Assessing the Effectiveness of Conservation Stocking for the Endangered White’s Seahorse Hippocampus whitei
- 1Fisheries Research, New South Wales (NSW) Department of Primary Industries, Port Stephens Fisheries Institute, Taylors Beach, NSW, Australia
- 2Fish Ecology Lab, School of Life Sciences, University of Technology Sydney, Sydney, NSW, Australia
The White’s seahorse Hippocampus whitei was listed as an Endangered species in 2020 on Australian state and federal legislation, as a result of population declines across its range attributed to habitat loss over the past decade. A captive-bred reintroduction program has been implemented as a possible management tool for species recovery, however, the viability of such a reintroduction program for seahorses has not been assessed to date. This study implemented a pilot captive-breeding program using adult H. whitei from Sydney Harbour, Australia, as brood stock. A total of 90 captive-bred seahorses were released into the wild on two different artificial habitat types (“seahorse hotels” and protective swimming net). Following release, a monthly post-release monitoring program was implemented for 12 months that involved underwater visual census surveys of the tagged seahorses. Sightings of captive-bred seahorse numbers were found to gradually decline over the 12-month period, with fewer seahorses found on the swimming net compared to the seahorse hotels and higher resighting probability of captive-bred animals on the seahorse hotels. After 12 months, 20% of the captive-bred seahorses were detected on the seahorse hotels, whilst two individuals were still observed 18 months after release on the hotels. Only 2% of captive-bred seahorses were observed on the swimming net after 12 months, with two individuals still detected on the net after two years. Nine of the captive-bred seahorses were found to reproduce in the wild, with two individuals observed mating with the wild population. This pilot study indicates that captive-bred seahorses can survive for up to two years in the wild, as well as contribute to local population recovery through reproductive success. However, while conservation stocking shows promise as a potential management tool to assist with threatened seahorse species recovery, there are several factors such as existing threats to the species that need to be addressed before such a program is implemented.
Introduction
The implementation of captive-breeding and stocking programs for terrestrial and aquatic species is a widely adopted method to increase species abundance and distribution in the wild and is typically implemented for those species which have experienced population declines (IUCN, 2021). Those species that have declined in abundance or in distribution across their range often end up listed as threatened species under international or local threatened species lists such as the IUCN Red List (IUCN, 2021). Whilst success stories for conservation stocking and translocation programs for threatened species, such as birds and mammals (Seddon et al., 2014; Thévenin et al., 2020) and freshwater fishes (Simons et al., 1989; Sayer et al., 2020; Welsh et al., 2020) are well documented, there are cases that are not always successful (Griffith et al., 1989; Pearson et al., 2021).
The conservation stocking and translocation of threatened species is considered the ‘last resort’ option and should not be implemented until threats are reduced or eliminated (IUCN, 2013). The reintroduction of a species needs to yield a measurable conservation benefit at the levels of a population, species or ecosystem; not just providing a benefit to the translocated or stocked individuals. (IUCN, 2013). In their comprehensive review of animal relocations, Fischer and Lindenmayer (2000) indicate that restocking initiatives are more likely to succeed when the source population was wild, large numbers of animals are released (n>100), and the threat that caused the population declines are removed.
Regarding aquatic species, little has been done on the restocking of threatened marine species. Instead, marine fish stocking is heavily focused on species that are either of commercial or recreational value or both (Bartley and Bell, 2008; Taylor et al., 2017; Kitada, 2018). Marine fisheries stock enhancement has been implemented for numerous species such as crabs, eels, salmon prawns and sharks (Feunteun, 2002; Halverson, 2008; Pratt and Threader, 2011; Lee et al., 2015; Taylor, 2017). The restocking of marine species that are listed as ‘threatened species’, through either translocation or release from breeding programs, is seldom done; however, there are some examples of restocking for threatened marine invertebrates (Baldacconi et al., 2010; Rogers-Bennett et al., 2016; Cabaitan and Conaco, 2017).
One group of marine species that are considered threatened worldwide are seahorses (Hippocampus spp.), and as a result the entire genus is listed on Appendix II of CITES (Convention on International Trade in Endangered Species of Wild Fauna and Flora). CITES listing compels signatory nations to ensure that harvest of seahorses is undertaken in a sustainable manner, however, some countries are failing to enforce this (Foster and Vincent, 2021). Even with the CITES listing, many species are currently experiencing population declines as a result of over-fishing, bycatch and habitat loss (Vincent et al., 2011; Pollom et al., 2021).
In 2020, the White’s seahorse Hippocampus whitei, a species endemic to the east coast of Australia (Short et al., 2019), was listed as an Endangered species on both Australia’s National Environment Protection and Biodiversity Conservation Act 1999 and the state New South Wales (NSW) Fisheries Management Act 1999 as a result of population declines across its range. It is also listed on the IUCN Red List as an Endangered species (Harasti and Pollom, 2017). The seahorse population declines were attributed to loss of essential habitats that it lives within, such as soft corals, sponges and seagrass (Harasti, 2016; Harasti and Pollom, 2017) that have been impacted on by threats such as vessel anchoring, installation of boat moorings and sand inundation (Harasti, 2016; Larkin et al., 2021).
Whilst habitats have declined across its range, H. whitei has shown an ability to adapt by utilising artificial habitats such as protective swimming nets (Hellyer et al., 2011; Simpson et al., 2021a; Simpson et al., 2021b) and purposefully designed artificial habitats known as seahorse hotels (Simpson et al., 2020). It was recently shown that adult H. whitei select artificial habitats over natural habitats (Simpson et al., 2019), similar to that of the only other Endangered seahorse species, Hippocampus capensis in South Africa (Claassens et al., 2018). Seahorse hotels have been installed in areas in NSW where H. whitei have declined as a result of habitat loss to provide them with refuge and to help promote population recovery (Simpson et al., 2020).
To manage the decline in H. whitei populations, a ‘Priority Action Statement’(PAS) was developed by the NSW Department of Primary Industries, outlining management actions to be implemented to assist in recovery of the species abundance and the habitats that it is known to use (DPI, 2020). The PAS suggests actions that can be undertaken to reduce threats to H. whitei in the wild, as well as suggesting other conservation and research measures that can be implemented to assist with recovery of populations across its range. One of the PAS recommended recovery actions is to “Develop and implement a captive breeding program to produce and release captive bred animals back into the wild to assist recovery of White’s seahorse populations”. The use of captive-breeding programs to help recover threatened fish has been undertaken for freshwater fish in NSW (Koehn et al., 2013; Zukowski et al., 2021), but not for marine fish. Numerous studies have demonstrated that seahorses can be raised in captivity under the right aquaria conditions (Woods, 2003; Koldewey and Martin-Smith, 2010; Koning and Hoeksema, 2021; Luzzatto and Estalles, 2021) with Wong and Benzie (2003) demonstrating that H. whitei is a suitable seahorse species for rearing in captivity.
The use of conservation stocking as a method to help repopulate declining seahorse populations has not previously been tested. It is unknown if seahorses raised in aquaria survive or perish when released back into the wild, nor is it known if they will remain at the release location. As H. whitei is a species that is known to exhibit small home ranges and displays strong site fidelity (Vincent et al., 2005; Harasti et al., 2014b; Manning et al., 2018), not only is it an ideal seahorse species to monitor following release back into the wild, its strong site fidelity and habitat dependence make it a suitable marine fish species to trial and assess conservation stocking methodology.
The IUCN ‘Guidelines for reintroductions and other conservation translocations’ state that post-monitoring of released animals needs to be implemented as the monitoring results will influence the need for either continuing or changing management regimes (IUCN, 2013). The implementation of a conservation stocking program for the White’s seahorse, including a post-monitoring program, provides an opportunity to assess the feasibility and effectiveness of this strategy as a recovery tool. Therefore, the aim of this research was to implement a pilot study for conservation stocking of the Endangered seahorse H. whitei and conduct a post-release monitoring program to assess whether captive-bred seahorses survive over time when released into the wild and assess if their habitat persistence and resighting probability at the release location was influenced by the type of artificial habitat that they were released on.
Methods
In October 2019, four pairs (male and female) of adult Hippocampus whitei were collected from the protective swimming net at Clifton Gardens, New South Wales (NSW), Australia (33° 50’ 21” S; 151° 15’ 12” E) in Sydney Harbour and transferred to SEA LIFE Sydney Aquarium located at Darling Harbour. Four pairs was the initial number allowed to be collected by SEA LIFE Sydney Aquarium issued under NSW DPI permit. Animals were collected by hand from the protective swimming net installed underneath the jetty and placed in underwater mesh bags before being transferred into aerated large plastic tubs on shore for transportation. At the time of collection, each of the collected males were pregnant and subsequently gave birth in the aquarium within two weeks and continued reproducing in captivity up until March 2020. The first batch of fry was born on 17/10/2019.
Seahorse fry were raised in captivity in a flow through oceanic system using water directly from Sydney Harbour. Juveniles were housed in 20L kreisels (cylindrical tanks) until 2 months old, then transferred to larger tanks of either 150L or 300L. All tanks had holdfasts provided, including netting and artificial plastic seagrass. Tanks did not have substrate (i.e., sand or gravel) on the bottom. All tanks were siphoned at least three times daily to remove waste matter and detritus and scrubbed with sponge once daily. 30% water changes were completed at least once per day. Tanks had a diurnal light cycle (LED lighting) matching local (Sydney) daylight. Juveniles were raised initially on phytoplankton-enriched rotifers until approximately 30 days old and then transferred to a diet of newly-hatched Artemia nauplii (SEP-ART GSL Artemia cysts) until approximately 90 days old, and then a combination of Artemia nauplii and wild-caught (Sydney Harbour) live mysids shrimp until time of release. Live food was constantly available within each tank.
Five days prior to the release of the captive-bred seahorses, the juveniles were marked using Visible Implant Fluorescent Elastomer (VIFE) (https://www.nmt.us). The marking of the captive-bred seahorses was essential to ensure that the captive-bred animals could be recognised from unmarked seahorses in the existing wild population. The use of VIFE is considered the most suitable marking method for seahorses (Woods and Martin-Smith, 2004) and it has been shown that VIFE tags can last in H. whitei for at least seven years in the wild (Harasti, 2021). Each of the juveniles were given between 1-3 elastomer marks depending on their size; the smaller animals (< 6 cm) were only given 1 VIFE mark and even some of the larger animals may have received only 1-2 marks if their body size was considered too small for additional tags. On subsequent resighting of the captive-bred animals with single marks in the wild, additional marks were added as the captive-bred seahorses grew larger. The location of the marks on the seahorse’s body and the different VIFE colours used allowed for individual identification of each of the 90 captive-bred seahorses.
On 07/05/2020, 90 juvenile Hippocampus whitei that were raised in SEA LIFE Sydney Aquarium were released back into the same location (Clifton Gardens) that their parents had been collected from. These juveniles were of various ages and sizes (aged 140 - 203 days old) and the mean total length of the 90 released juveniles was 58.4 mm ± 0.9 mm S.E [range: 40.2 mm (140 d old) to 78.1 mm (203 d old)]. The total length is taken from two combined measurements: from the top of the seahorse coronet to the base of the abdomen and from the base of the abdomen to the bottom of the tail. However, these total lengths are estimates as it was rather difficult to accurately measure the small sized seahorses without causing unnecessary stress to the animals, as they tend to curl into ‘balls’ when being handled. Of the 90 released seahorses, 18 were identified as males through the presence of a developing brood pouch, however, confirmation of whether they had matured into a male was not always possible as determining if the brood pouch was present is difficult at such a small size (> 60 mm) (Harasti et al., 2012).
For the release, seahorses were transported underwater by scuba divers in large plastic bags and 45 juveniles were released onto the middle of the Clifton Gardens protective swimming net at two locations on the net separated by ~5 m. This section of net that is parallel to the shore is approximately 50 m in length and is heavily covered in marine growth, particularly sponges and kelp Ecklonia radiata. However, this 50 m section of net was not continuous, as there were several large holes (~2-3 m width) along its length where the net had broken away. The reason that this section of net was chosen for the seahorse release is that it’s a known area for wild H. whitei to occur. Other areas of the net were considered unsuitable for H. whitei, as a result of shallow depth and insignificant marine growth on the net, as H. whitei is known to avoid nets devoid of marine growth (Harasti et al., 2010). The other 45 juveniles were released onto nine seahorse hotels (5 individuals per hotel) that were located approximately 25 m from the net (Figure 1). For this study, a limit of 10 seahorse hotels could be installed at the site, as per the license conditions from Transport NSW. The depth of the release site of both the protective swimming net and the seahorse hotels was ~3 - 4 m.
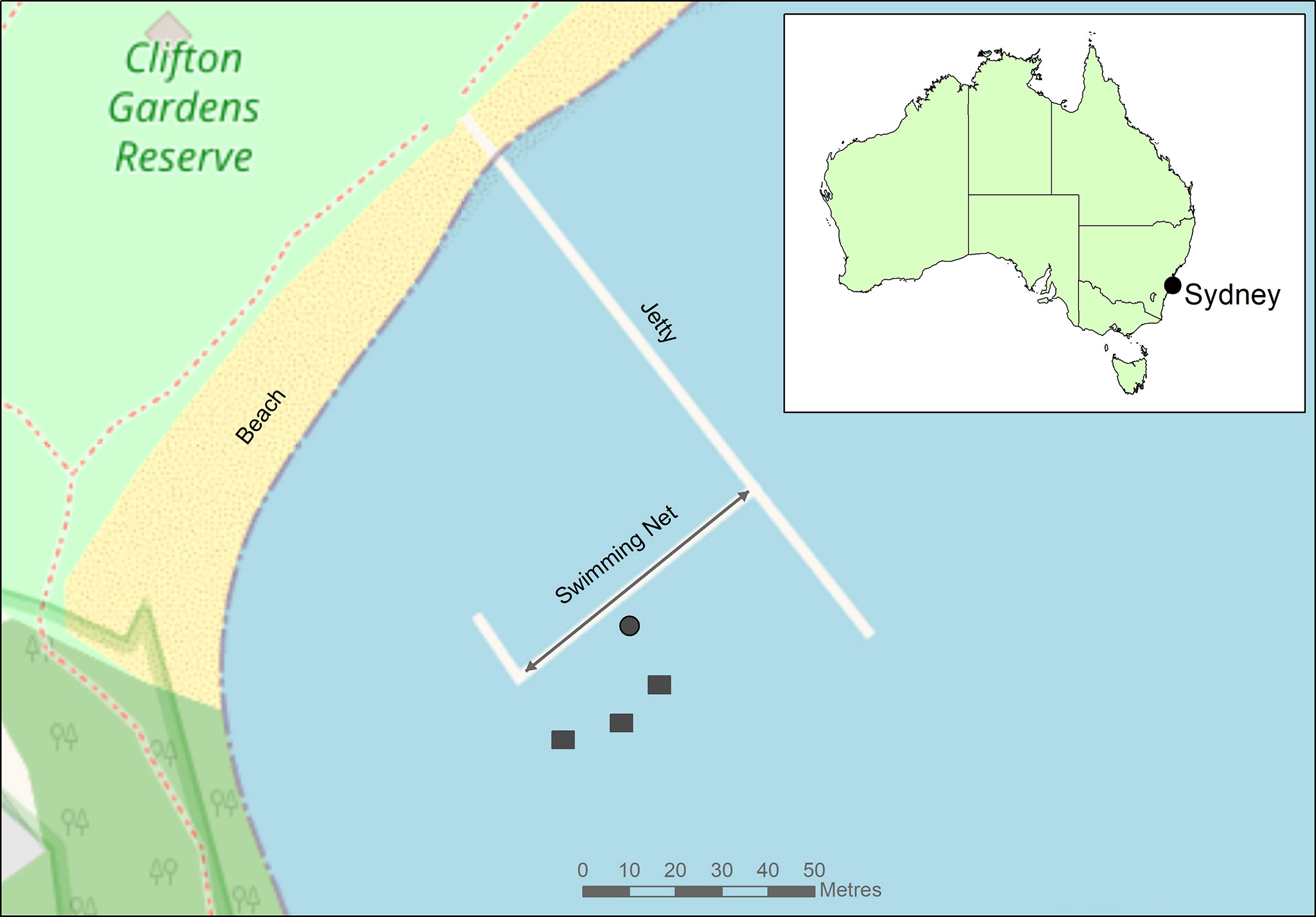
Figure 1 Location of the protective swimming net at Clifton Gardens in Sydney, New South Wales, Australia and the location of the installed seahorse hotels. ● = release location for seahorses on swimming net and ਕ= Three seahorse hotels with each of the three designs (9 in total). The area indicated by the arrowed line is the survey area on the net for the monthly surveys of the captive-bred seahorses.
The seahorse hotels were cube-shaped and constructed from galvanised steel mesh with a reinforcing-bar metal frame that measured 1 m x 1 m x 50 cm height. They were secured to the sea floor with star pickets driven through the sides to hold the hotels in place. To reduce the impact of potential known predators of H. whitei that are known to occur in the release location (Harasti et al., 2014a), unlike the initial seahorse hotels design of Simpeson et al. (2020), these hotels were completely enclosed to minimise the opportunity for predators to prey on the seahorses (Figure 2).
The seahorse hotels were placed in three groups with each group containing three different seahorse hotel designs. The first hotel design contained a panel of dark blue netting (Figure 2), which was made of compressed continuous twine with 9 strands of stainless steel and was stretched across the inside of the hotel to act as a holdfast. The second design contained white netting material approximately 6 mm diameter made from polypropylene rope material and the third design had no netting inside the hotel. The netting used was the same netting material used in the protective swimming nets around Sydney Harbour. Within each group, the hotels were placed ~1 m from each other and the three groups were separated by ~ 10 m. The seahorse hotels were installed at the site in March 2020, six weeks prior to captive-bred seahorse release, to allow marine epibiota growth to develop on the structures providing suitable habitat for the juveniles as per the suggestions of Simpeson et al. (2020).
Post-Release Monitoring
Following release of the captive-bred seahorses (07/05/2020), a post-release monitoring program was implemented that involved scuba diving surveys at the release site to search for and record any captive-bred seahorses on the two different habitat types. A survey was done each month from May 2020 to April 2021. A diving survey consisted of two divers searching the nine seahorse hotels for the presence of the captive-bred seahorses with a search on the hotels initially taking approximately 40 mins to complete due to the number of seahorses on initial release. The search time gradually reduced through time as a result of reduced seahorse numbers on the hotels (see Results) with surveys over the last three months taking ~20 mins. The same method was used to search the protective swimming net for captive-bred seahorses with two divers searching the net for approximately 40 mins with the area of net being searched ~40 m length x 3 m height. The actual time spent searching each habitat type varied based on tidal height, as a low tide survey meant less net area to search, whilst surveys of the seahorse hotels could take longer because of time spent trying to catch and identify seahorses within the hotels and the increased numbers of seahorses on hotels. Importantly, the search effort for both habitat types was consistent across all twelve surveys with every hotel being individually searched and the same start and end point on the net being searched each survey occasion.
For every captive-bred seahorse encountered, tag ID, location, holdfast (for seahorse hotels), sex and reproductive status were recorded. Male pregnancy was determined by the presence of an inflated brood pouch. The 90 captive-bred seahorses were recorded as either present or absent for each monthly survey. Following the last survey in April 2021, ad hoc surveys (n=5) were done to assess if any of the captive-bred seahorses remained on the site with the last survey conducted 18/05/2022. These surveys were ad hoc as a result of Covid-19 lockdowns in NSW that prevented access to the site and diving operations. These ad hoc surveys also included searches of other areas of the swimming net, however, no captive-bred seahorses were recorded outside the 50 m section of net used for the monthly surveys.
Data Analysis
Using mark–resight data of tagged captive-bred seahorses, recapture probability (p) over a period of 12 months was estimated using a Cormack–Jolly–Seber (CJS) model analysed in Program MARK. For seahorses, the term ‘resighting probability’ is generally used instead of recapture probability, as seahorses are not actually captured; they are resighted. Mark-resight methods have been used in various other seahorse studies to gain an understanding of population abundance, mortality and resighting probability (Curtis and Vincent, 2006; Harasti et al., 2012; Claassens and Harasti, 2021). Candidate models were developed to determine if p (resighting probability) varied across habitats and time. Model selection was done using Akaike’s information criteria (AIC) that assessed time and habitat dependence.
To determine whether habitat persistence of the captive-bred animals differed between the two different artificial habitat types on which they were released, a Kaplan-Meier log rank test (Kaplan and Meier, 1958) was performed in SPSS v28 based on a previous method assessing site persistence in H. whitei (Harasti and Gladstone, 2013). Whilst it was initially aimed to assess the actual survival rate of the captive-bred seahorses, estimates of survival were not considered suitable as it could not be conclusive determined that an end point of death for the seahorse had occurred, rather than an individual seahorse just emigrating from the release location and not being seen again.
To test whether the type of seahorse hotel design (n=3) influenced the number of captive-bred seahorses found using the hotels, analysis was undertaken on monthly survey data (n=12) collected from May 2020 to April 2021 on the abundance of seahorses across the different hotel types. Homogeneity of variance was found to be equal across groups and a one factor ANOVA was conducted with hotel design as a fixed factor (3 designs). ANOVA was conducted in SPSS v28.
Results
Following the release of the 90 captive-bred seahorses, a survey was undertaken two hours after their initial release to assess whether they stayed on the artificial habitats on which they were released. 70 of the released seahorses were detected in this initial survey; 37 were detected of the 45 released on the hotels and 33 of the 45 released on the swimming net. Only one individual, released onto the swimming net, was never seen in any subsequent surveys.
Monthly surveys were implemented from May 2020 to April 2021 with the first monthly survey undertaken two weeks after initial release and observations of the captive-bred seahorses gradually declined from June 2020 to April 2021 (Figure 3).
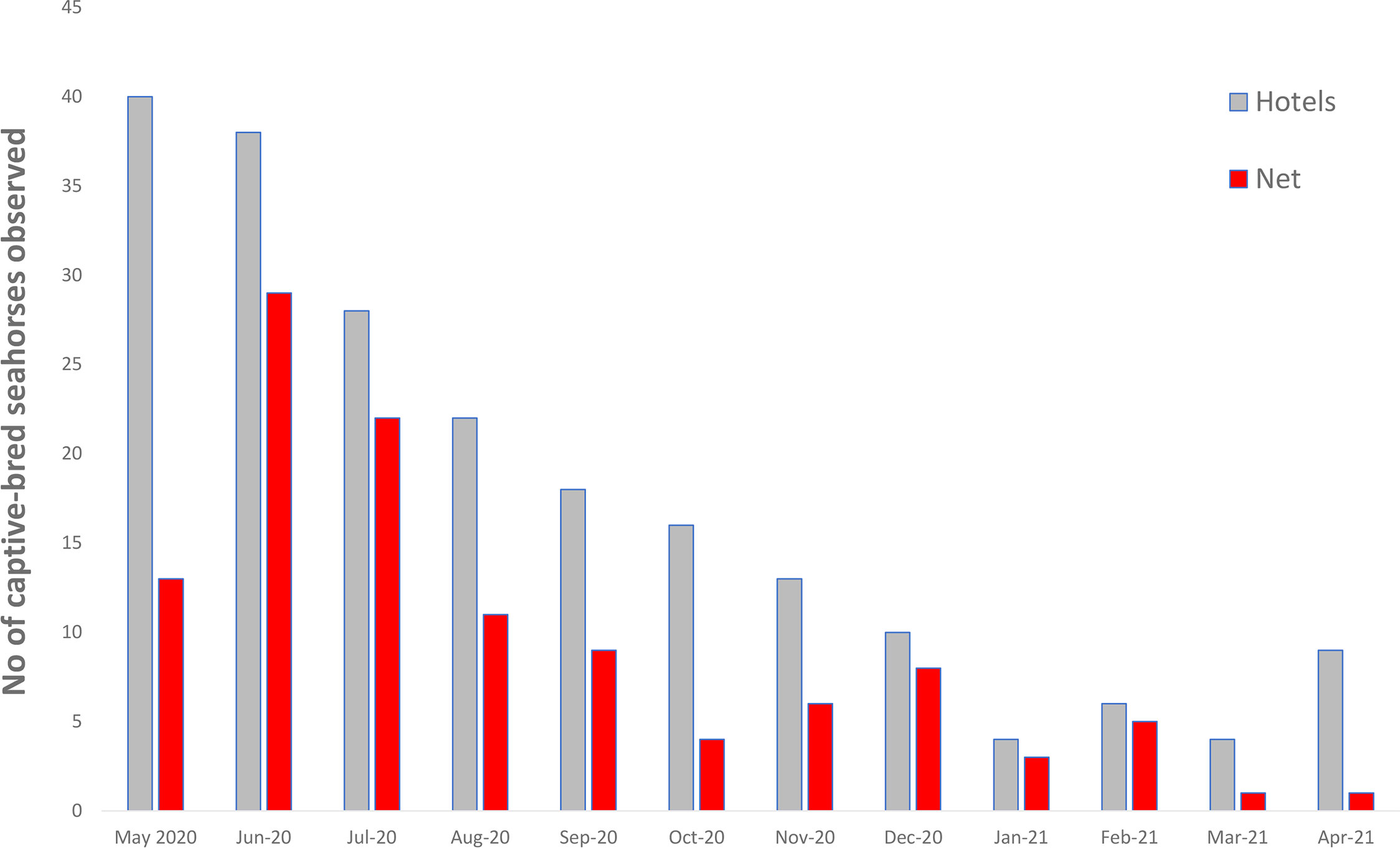
Figure 3 Number of captive-bred Hippocampus whitei observed during each monthly survey from May 2020 to April 2021 on the protective swimming net and seahorse hotels.
Resighting Probability
Based on mark-resight data of the 90 captive-bred seahorses, resighting probability from May 2020 to March 2021 varied between the two habitat types (net and seahorse hotels) with the most suitable CJS model (AICC = 1002.3, likelihood = 1·00, deviance = 382.8, parameters = 25) being constant for ϕ and time and habitat dependent for p. The mean probability of resighting a tagged captive-bred seahorse across all surveys was 0.54 ± 0.07 for seahorses released onto the seahorse hotels compared with only 0.26 ± 0.06 for seahorses released onto the swimming net (Figure 4).
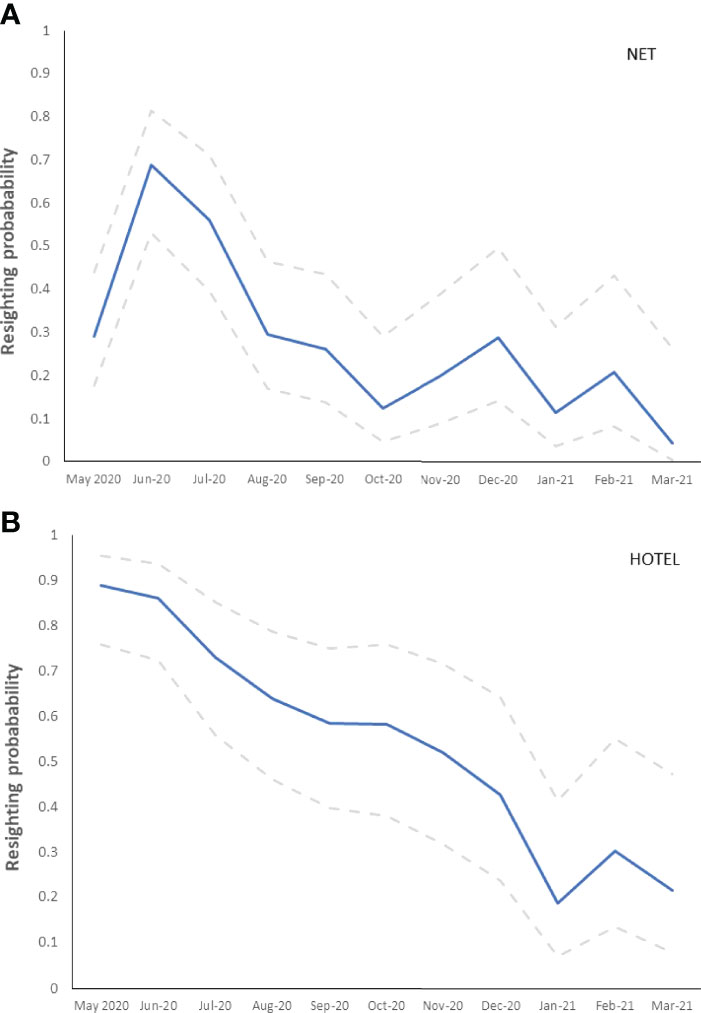
Figure 4 Variability in resighting probability based on mark-resight data of captive-bred tagged seahorses from May 2020 – March 2021 between (A) protective swimming net and (B) seahorse hotels. Dashed lines indicate the 95% upper and lower confidence limits.
Habitat Persistence Analysis
Kaplan-Meier comparison of habitat persistence between the two release artificial habitats (swimming net and seahorse hotels) found that there was a significant difference in the number of observed captive-bred seahorses (x2 = 5.5, df = 1, P < 0.05) from May 2020 – April 2021 (Figure 5). After 6 months, the number of seahorses remaining on the net was 38% compared with 47% on the hotels, whilst the last survey at 12 months from release found only 2% of captive-bred animals remaining on the net compared with 20% on the seahorse hotels.
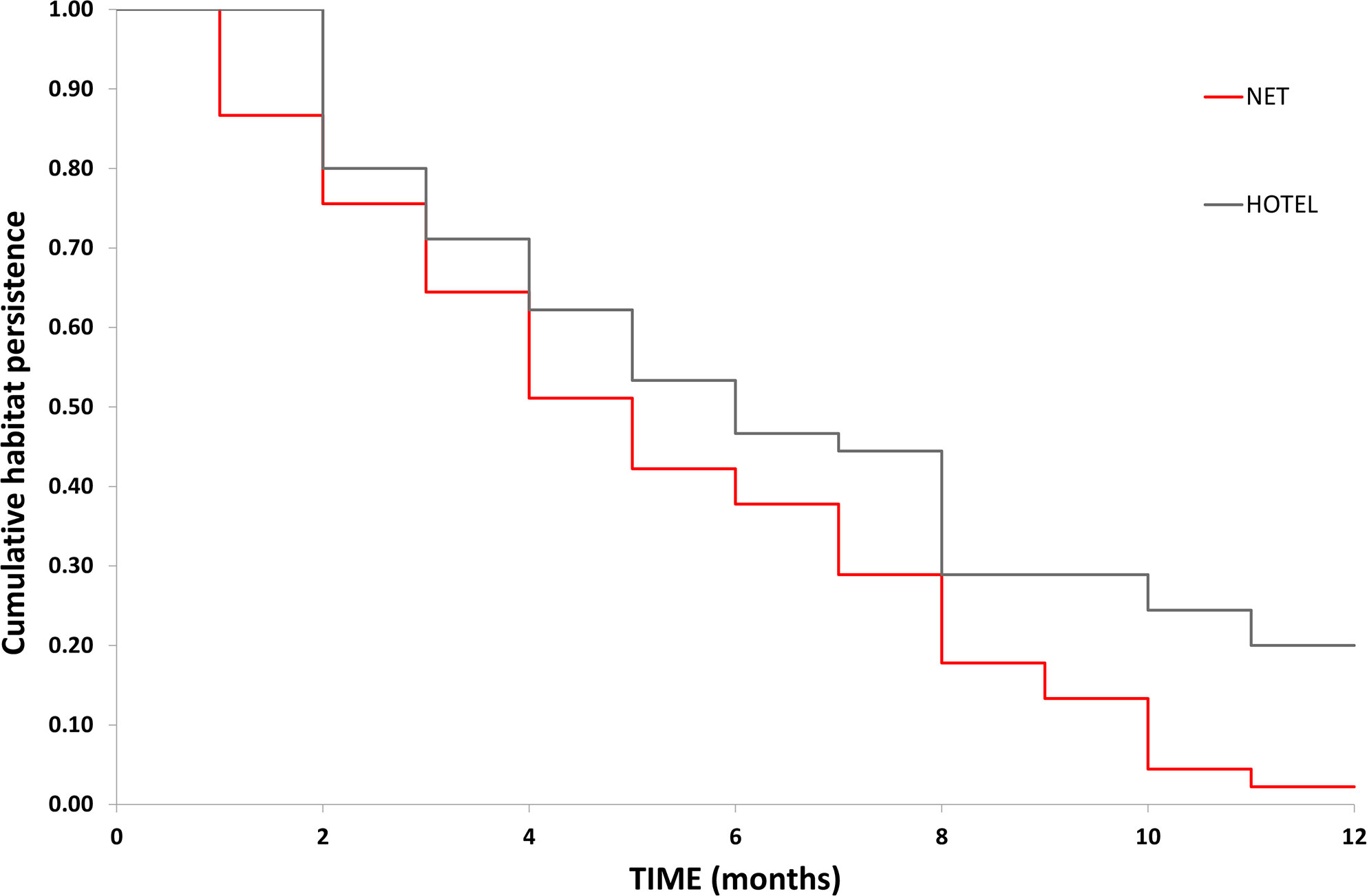
Figure 5 Kaplan-Meier cumulative habitat persistence estimates for captive-bred seahorses released onto the protective swimming net and seahorse hotels.
Of the captive released juveniles, a total of nine males were found to become pregnant six months after the release. The first time an individual was observed pregnant, determined through the swelling of the males’ brood pouch, was on 04/11/2020 with this individual, found on the net, being 384 days old. One male was observed to be pregnant on three separate monthly surveys (13/11/2020, 15/01/2021 and 23/02/2021) indicating that it experienced at least three separate pregnancies given the gestation period for H. whitei is 20-22 days (Vincent and Sadler, 1995). Two of the pregnant male captive-bred seahorses were confirmed to be paired up with wild female seahorses through repeated observations of them together.
The last observations for any of the captive-bred seahorses released onto the swimming net was 742 days (2 year 12 days) after release for two females that were observed on 18/05/2022. The last observations of captive-bred seahorses released onto the seahorse hotels was two females on 09/12/2021: 581 days after release.
Habitat Use
Whilst the mean number of captive-bred seahorses over the 12 monthly surveys were observed to be highest on the hotel design that contained the white netting (mean 2.3 ± 0.4 seahorse per hotel), it was not significantly different (F8,23 = 1.22, P > 0.05) than the hotel design without any netting (1.7 ± 0.3) and the hotel design containing blue netting (1.3 ± 0.3). Therefore, the type of hotel design was not found to significantly influence the number of captive-bred seahorses using the hotels.
Movements between the swimming net and hotel habitats were rare, with only three individuals released onto the seahorse hotels subsequently being seen on the net during monthly surveys; none of the three were found to return to the seahorse hotels. There was no observation of seahorses originally released onto the net moving to the hotels. Ad hoc searches conducted whilst swimming between the swimming net and hotels, and between the three groups of hotels, identified some captive-bred individuals within the vicinity (approx. 3 m) of both artificial habitats. These seahorses were observed to use natural habitats as holdfasts such as sponges, Ecklonia radiata and Sargassum sp. Regular movement was recorded of the captive-bred seahorses between the three clusters of hotels (located ~ 10 m apart) with 11 individuals observed moving between all three groups of hotels over the 12-month period.
Discussion
The use of conservation stocking to assist with recovery of threatened marine species is rare and this is the first documented study that assesses the effectiveness of conservation stocking for a threatened marine fish through implementation of a comprehensive post-release monitoring program. This study demonstrates that captive-bred Hippocampus whitei can survive for at least two years and reproduce when released back into the wild, however, their habitat persistence was influenced by the type of artificial habitat they were placed on and their occurrence and resighting probability declined over a period of 12 months. Habitat persistence was found to decrease through time, which is not uncommon in studies involving release of captive-bred animals back into the wild. It has previously been shown in terrestrial studies, that the detection of animals declines through time following species release (Peignot et al., 2008; Maran et al., 2009; White et al., 2021). Often species mortality occurs soon after release, often within the first month, as has been shown for bird and mammal species (Grey-Ross et al., 2009; Tavecchia et al., 2009; Taggart et al., 2016). In this study, it was found that their occurrence at the site gradually decreased over the 12 months; with no single monthly period corresponding to a dramatic decline in seahorse numbers. However, sightings of two captive-bred individuals two years after initial release provides a good indication that long-term occurrence of captive-bred H. whitei to remain at the release location is feasible.
One of the key issues regarding survival and site persistence of captive-bred animals released into the wild is their ability to evade predators. Mortality from predation is one of the main reasons that reintroductions to the wild are unsuccessful (Griffith et al., 1989; Bertolero et al., 2007; Aaltonen et al., 2009; Moseby et al., 2011). The occurrence of the captive-bred seahorses was found to be higher on seahorse hotels than on the nets and after 12 months there were significantly more captive-bred seahorses living on the hotels than the net. One of the factors that possibly influenced the habitat persistence between these two habitat types was predation, with hotels providing more protection from predators due to their fully enclosed construction. However, as actual death of seahorses was impossible to confirm, it is unknown if seahorses were predated on either of the habitat types, or instead had emigrated from the site. Ad hoc searches done throughout the study period by the authors and other local divers, never encountered any of the captive-bred seahorses far away from the seahorse hotels or on the section of the net they were initially released (authors personal observations).
Whilst there were no captive-bred seahorse mortalities attributed to predation in this study, with no dead seahorses found, there were several observations of predators trying to grab or attack seahorses hiding inside the seahorse hotels and on the swimming net. The predators observed attacking the captive-bred seahorses included three species of cephalopods (Octopus tetricus, Sepia plangon and Sepioteuthis australis) and juveniles of the fish Nelusetta ayraud. It has previously been documented that O. tetricus is a predator of H. whitei (Muller et al., 2022), as are S. plangon and N. ayraud (Harasti et al., 2014a). As the hotels were designed to exclude predators by providing an enclosure for the seahorses to hide in, they provided a higher level of protection compared to the swimming net where predators have ‘open access’ to the seahorses. Similar to the findings of Simpson et al. (2020), there was no difference in seahorse abundance between the different hotel designs indicating the addition of netting inside the hotel was not necessary to attract seahorses.
In addition to the seahorse hotels providing better protection from predators, another benefit was that resighting probability of captive-bred seahorses was significantly higher on the hotels than the swimming net, although this did decline through time for both habitats. It was considered more challenging to find the captive-bred seahorses on the net as the net had a larger search area and considerably more marine growth than the seahorse hotels. The seahorse hotels were easier to survey as they aggregated the seahorses to a confined area, and as they had only been installed a few months prior to the release of the captive-bred seahorses, marine growth was not dense compared to the swimming nets, making the hotels easier to search through initially. However, over the 12 month surveys, it became increasingly difficult to search inside the hotels for seahorses as the density of marine growth increased, with similar observations of epibiotic growth increasing over time recorded in the initial seahorse hotel experiments (Simpson et al., 2020).
For any future seahorse stocking programs, artificial habitats such as seahorse hotels could be considered as a suitable release habitat as they allow seahorses to aggregate in the one location, which would therefore increase the likelihood of successful reproduction if they are living in proximity making it easier to find a mate. To reduce the impact of a single threat/event causing a localised impact, it would be perhaps beneficial to have seahorses released at more than one location in a region to help improve chances of population recovery.
Releasing captive-bred seahorses into seahorse hotels also provides an increased level of protection for seahorses from predators. For post-monitoring seahorse surveys, it would be useful to modify the seahorse hotels so that a panel could be temporarily removed, allowing divers easier access inside the hotel to detect seahorses. Given that there were some sightings of captive-bred seahorses on natural habitats, it would be worthwhile to investigate if releasing captive-bred seahorses onto preferred natural habitats (i.e. seagrass, sponges) improves their habitat persistence and resighting probability compared to artificial habitats designed for seahorses (Correia et al., 2013; Correia et al., 2015; Simpson et al., 2020).
In other captive-release studies, it has been shown that factors such as age (Lee et al., 2015; Kyle et al., 2017; Efrat et al., 2020) and sex (Campbell-Thompson et al., 2012) can influence species survival and behaviour when released into the wild. In this study, it was not possible to assess if size influenced the habitat persistence of the captive-bred seahorses as there were no distinct size classes with size estimates for the 90 captive-bred seahorses linearly distributed. Similarly, at the time of the release, the sex of the captive-bred seahorses could not be determined for the majority of individuals, hence an assessment of resighting probability and habitat persistence between males and females was not feasible. In any future seahorse stocking programs, further research is warranted to assess if age, size and sex influences the habitat persistence, survival and behaviour of captive-bred seahorses.
Determining whether this conservation stocking program was successful is challenging, as there are no other studies or guidelines for threatened marine fish species to compare against. One of the key criteria for assessing captive-bred release programs is the ability to monitor the survival of captive-bred animals released into the wild to their first breeding season (IUCN, 2013). Whilst actual survival rates were not determined in this project, habitat persistence was a good indication that captive-bred seahorses remained in their release location, which led to reproductive success with nine of the captive-bred seahorses observed reproducing in the wild, including mating with the wild population. This is a good indication that there has been a successful outcome from this restocking program. Additionally, the occurrence of 20% of captive-bred seahorses released onto the seahorse hotels still being detected at 12 months, and at least two individuals still occurring at the site after two years, indicates that captive-bred H. whitei can survive in the wild if suitable habitat conditions are available.
Whilst this study indicates that there is potential for conservation stocking to be used as a future tool to assist with the recovery of declining seahorse populations, caution is warranted before such a program is implemented. There are many ‘tools’ that can be used to assist with recovery of declining seahorse populations, and conservation stocking is one such tool; however, conservation stocking should not be considered as the ultimate recovery option, nor should it be done in isolation from other recovery efforts (IUCN, 2013). The reason for the decline of H. whitei has been attributed to loss of natural habitats as a result of anthropogenic impacts (Harasti, 2016; Harasti and Pollom, 2017) and its vital that efforts are made to halt the decline in natural habitats and if possible, to implement programs to assist with recovering declining habitats across the species range. There are several initiatives underway in NSW to help recover important seahorse habitats such as seagrasses (https://www.operationposidonia.com) and soft corals (Meryl Larkin unpublished data) and if successful, these habitat restoration efforts should benefit H. whitei populations.
The financial costs involved for a seahorse conservation stocking program would need to be considered prior to commencement to determine if such an operation is financially viable. SEA Life Sydney Aquarium estimate the current breeding program for H. whitei costs approximately $100 k (Australian dollars) for a period of 12 months. The costs associated with the program are aquaria operational costs (i.e., electricity, tank facilities, pumping of sea water), staff time (maintenance, feeding and veterinary support), food collection (live mysid shrimps from the wild) and food supplements. Similarly, if deploying artificial habitats such as seahorse hotels, the cost of materials and construction would also need to be budgeted with the cost for a single seahorse hotel costing ~$200 Aus dollars, however, this cost can vary depending on the type of materials used.
The seahorse H. whitei proved to be a good candidate to assess the viability of a marine conservation stocking program given its strong site attachment, resighting probability and ease of individual identification through VIFE marking techniques. This conservation stocking program provides a preliminary framework for future seahorse conservation stocking programs and the lessons learnt from this pilot study would be applicable to future conservation stocking programs for other threatened marine species. Particularly, the importance of implementing a post-monitoring program to determine if releasing captive-bred animals back into the wild was a success.
There is no right or wrong answer to whether conservation stocking of seahorses is a viable conservation method as it will likely vary between Hippocampus spp., however, there are numerous issues that should be considered in the planning stage for any seahorse stocking program. The IUCN ‘Guidelines for reintroductions and other conservation translocations’ (IUCN, 2013) provides valuable information that should be referred to in the planning stage. Whilst there are no specific guidelines for conservation stocking or translocations of seahorses, the following issues should be considered at a minimum:
1) Is there are an actual need for a captive-breeding program for the proposed Hippocampus spp.? Is the species abundance considered to be that depleted in the wild that a captive-breeding program needs to be implemented to ensure the species ongoing survival?
2) It needs to be considered if collecting seahorses from the wild for brood stock could actually have a detrimental effect on the wild population, as has been demonstrated in terrestrial species (IUCN, 2013; McCleery et al., 2014);
3) Consideration must be given to any potential unintended consequences of introductions back into the wild (Pearson et al., 2021);
4) Are the threats that have caused the seahorse to decline in the receiving location been reduced or eliminated, as releasing captive-bred animals whilst threats remain will likely reduce their survival (Moseby et al., 2011);
5) Brood stock selection should aim to provide adequate genetic diversity, selecting seahorses from the same area/habitats as the proposed release site and ensuring that only fit captive-bred individuals are released into the wild to maintain genetic diversity (IUCN, 2013; Willoughby and Christie, 2019); and
6) Finally, a seahorse stocking program must involve post-monitoring to assess the success of the release, therefore, enough resources and time need to be invested to assess variables such as seahorse survival, movement and reproduction. This study indicated that post-monitoring should be implemented for at least 12 months to assess the potential success or failure of a conservation stocking program for seahorses.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
This research was undertaken in accordance with NSW Department of Primary Industries (NSW DPI) Animal Research Authority 15/01 issued by the NSW Animal Care and Ethics Committee and NSW DPI scientific research permit P01/0059(A)-4.0. The collection of seahorses from the wild by SEA Life Sydney Aquarium was under NSW DPI permit (OUT21/12000) and the installation of seahorse hotels in Sydney Harbour was completed under a Transport for NSW licence to NSW DPI issued on 12 February 2020.
Author Contributions
DH, MB, and DB contributed to conception and design of the study. DH performed the statistical analysis. DH wrote the first draft of the manuscript. DH, MB, and DB wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This research was undertaken in accordance with NSW Department of Primary Industries (NSW DPI) Animal Research Authority 15/01 issued by the NSW Animal Care and Ethics Committee and NSW DPI scientific research permit P01/0059(A)-4.0. The collection of seahorses from the wild by SEA Life Sydney Aquarium was under NSW DPI permit (OUT21/12000) and the installation of seahorse hotels in Sydney Harbour was completed under a Transport for NSW license to NSW DPI issued on 12 February 2020. We are grateful to SEA LIFE Sydney Aquarium staff that implemented the captive breeding program within their facility, particularly Robbie McCracken, Chris Brown, Laura Simmons, Patrick Noble, Taylah Starc and Jillian Chambers. Thanks to the Threatened Species and Aquatic conservation managers within NSW Department of Primary Industries (Trevor Daly, Jillian Keating and Sarah Conacher) that were responsible for approvals and permits that allowed this project to be implemented. Thanks to the various scuba divers that assisted with the diving surveys, particularly Bee Kyle who helped with the initial surveys for several months. Thanks also to Dr Louw Claassens for assistance with mark-resight analysis and initial review of the manuscript.
References
Aaltonen K., Bryant A. A., Hostetler J. A., Oli M. K. (2009). Reintroducing Endangered Vancouver Island Marmots: Survival and Cause-Specific Mortality Rates of Captive-Born Versus Wild-Born Individuals. Biol. Conserv. 142 (10), 2181–2190. doi: 10.1016/j.biocon.2009.04.019
Baldacconi R., Cardone F., Longo C., Mercurio M., Marzano C. N., Gaino E., et al. (2010). Transplantation of Spongia Officinalis L.(Porifera, Demospongiae): A Technical Approach for Restocking This Endangered Species. Marine Ecol. 31 (2), 309–317. doi: 10.1111/j.1439-0485.2009.00299.x
Bartley D. M., Bell J. D. (2008). Restocking, Stock Enhancement, and Sea Ranching: Arenas of Progress. Rev. Fish Sci. 16 (1-3), 357–365. doi: 10.1080/10641260701678058
Bertolero A., Oro D., Besnard A. (2007). Assessing the Efficacy of Reintroduction Programmes by Modelling Adult Survival: The Example of Hermann’s Tortoise. Anim. Conserv. 10 (3), 360–368. doi: 10.1111/j.1469-1795.2007.00121.x
Cabaitan P. C., Conaco C. (2017). Bringing Back the Giants: Juvenile Tridacna Gigas From Natural Spawning of Restocked Giant Clams. Coral Reefs 36 (2), 519–519. doi: 10.1007/s00338-017-1558-9
Campbell-Thompson E., Vargas F. H., Watson R. T., Muela A., Cáceres N. C. (2012). Effect of Sex and Age at Release on the Independence of Hacked Harpy Eagles. J. Raptor Res. 46 (2), 158–167. doi: 10.3356/JRR-10-74.1
Claassens L., Booth A. J., Hodgson A. N. (2018). An Endangered Seahorse Selectively Chooses an Artificial Structure. Environ. Biol. Fish 101 (5), 723–733. doi: 10.1007/s10641-018-0732-4
Claassens L., Harasti D. (2021). Life History and Population Dynamics of an Endangered Seahorse (Hippocampus Capensis) Within an Artificial Habitat. J. Fish Biol 97 (4), 974–986. doi: 10.1111/jfb.14452
Correia M., Koldewey H., Andrade J. P., Palma J. (2015). Effects of Artificial Holdfast Units on Seahorse Density in the Ria Formosa Lagoon, Portugal. J. Exp. Marine Biol. Ecol. 471, 1–7. doi: 10.1016/j.jembe.2015.05.012
Correia M., Palma J., Koldewey H., Andrade J. P. (2013). Can Artificial Holdfast Units Work as a Habitat Restoration Tool for Long-Snouted Seahorse (Hippocampus Guttulatus Cuvier)? J. Exp. Marine Biol. Ecol. 448, 258–264. doi: 10.1016/j.jembe.2013.08.001
Curtis J. M. R., Vincent A. C. J. (2006). Life History of an Unusual Marine Fish: Survival, Growth and Movement Patterns of Hippocampus Guttulatus Cuvier 1829. J. Fish Biol. 68 (3), 707–733. doi: 10.1111/j.0022-1112.2006.00952.x
DPI N. (2020) Priorities Action Statement - Draft Actions for White’s Seahorse (Hippocampus Whitei (NSW Department of Primary Industries). Available at: https://www.dpi.nsw.gov.au/fishing/threatened-species/what-current/endangered-species2/whites-seahorse/priorities-action-statement-draft-actions-for-whites-seahorse-hippocampus-white (Accessed 20/01/2022).
Efrat R., Hatzofe O., Miller Y., Berger-Tal O. (2020). Determinants of Survival in Captive-Bred Griffon Vultures Gyps Fulvus After Their Release to the Wild. Conserv. Sci. Pract. 2 (12), e308. doi: 10.1111/csp2.308
Feunteun E. (2002). Management and Restoration of European Eel Population (Anguilla Anguilla): An Impossible Bargain. Ecol. Eng. 18 (5), 575–591. doi: 10.1016/S0925-8574(02)00021-6
Fischer J., Lindenmayer D. B. (2000). An Assessment of the Published Results of Animal Relocations. Biol. Conserv. 96 (1), 1–11. doi: 10.1016/S0006-3207(00)00048-3
Foster S. J., Vincent A. C. J. (2021). Holding Governments Accountable for Their Commitments: CITES Review of Significant Trade for a Very High-Volume Taxon. Global Ecol. Conserv. 27, e01572. doi: 10.1016/j.gecco.2021.e01572
Grey-Ross R., Downs C. T., Kirkman K. (2009). Reintroduction Failure of Captive-Bred Oribi (Ourebia Ourebi). South Afr. J. Wildlife Research-24-month delayed Open Access 39 (1), 34–38. doi: 10.3957/056.039.0104
Griffith B., Scott J. M., Carpenter J. W., Reed C. (1989). Translocation as a Species Conservation Tool: Status and Strategy. Science 245 (4917), 477–480. doi: 10.1126/science.245.4917.477
Halverson M. A. (2008). Stocking Trends: A Quantitative Review of Governmental Fish Stocking in the United State to 2004. Fisheries 33 (2), 69–75. doi: 10.1577/1548-8446-33.2.69
Harasti D. (2016). Declining Seahorse Populations Linked to Loss of Essential Marine Habitats. Marine Ecol. Prog. Ser. 546, 173–181. doi: 10.3354/meps11619
Harasti D. (2021). Getting Old: An Endangered Seahorse (Hippocampus Whitei) Lives for Up to 7 Years in the Wild. J. Fish Biol. 99 (5), 1752–1754. doi: 10.1111/jfb.14859
Harasti D., Gladstone W. (2013). Does Underwater Flash Photography Affect the Behaviour, Movement and Site Persistence of Seahorses? J. Fish Biol. 83, 1344–1353. doi: 10.1111/jfb.12237
Harasti D., Glasby T. M., Martin-Smith K. M. (2010). Striking a Balance Between Retaining Populations of Protected Seahorses and Maintaining Swimming Nets. Aquat. Conservation: Marine Freshwater Ecosystems 20 (2), 159–166. doi: 10.1002/aqc.1066
Harasti D., Martin-Smith K., Gladstone W. (2012). Population Dynamics and Life History of a Geographically Restricted Seahorse, Hippocampus Whitei. J. Fish Biol. 81 (4), 1297–1314. doi: 10.1111/j.1095-8649.2012.03406.x
Harasti D., Martin-Smith K., Gladstone W. (2014a). Does a No-Take Marine Protected Area Benefit Seahorses? PLos One 9 (8), e105462. doi: 10.1371/journal.pone.0105462
Harasti D., Martin-Smith K., Gladstone W. (2014b). Ontogenetic and Sex-Based Differences in Habitat Preferences and Site Fidelity of White’s Seahorse Hippocampus Whitei. J. Fish Biol. 85 (5), 1413–1428. doi: 10.1111/jfb.12492
Harasti D., Pollom R. A. (2017). “Hippocampus Whitei,” in The IUCN Red List of Threatened Species (IUCN). Available at: http://https://www.iucnredlist.org/species/10088/46721312
Hellyer C. B., Harasti D., Poore A. G. B. (2011). Manipulating Artificial Habitats to Benefit Seahorses in Sydney Harbour. Aquat. Conservation: Marine Freshwater Ecosystems 21, 582–589. doi: 10.1002/aqc.1217
IUCN (2013). Guidelines for Reintroductions and Other Conservation Translocations. Available at: https://www.iucn.org/content/guidelines-reintroductions-and-other-conservation-translocations
IUCN (2021) 2021 IUCN Red List of Threatened Species. Available at: http://www.iucnredlist.org (Accessed 20 January 2022).
Kaplan E. L., Meier P. (1958). Nonparametric Estimation From Incomplete Observations. J. Am. Stat. Assoc. 53 (282), 457–481. doi: 10.1080/01621459.1958.10501452
Kitada S. (2018). Economic, Ecological and Genetic Impacts of Marine Stock Enhancement and Sea Ranching: A Systematic Review. Fish Fish 19 (3), 511–532. doi: 10.1111/faf.12271
Koehn J. D., Lintermans M., Lyon J. P., Ingram B. A., Gilligan D. M., Todd C. R., et al. (2013). Recovery of the Endangered Trout Cod, Maccullochella Macquariensis: What Have We Achieved in More Than 25 Years? Marine Freshwater Res. 64 (9), 822–837. doi: 10.1071/MF12262
Koldewey H. J., Martin-Smith K. M. (2010). A Global Review of Seahorse Aquaculture. Aquaculture 302 (3-4), 131–152. doi: 10.1016/j.aquaculture.2009.11.010
Koning S., Hoeksema B. W. (2021). Diversity of Seahorse Species (Hippocampus Spp.) in the International Aquarium Trade. Diversity 13 (5), 187. doi: 10.3390/d13050187
Kyle R., Reid N., O’Connor N., Roberts D. (2017). Development of Release Methods for Captive-Bred Freshwater Pearl Mussels (Margaritifera Margaritifera). Aquat. Conservation: Marine Freshwater Ecosystems 27 (2), 492–501. doi: 10.1002/aqc.2704
Larkin M. F., Davis T. R., Harasti D., Cadiou G., Poulos D. E., Smith S. D. A. (2021). The Rapid Decline of an Endangered Temperate Soft Coral Species. Estuarine Coastal Shelf Sci. 255, 107364. doi: 10.1016/j.ecss.2021.107364
Lee K. A., Huveneers C., Peddemors V., Boomer A., Harcourt R. G. (2015). Born to be Free? Assessing the Viability of Releasing Captive-Bred Wobbegongs to Restock Depleted Populations. Front. Marine Sci. 2 (18). doi: 10.3389/fmars.2015.00018
Luzzatto D. C., Estalles M. L. (2021). Ex Situ Growing Out of Early Stages of Wild Seahorses: Envisioning Conservation Options for Threatened Populations. Aquat. Conservation: Marine Freshwater Ecosystems. 31 (9), 2666–2670. doi: 10.1002/aqc.3611
Manning C. G., Foster S. J., Harasti D., Vincent A. C. (2018). A Holistic Investigation of the Ecological Correlates of Abundance and Body Size for the Endangered White’s Seahorse Hippocampus Whitei. J. fish Biol. 93 (4), 649–663. doi: 10.1111/jfb.13745
Maran T., Põdra M., Põlma M., Macdonald D. W. (2009). The Survival of Captive-Born Animals in Restoration Programmes – Case Study of the Endangered European Mink Mustela Lutreola. Biol. Conserv. 142 (8), 1685–1692. doi: 10.1016/j.biocon.2009.03.003
McCleery R., Hostetler J. A., Oli M. K. (2014). Better Off in the Wild? Evaluating a Captive Breeding and Release Program for the Recovery of an Endangered Rodent. Biol. Conserv. 169, 198–205. doi: 10.1016/j.biocon.2013.11.026
Moseby K. E., Read J. L., Paton D. C., Copley P., Hill B. M., Crisp H. A. (2011). Predation Determines the Outcome of 10 Reintroduction Attempts in Arid South Australia. Biol. Conserv. 144 (12), 2863–2872. doi: 10.1016/j.biocon.2011.08.003
Muller E., Harasti D., Hoeksema B. W. (2022). Seahorse Predation by Octopuses in the Caribbean and the West Pacific. Diversity 14, 125. doi: 10.3390/d14020125
Pearson D. E., Clark T. J., Hahn P. G. (2021). Evaluating Unintended Consequences of Intentional Species Introductions and Eradications for Improved Conservation Management. Conserv. Biol. 36 (1), e13734. doi: 10.1111/cobi.13734
Peignot P., Charpentier M. J., Bout N., Bourry O., Massima U., Dosimont O., et al. (2008). Learning From the First Release Project of Captive-Bred Mandrills Mandrillus Sphinx in Gabon. Oryx 42 (1), 122–131. doi: 10.1017/S0030605308000136
Pollom R. A., Ralph G. M., Pollock C. M., Vincent A. C. (2021). Global Extinction Risk for Seahorses, Pipefishes and Their Near Relatives (Syngnathiformes). Oryx, 55 (4), 1–10. doi: 10.1017/S0030605320000782
Pratt T. C., Threader R. W. (2011). Preliminary Evaluation of a Large-Scale American Eel Conservation Stocking Experiment. North Am. J. Fish Manage. 31 (4), 619–628. doi: 10.1080/02755947.2011.609003
Rogers-Bennett L., Aquilino K. M., Catton C. A., Kawana S. K., Walker B. J., Ashlock L. W., et al. (2016). Implementing a Restoration Program for the Endangered White Abalone (Haliotis Sorenseni) in California. J. Shellfish Res. 35611–618(3), . doi: 10.2983/035.035.0306
Sayer C. D., Emson D., Patmore I. R., Greaves H. M., West W. P., Payne J., et al. (2020). Recovery of the Crucian Carp Carassius Carassius (L.): Approach and Early Results of an English Conservation Project. Aquat. Conservation: Marine Freshwater Ecosystems 30 (12), 2240–2253. doi: 10.1002/aqc.3422
Seddon P. J., Griffiths C. J., Soorae P. S., Armstrong D. P. (2014). Reversing Defaunation: Restoring Species in a Changing World. Science 345 (6195), 406–412. doi: 10.1126/science.1251818
Short G., Harasti D., Hamilton H. (2019). Hippocampus Whitei Bleeke a Senior Synonym of the Southern Queensland Seahorse H. Procerus Kuite: Molecular and Morphological Evidence (Teleostei, Syngnathidae). ZooKeys 824), 109. doi: 10.3897/zookeys.824.30921
Simons L. H., Hendrickson D. A., Papoulias D. (1989). Recovery of the Gila Topminnow: A Success Story? Conserv. Biol. 3 (1), 11–15. doi: 10.1111/j.1523-1739.1989.tb00218.x
Simpson M., Coleman R. A., Morris R. L., Harasti D. (2020). Seahorse Hotels: Use of Artificial Habitats to Support Populations of the Endangered White’s Seahorse Hippocampus Whitei. Marine Environ. Res. 157, 104861. doi: 10.1016/j.marenvres.2019.104861
Simpson M., Morris R. L., Harasti D., Coleman R. A. (2019). The Endangered White’s Seahorse Hippocampus Whitei Chooses Artificial Over Natural Habitats. J. fish Biol. 95 (2), 555–561. doi: 10.1111/jfb.14002
Simpson M., Morris R. L., Harasti D., Coleman R. A. (2021a). Assessing the Effects of Swimming Net Material on Populations of an Endangered Seahorse. Marine Freshwater Res. 72 (6), 800–810. doi: 10.1071/MF20022
Simpson M., Morris R. L., Harasti D., Coleman R. A. (2021b). Swimming Nets Have Positive Effects on Populations of the Endangered White’s Seahorse Hippocampus Whitei. Aquat. Conservation: Marine Freshwater Ecosystems 31 (1), 60–73. doi: 10.1002/aqc.3451
Taggart D. A., Schultz D. J., Corrigan T. C., Schultz T. J., Stevens M., Panther D., et al. (2016). Reintroduction Methods and a Review of Mortality in the Brush-Tailed Rock-Wallaby, Grampians National Park, Australia. Aust. J. Zoology 63 (6), 383–397. doi: 10.1071/ZO15029
Tavecchia G., Viedma C., Martínez-Abraín A., Bartolomé M.-A., Gómez J. A., Oro D. (2009). Maximizing Re-Introduction Success: Assessing the Immediate Cost of Release in a Threatened Waterfowl. Biol. Conserv. 142 (12), 3005–3012. doi: 10.1016/j.biocon.2009.07.035
Taylor M. D. (2017). Preliminary Evaluation of the Costs and Benefits of Prawn Stocking to Enhance Recreational Fisheries in Recruitment Limited Estuaries. Fish Res. 186, 478–487. doi: 10.1016/j.fishres.2016.05.030
Taylor M. D., Chick R. C., Lorenzen K., Agnalt A.-L., Leber K. M., Blankenship H. L., et al. (2017). Fisheries Enhancement and Restoration in a Changing World. Fish Res. 186, 407–412. doi: 10.1016/j.fishres.2016.10.004
Thévenin C., Morin A., Kerbiriou C., Sarrazin F., Robert A. (2020). Heterogeneity in the Allocation of Reintroduction Efforts Among Terrestrial Mammals in Europe. Biol. Conserv. 241, 108346. doi: 10.1016/j.biocon.2019.108346
Vincent A. C. J., Evans K. L., Marsden A. D. (2005). Home Range Behaviour of the Monogamous Australian Seahorse, Hippocampus Whitei. Environ. Biol. Fish 72 (1), 1–12. doi: 10.1007/s10641-004-4192-7
Vincent A. C. J., Foster S. J., Koldewey H. J. (2011). Conservation and Management of Seahorses and Other Syngnathidae. J. Fish Biol. 78 (6), 1681–1724. doi: 10.1111/j.1095-8649.2011.03003.x
Vincent A. C. J., Sadler L. M. (1995). Faithful Pair Bonds in Wild Seahorses, Hippocampus Whitei. Anim. Behav. 50, 1557–1569. doi: 10.1016/0003-3472(95)80011-5
Welsh A. B., Carlson D. M., Schlueter S. L., Jackson J. R. (2020). Tracking Stocking Success in a Long-Lived Species Through Genetics and Demographics: Evidence of Natural Reproduction in Lake Sturgeon After Twenty-Two Years. Trans. Am. Fish Soc. 149 (1), 121–130. doi: 10.1002/tafs.10214
White T. H., Abreu W., Benitez G., Jhonson A., Lopez M., Ramirez L., et al. (2021). Minimizing Potential Allee Effects in Psittacine Reintroductions: An Example From Puerto Rico. Diversity 13 (1), 13. doi: 10.3390/d13010013
Willoughby J. R., Christie M. R. (2019). Long-Term Demographic and Genetic Effects of Releasing Captive-Born Individuals Into the Wild. Conserv. Biol. 33 (2), 377–388. doi: 10.1111/cobi.13217
Wong J. M., Benzie J. A. H. (2003). The Effects of Temperature, Artemia Enrichment, Stocking Density and Light on the Growth of Juvenile Seahorses, Hippocampus Whitei (Bleeke, From Australia. Aquaculture 228 (1-4), 107–121. doi: 10.1016/S0044-8486(03)00320-X
Woods C. (2003). Growth and Survival of Juvenile Seahorse Hippocampus Abdominalis Reared on Live, Frozen and Artificial Foods. Aquaculture 220 (1-4), 287–298. doi: 10.1016/S0044-8486(02)00227-2
Woods C. M. C., Martin-Smith K. M. (2004). Visible Implant Fluorescent Elastomer Tagging of the Big-Bellied Seahorse, Hippocampus Abdominalis. Fish Res. (Amsterdam) 66 (2-3), 363–371. doi: 10.1016/S0165-7836(03)00183-8
Zukowski S., Whiterod N., Ellis I., Gilligan D., Kerezsy A., Lamin C., et al. (2021). Conservation Translocation Handbook for New South Wales Threatened Freshwater Fishes. Available at: https://www.freshwateranglers.com.au/wp-content/uploads/2021/04/Zukowskietal-2021-NSW-conservation-translocation-handbook-FINAL.pdf
Keywords: captive-bred, mark-resight, restocking, reintroduction, threatened species, syngnathidae
Citation: Harasti D, Brennan M and Booth DJ (2022) Assessing the Effectiveness of Conservation Stocking for the Endangered White’s Seahorse Hippocampus whitei. Front. Mar. Sci. 9:867352. doi: 10.3389/fmars.2022.867352
Received: 01 February 2022; Accepted: 13 May 2022;
Published: 22 June 2022.
Edited by:
Joseph D. DiBattista, Australian Museum, AustraliaReviewed by:
Maarten De Brauwer, Commonwealth Scientific and Industrial Research Organisation (CSIRO), AustraliaFelipe Cohen, University of São Paulo, Brazil
Copyright © 2022 Harasti, Brennan and Booth. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David Harasti, david.harasti@dpi.nsw.gov.au
 David Harasti
David Harasti Mitchell Brennan
Mitchell Brennan David J. Booth
David J. Booth