANN modelling
For the removal of cyanide, ANN has been trained with 85%, 10% and 5% data for training, validation and testing, respectively. The number of hidden neurons has been chosen as 10. Once ANN is trained with the reasonable coefficient of determination (\(R^{2}\)) and mean square error (MSE), it provides equation no. 3, which relates % removal of cyanide and phenol to the input variables. The values of weights (\(W_{i,j}\), \(W_{j,k}\)) are shown in Tables 3 and 4.
One can find the complete equation using these weights, and thus the removal of pollutants (%) can be determined with Eq. (4) (Lahiri and Ghanta 2010).
where \(U_{i}\) = input variable and \(H_{j}\) = Hidden layer output.
The basic purpose of using data-driven ANN modelling techniques in this study is to find a relationship between per cent removal and the input parameters, as shown in Eqs. (3) and (4). For the present adsorption study, this relationship is not known and a reliable phenomenological model is not available in the literature.
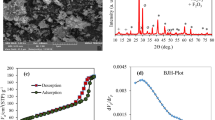
Table 5 depicts some typical input–output data for cyanide and phenol removal using ANN training. The coefficient of determination (\(R^{2}\)) and mean square error (MSE) are used to evaluate the model performance. Figure 9a, b illustrates the values of \(R^{2}\), MSE and experimental vs predicted responses for the removal of cyanide and phenol, respectively. Both the figures show the close fit of experimental date with model predicted one for the removal of cyanide and phenol individually. Very high \(R^{2}\) (0.99 for cyanide, 0.99 for phenol) and very low MSE (2.16 for cyanide, 0.71 for phenol) (Fig. 9a, b) for the removal of cyanide and phenol indicates that both the ANN models are very accurate.
Particle swarm optimization implementation
Once the ANN has captured a relationship, the PSO optimization technique is utilized to identify the best value of input parameters in order to optimize the removal of pollutants in this study. It is difficult to obtain the best values of these parameters using trial and error approaches during experimentation, and it may take a lot of runs. However, PSO does this optimization mathematically and provides the optimum value quickly, and thus avoids a lot of experimentations.
While input parameters were restricted between their low and high limits, a Matlab algorithm for PSO was used to maximize the removal of cyanide and phenol. The removal after PSO exposure was found to be 99.99% in both situations. As illustrated in Tables 6 and 7, there are a variety of input combinations that can result in nearly 100% removal of cyanide and phenol. This is the biggest advantage of using PSO to figure out the best input combinations to achieve almost 100% removal of pollutants that are difficult to detect during the experimental phase.
Separate treatments of cyanide and phenol were used in this study. However, in the near future, a unified operating condition for the removal of both cyanide and phenol will be required for the treatment of real coke-oven wastewater. Values of input parameters such as IC and pH of the solution were set for this run based on the state of conventional coke-oven wastewater. To keep the operating cost low, the adsorbent dose and contact time for treating cyanide and phenol from wastewater with adsorbent CIABT were reduced to a minimum. The goal is to determine the most cost-effective way to adsorb cyanide and phenol from wastewater using the CIABT adsorbent. The optimum conditions as found by the PSO algorithm with input variables are shown in Tables 8 and 9. According to the tables, PSO is capable of determining the most cost-effective optimum condition for removing 99.99% of cyanide and 99.99% of phenol.
However, for large-scale real-life implementations, this ANN model needs to be retrained with actual operating data. It is imperative that the present ANN model is developed with experimental data in a controlled laboratory environment. In large-scale operations, the contaminant removal may depend on various other parameters like the presence of other contaminants in actual wastewater, which can severely impact the adsorption phenomena. In actual implementations, ANN can be newly configured and retrained with a lot of actual operating parameters, and it is expected that the developed ANN model will be more accurate due to the vast quantity of training data sets available. After developing the new ANN model with actual operating data, the PSO algorithm will be re-applied to get the new set of optimum parameters. The methodology and algorithm will remain the same, and only they need to be retrained and executed with actual operating data. The developed hybrid ANN–PSO methodology is generic and can be extended to real-life plant implementation.
Conclusions
In the present study, pseudo-stem of banana tree, an agricultural waste, has been used as a precursor to prepare adsorbent for removal of cyanide and phenol, the common pollutants as present in coke-oven wastewater from synthetic wastewater. Copper has been impregnated, and optimum condition for impregnation vis-a-vis carbonization condition has been assessed using TGA, and the adsorbent thus formed is termed as copper impregnated activated banana tree (CIABT). A thorough investigation of the effect of operational variables on the efficacy of CIABT in removing cyanide and phenol from synthetic solution was carried out. Initial concentrations of both the pollutants have been varied as per real coke-oven wastewater. Maximum removal of 95.99 ± 1.03% and 97.33 ± 0.04% for cyanide and phenol, respectively, have been achieved when 100 mL of 100 ppm of cyanide solution and 450 ppm phenol solutions have been contacted separately with 1.0 g of CIABT at 25 °C for 150 min. Therefore, the current study confirms CIABT's high potential for cyanide and phenol removal. An accurate ANN model has been developed based on the experimental data to forecast the adsorption efficiency in terms of various input parameters. The PSO algorithm was used to determine the most cost-effective input conditions for cyanide and phenol removal. The modelling and optimization techniques used in this study can be applied to other adsorption studies with unknown process phenomenology.
Data availability
Some or all data and models that support the findings of the study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
Abbreviations
- N :
-
Number of experimental data
- Y i ,pred :
-
ith data point experimental ANN-predicted output
- Y i ,exp :
-
ith data point experimental output
- \(\overline{Y}_{{{\text{pred}}}}\) :
-
Mean value of ANN-predicted output
- \(\overline{{Y_{\exp } }}\) :
-
Mean value of experimental output
- \(q^{0}\) :
-
Adsorption equilibrium constant (L/mg adsorbate)
- \(C_{{\text{e}}}\) :
-
Concentration of cyanide and phenol at equilibrium after adsorption (ppm)
- \(k_{{\text{L}}}\) :
-
Langmuir isotherm equilibrium concentration for adsorption (L/mg)
- \(n\) :
-
Parameter for Freundlich intensity
- \(k_{{\text{F}}}\) :
-
The adsorption equilibrium constant as used in Freundlich adsorption isotherm model ((mg/g)/(mg/L))1/\(n\)
- \(R^{2}\) :
-
Coefficient of determination
- \({\text{MSE}}\) :
-
Mean square error
- \(W_{i,j}\) :
-
Input weights in ANN model
- \(W_{j,k}\) :
-
Output weights in ANN model
- U i :
-
Input variable in ANN model
- H j :
-
Hidden layer output in ANN model
References
Abbas NM, Abbas SF, Ibrahim AS (2014) Cyanide removal from wastewater by using banana peel. J Asian Sci Res 4(5):239–247
Abdullah N, Sulaiman F, Miskam MA, Taib RM (2014) Characterization of banana (Musaspp.) pseudo-stem and fruit-bunch-stem as a potential renewable energy resource. Int J Biol Vet Agric Food Eng 8:815–819
Agarwal B, Balomajumder C, Thakur PK (2013a) Simultaneous co-adsorptive removal of phenol and cyanide from binary solution using granular activated carbon. Chem Eng J 228:655–664. https://doi.org/10.1016/j.cej.2013.05.030
Agarwal B, Thakur PK, Balomajumder C (2013b) Use of iron-impregnated granular activated carbon for co-adsorptive removal of phenol and cyanide: insight into equilibrium and kinetics. Chem Eng Commun 200:1278–1292. https://doi.org/10.1080/00986445.2012.744749
Agarwal S, Pramanick S, Rahaman SA, Ghanta KC, Dutta S (2019) A cost-effective approach for abatement of cyanide using iron impregnated activated carbon: kinetic and equilibrium study. Appl Water Sci 9(4):74. https://doi.org/10.1007/s13201-019-0953-5
Chowdhury R, Ghosh S, Debnath B, Manna D (2018) Indian agro-wastes for 2G biorefineries: strategic decision on conversion processes. Sustain Energy Technol Policies. https://doi.org/10.1007/978-981-10-7188-1_16
Depci T, Onal Y, Prisbrey KA (2014) Apricot stone activated carbons adsorption of cyanide as revealed from computational chemistry analysis and experimental study. J Taiwan Inst Chem Eng 45(5):2511–2517. https://doi.org/10.1016/j.jtice.2014.05.015
Dwivedi N, Balomajumder C, Mondal P (2016) Comparative investigation on the removal of cyanide from aqueous solution using two different bioadsorbents. Water Resour Ind 15:28–40. https://doi.org/10.1016/j.wri.2016.06.002
Fernandes ER, Marangoni C, Medeiros S H, Souza O, Sellin N (2012) Slow pyrolysis of banana culture waste: leaves and pseudostem. In: 3rd international conference on industrial and hazardous waste management
Gebresemati M, Gabbiye N, Sahu O (2017) Sorption of cyanide from aqueous medium by coffee husk: response surface methodology. J Appl Res Technol 15(1):27–35. https://doi.org/10.1016/j.jart.2016.11.002
Kang C, Wang Y, Li R, Du Y, Zhang LJ, B, Zhou L, Du Y, (2000) A modified spectrophotometric method for the determination of trace amounts of phenol in water. Microchem J 64:161–171. https://doi.org/10.1016/S0026-265X(99)00022-3
Karri RR, Sahu JN (2018) Modeling and optimization by particle swarm embedded neural network for adsorption of zinc (II) by palm kernel shell based activated carbon from aqueous environment. J Environ Manag 206:178–191. https://doi.org/10.1016/j.jenvman.2017.10.026
Kulkarni JS, Tapare RW, Patil SV, Sawarkar MB (2013) Adsorption of phenol from wastewater in fluidized bed using coconut shell activated carbon. Procedia Eng 51:300–307. https://doi.org/10.1016/j.proeng.2013.01.040
Kumar R, Pal P (2012) Response surface-optimized Fenton’s pre-treatment for chemical precipitation of struvite and recycling of water through downstream nanofiltration. Chem Eng J 210:33–44. https://doi.org/10.1016/j.cej.2012.08.036
Kuyucak N, Akcil A (2013) Cyanide and removal options from effluents in gold mining and metallurgical processes. Miner Eng 50–51:13–29. https://doi.org/10.1016/j.mineng.2013.05.027
Lahiri SK, Ghanta KC (2010) Artificial neural network model with parameter tuning assisted by genetic algorithm technique: study of critical velocity of slurry flow in pipeline. Asia Pac J Chem Eng 5:763–777. https://doi.org/10.1002/apj.403
Mishra L, Paul KK, Jena S (2018) Characterization of coke oven wastewater. Earth Environ Sci 167:012011. https://doi.org/10.1088/1755-1315/167/1/012011
Mohsen A, Neda M, Masoud A (2015) Negative effects of cyanide on health and its removal options from industrial wastewater. Int J Epidemiol 2(1):44–49
Ochoa-Estopier LM, Jobson M, Smith R (2013) Operational optimization of crude oil distillation systems using artificial neural networks. Comput Chem Eng 59:178–185. https://doi.org/10.1016/j.compchemeng.2013.05.030
Pramanik S, Ahamed F, Ghanta KC, Dutta S (2019) Treatment of coke oven effluent using copper impregnated activated carbon: experiment and modelling. Indian Chem Eng. https://doi.org/10.1080/00194506.2019.1573707
Prata DM, Schwaab M, Lima EL, Pinto JC (2009) Nonlinear dynamic data reconciliation and parameter estimation through particle swarm optimization: application for an industrial polypropylene reactor. Chem Eng Sci 64(18):3953–3967. https://doi.org/10.1016/j.ces.2009.05.028
Rai A, Chakrabarty J, Dutta S (2020) Phycoremediation of pollutants from coke-oven wastewater using Tetraspora sp. NITD 18 and estimation of macromolecules from spent biomass. J Water Process Eng 1:2214–7144. https://doi.org/10.1016/j.jwpe.2020.101746
Sadhu K, Mukherjee A, Shukla SK, Adhikari K, Dutta S (2013) Adsorptive removal of phenol from coke-oven wastewater using Gondwana shale, India: experiment, modeling and optimization. Desalin Water Treat 52(34–36):6492–6504. https://doi.org/10.1080/19443994.2013.815581
Sarkar B, Sharma U, Adhikari K, Lahiri SK, Baltrėnaitė E, Baltrėnas P, Dutta S (2021) Application of artificial neural network and particle swarm optimization for modelling and optimization of biosorption of Lead(II) and Nickel(II) from wastewater using dead cyanobacterial biomass. J Indian Chem Soc 98(3):1–15
Sing N, Balomajumder C (2015) Removal of cyanide from aqueous media by adsorption using AL-activated carbon: parametric experiments, equilibrium, kinetics, and thermodynamic analysis. Int J Adv Technol Eng Sci 3(1):23–35
Singh N, Kumari A, Balomajumder C (2018) Modeling studies on mono and binary component biosorption of phenol and cyanide from aqueous solution onto activated carbon derived from saw dust. Saudi J Biol Sci. 25(7):1454–1467. https://doi.org/10.1016/j.sjbs.2016.01.007
Upendar G, Singh S, Chakrabarty J, Ghanta KC, Shahnawaz M, Lahiri SK, Dutta S (2020) Parametric study on CO sequestration using cyanobacterial consortium and production of macromolecules: experimentation, modelling and optimization. J Water Environ 35(2):500–513. https://doi.org/10.1111/wej.12646
Funding
The authors received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design. Material preparation, data collection and analysis were performed by Mr. SP and Mr. BS. ANN model was performed by Mr. SP. The first draft of the manuscript was written by Mr. SP and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pramanik, S., Sarkar, B., Lahiri, S. et al. Application of hybrid artificial neural network (ANN)–particle swarm optimization (PSO) for modelling and optimization of the adsorptive removal of cyanide and phenol from wastewater using agro-waste-derived adsorbent. Appl Water Sci 12, 184 (2022). https://doi.org/10.1007/s13201-022-01706-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-022-01706-3