Hydrodynamic Interactions Between Macroalgae and Their Epibionts
- 1Department of Integrative Biology, University of California, Berkeley, Berkeley, CA, United States
- 2Department of Biology, University of Washington, Seattle, WA, United States
Macroalgae provide surfaces where other organisms live. Unlike organisms on rigid substrata, epibionts on host macroalgae sit on flexible surfaces that bend, stretch, and move in turbulent water currents and waves. We used blade-like red algae, Mazzaella splendens, and encrusting bryozoans, Membranipora membranacea, to investigate the biomechanical and hydrodynamic effects of encrusting epibionts on macroalgae, and of flexible hosts on epibiotic bryozoans. Passive flapping by algae in wave-driven ambient flow enhanced renewal of water near hosts and epibionts. Wave exposure and the presence of a surrounding canopy of flexible algae altered the locations along algal blades where bryozoans encountered the highest time-averaged boundary shear velocities. Hydrodynamic forces on flexible algae moving back-and-forth with the water were lower in waves than in unidirectional flow. Bryozoan epibionts increased hydrodynamic forces on host algae by affecting their reconfiguration in moving water. Encrusting bryozoans increased the flexural stiffness of algal blades, but the elastic modulus, extensibility, and strength of blade tissue was unaffected by bryozoan epibionts. Algal blades were more extensible and stronger than bryozoans, so bryozoans fractured or popped off stretched algae. Algae in rapid-flow habitats had few epibionts, and encrusted algae transplanted from a protected to a wave-exposed habitat lost their epibionts.
1 Introduction
In many benthic communities, macroalgae and seagrasses (“macrophytes”) provide surfaces on which other organisms live (reviewed by Seed and O’Connor, 1981; Wahl, 1989; Christie et al., 2009; Harder, 2009). Therefore, an important aspect of understanding the hydrodynamic forces on, and mass transport to and from these organisms depends on how the host (“basibiont”) macrophyte and the organisms living on its surfaces (“epibionts”) affect each other’s hydrodynamics. While organisms living on the substratum or on rigid basibionts such as corals are located on solid surfaces that are fixed relative to the ambient flow, epibionts on macrophytes sit on flexible surfaces that bend, stretch, and move in turbulent water currents and waves.
The epibionts living on macrophyte hosts include bacteria, micro- and macroalgae, sessile suspension-feeding invertebrates (e.g. bryozoans, hydroids, sponges, tunicates), and motile fauna (e.g. snails, amphipods, isopods, crabs) (Wing and Clendenning, 1971; Seed and O’Connor, 1981; Stewart, 1982; Schultze et al., 1990; Wahl, 2008; Christie et al., 2009; Burnett and Koehl, 2018). Older, larger macrophytes, and those with complex architecture tend to have more abundant epibionts (Seed and O’Connor, 1981; Wahl, 2008; Christie et al., 2009; Burnett and Koehl, 2019). The spatial distribution of epibionts on their hosts depends on species and is determined by factors such as larval settlement, growth, competition, and mortality of the epibionts (Bernstein and Jung, 1979; Seed and O’Connor, 1981; Durante and Chia, 1991; Harder, 2009; Arkema and Samhouri, 2019). The ecological interactions of the members of epibiotic communities are well-studied, as are epibiont effects on the roles played by their macrophyte hosts in benthic communities (Seed and O’Connor, 1981; Scheibling et al., 1999; Wahl, 2008; Harder, 2009; da Gama et al., 2014). Here we complement that ecological work with a focus on the physical mechanisms by which flexible macrophyte hosts and epibionts affect each other’s biomechanical properties and hydrodynamic performance.
1.1 Effects of Macrophyte Hosts on Their Epibionts
Marine macrophyte hosts are “ecosystem engineers”(sensu Jones et al., 1994; Arkema and Samhouri, 2019) that alter the physical and chemical environment of their epibionts. In addition to providing surfaces on which epibionts live (e.g. Seed and O’Connor, 1981; Wahl, 1989; Christie et al., 2009; Harder, 2009), host macrophytes also provide a number of benefits to their epibionts. For example, epiphytes that photosynthesize can encounter more light if sitting on hosts that hold them higher in the water column (Wahl, 1989). In addition, exudates from host macrophytes can contribute to the nutrition of epibionts and enhance their growth and survival (de Burgh and Fankboner, 1978; Oswald and Seed, 1986; Williams and Seed, 1992; Manriquez and Cancino, 1996; Harder, 2009). Host exudates also encourage the growth of microorganisms that are eaten by epibiotic animals (Christie et al., 2009). Some epibionts are herbivores that eat host tissue (Seed and O’Connor, 1981; Christie et al., 2009; Burnett and Koehl, 2019; Koehl, 2022). Macrophytes can provide refuges for epibionts from predators (Seed and O’Connor, 1981; Wahl, 1989), and chemical defenses of host macroalgae against grazers can also protect their epibionts from being eaten (Harder, 2009). Furthermore, rafting on broken drifting macrophytes is an important mechanism of dispersal to new habitats for epibionts (Worcester, 1994; Wahl, 2008; Avila et al., 2020).
Local water motion near macroalgal surfaces can affect epibionts in a several important ways. For example, it has long been recognized that moving water imposes hydrodynamic forces on epibionts that could break or dislodge them (Fenwick, 1976; Seed and O’Connor, 1981; Walters et al., 2003), but also that flow near macrophytes transports water-borne nutrients, prey, and gases to epibionts, and removes wastes and silt (e.g. Seed and O’Connor, 1981; Wahl, 1989; Harder, 2009). Moreover, ambient water motion near macrophytes can disperse the gametes and propagules of epibionts. In addition, the food-capture rates of suspension-feeding animals such as bryozoans that live on macrophytes are reduced if the water motion across them is too fast or too slow (Okamura, 1985; Okamura, 1990; Okamura, 1992; Pratt, 2008).
Despite the importance of water flow to epibiont function, the effects of flexible macrophytes on the water motion encountered by epibionts are poorly understood. For example, it has been suggested that macrophytes with branching or complex morphologies provide refuges for epibionts from rapid flow (Fenwick, 1976; Seed and O’Connor, 1981; Anderson and Martone, 2014; Burnett and Koehl, 2018). However, it has also been suggested that sitting on a host places epibionts in the more rapidly-moving water away from the substratum where the transport of water-borne materials is faster (Wahl, 1989; Harder, 2009). Some investigators propose that such enhanced transport is the mechanism responsible for higher growth rates of epibionts near the tips of macrophyte blades (Keough, 1986; Harder, 2009), whereas others argue that blade tips provide a refuge where epibionts are protected from very rapid flow as flexible macrophytes are bent over by currents and blade tips are sheltered in the wake behind upstream parts of the macrophyte (Anderson and Martone, 2014). Thus, the effects of the deformation of flexible hosts on the fine-scale hydrodynamic environments of their epibionts remains and open and interesting problem and should be measured.
Little is known about effects of habitat type on the water motion experienced by organisms living on flexible macroalgae and seagrasses. Although there have been many reports of greater diversity (Norton, 1973; Fenwick, 1976; Schultze et al., 1990; Bueno et al., 2016) and higher abundance (Peteiro and Freire, 2013) of epibionts on macrophytes in habitats protected from fast ambient flow, there are other cases where epibiont abundance is higher in habitats exposed to rapid, turbulent water motion (Seed and O’Connor, 1981). The shallow coastal marine habitats where macrophytes live, from wave-swept rocky shores to protected estuaries, are exposed to turbulent currents and waves. In areas with wave-driven water flow, macrophytes are subjected to back-and-forth water motion with a period of seconds when a wave passes, but the net transport of water and water-borne materials through such habitats is slow as the water sloshes back and forth (Koehl et al., 1993; Koehl and Powell, 1994; Koehl, 2022). As a flexible macrophyte is flapped back and forth in wavy flow, it moves with the water (hence there is no flow relative to its surfaces) until it reaches the end of its tether and the water moves past it (Koehl, 1999; Koehl, 2022). Therefore, epibionts near the bases of macrophytes in waves should encounter more water motion relative to their surfaces than those riding with the flow on more distal regions of the host. However, flexible macrophytes in water currents can flutter like flags, and such fluttering might increase the local water motion encountered by epibionts on distal regions of the host. Fluttering also stirs the water near macrophytes, thereby replacing depleted water with undepleted water (Koehl and Alberte, 1988; Koch et al., 2006; Jabbari et al., 2021). Another important aspect of the environments of epibionts is that macroalgae and seagrasses usually occur in aggregations (e.g. seagrass meadows, kelp forests, algal beds). Such canopies reduce the speed, damp the waves, and alter the turbulence spectrum of the water flowing through them (Arkema and Samhouri, 2019; Zhu et al., 2021; Koehl, 2022), which should affect both the transport and hydrodynamic forces experienced by epibionts on hosts in the canopy. Thus, studies of hydrodynamic effects of flexible hosts on their epibionts should be conducted in the field and in laboratory conditions that mimic flow measured in the field.
1.2 Effects of Epibionts on Their Macrophyte Hosts
Heavy fouling by epibionts can decrease growth rates of macrophytes (Seed and O’Connor, 1981; Brush and Nixon, 2002; da Gama et al., 2008; Harder, 2009) and reduce their reproductive output (Seed and O’Connor, 1981; Brush and Nixon, 2002; Saier and Chapman, 2004; da Gama et al., 2008; da Gama et al., 2014). Such reduced growth can be due to the deleterious effects of epibionts on mass exchange and photosynthesis by the host. Macroalgae have no roots and thus depend on uptake through their surfaces to acquire nutrients, dissolved gases, and other essential substances from the surrounding water (reviewed by Hurd et al., 2000), while seagrasses rely on both root uptake and transport across the surfaces of their leaves (Koch et al., 2006). Epibionts can interfere with uptake by macrophytes by covering uptake surfaces or by depleting needed substances from the water (Wahl, 1989; Fletcher, 1995; Manriquez and Cancino, 1996; Hurd et al., 2000; Brush and Nixon, 2002; Koch et al., 2006; Harder, 2009; da Gama et al., 2014). Epibionts coating macrophyte surfaces can reduce photosynthesis by blocking sunlight (Wing and Clendenning, 1971; Oswald et al., 1984; Wahl, 1989; Fletcher, 1995; Brush and Nixon, 2002; da Gama et al., 2008; Wahl, 2008; Harder, 2009; da Gama et al., 2014), and by weighing down hosts, which sink to depths where they encounter lower light (Dixon et al., 1981; Seed and O’Connor, 1981; Wahl, 1989; Wahl, 2008; Wong and Vercaemer, 2012). Shading by epibionts can also lead to decreased production of antifouling compounds by the host (Wahl, 2008).
Some epibionts can provide benefits to host macrophytes. For example, herbivores that graze on epiphytic algae can reduce their deleterious effects on the host (Hughes et al., 2004; Heck and Valentine, 2006). Furthermore, the wastes of epibiotic animals can provide nutrients to macrophytes (Wahl, 1989; Hepburn et al., 2006; Wahl, 2008; Hepburn et al., 2012). A coating of epibionts can slow the rate of desiccation of intertidal macrophytes when exposed to air (Wahl, 1989; Wahl, 2008).
Epibionts can either increase or decrease the susceptibility of the host to being eaten (Wahl, 1989). Hosts can be damaged by predators on their epibionts (“shared doom” sensu Wahl and Hay, 1995) (Bernstein and Jung, 1979; Dixon et al., 1981; Wahl, 1989; Karez et al., 2000; Wahl, 2008; da Gama et al., 2008). Conversely, hosts can be protected from grazing if their epibionts repel herbivores or provide chemical camouflage for the host (“associational resistance” sensu Wahl and Hay, 1995) (Wahl, 1989; Durante and Chia, 1991; Karez et al., 2000; Wahl, 2008; da Gama et al., 2008). Macrophytes often break at wounds produced by herbivores (Black, 1976; Koehl and Wainwright, 1977; Scheibling et al., 1999; Krumhansl and Scheibling, 2011; Burnett and Koehl, 2019; Burnett and Koehl, 2020; Burnett et al., 2021). Sometimes such pruning reduces the hydrodynamic forces on the hosts, enabling them to better withstand dislodgement during times of high wave action (Black, 1976).
There are many reports that epibionts increase the defoliation or dislodgement of host macrophytes, especially during winter storms (e.g. Dixon et al., 1981; Lambert et al., 1992; Scheibling et al., 1999; Levin et al., 2002; Saier and Chapman, 2004; Saunders and Metaxas, 2008; Scheibling and Gagnon, 2009; Watanabe et al., 2010; Krumhansl and Scheibling, 2011; da Gama et al., 2014), leading to loss of biomass from macrophyte communities and seaweed farms and to an increase in the supply of detritus for benthic communities (Fletcher, 1995; Krumhansl and Scheibling, 2011). It has been suggested that epibionts cause this macrophyte loss by increasing the hydrodynamic forces the hosts must bear in flowing water (Dixon et al., 1981; Wahl, 1989; Wahl, 2008; Harder, 2009). Flexible macrophytes are passively reconfigured by ambient flow into compact streamlined shapes that reduce drag (Koehl, 1986; Koehl and Alberte, 1988; Martone et al., 2012; de Bettignies et al., 2013; Anderson and Martone, 2014; Koehl, 2022), so it has been suggested that epibionts that increase the effective size or stiffness of the host could increase drag on the host by interfering with such reconfiguration (Koch et al., 2006; Wahl, 2008; Harder, 2009). Measurements in unidirectional flow showed that the drag on intertidal red algae was higher when they were covered with bulbous algal epiphytes than when they bore no epibionts (Anderson and Martone, 2014). However, the effects on drag of crustose epibionts that appear to deform and stiffen macrophyte blades (Neushul and Haxo, 1963; Dixon et al., 1981; Wahl, 1989) have not yet been measured, nor have the hydrodynamic forces on fouled versus unfouled macrophytes in the oscillatory flow of waves.
Epibionts might also increase the chances that hosts are broken in waves and currents by damaging host tissues or affecting their mechanical behavior. Epibionts can damage host surfaces by altering the local pH (Wahl, 1989; Harder, 2009), by causing injuries with their anchoring devices (da Gama et al., 2014), or by grazing (e.g. Scheibling et al., 1999; Krumhansl and Scheibling, 2011; Burnett and Koehl, 2019; Koehl, 2022; Burnett et al., 2021). Epibionts might also affect the mechanical properties of the host’s tissues (“material properties”). Many reports describe macroalgal fronds encrusted with epibionts as being “brittle”, and thus susceptible to breaking during winter storms (Lobban, 1978; Dixon et al., 1981; Wahl, 1989; Forde et al., 2016). However, unfouled tissues of macroalgae also become weaker during the winter when growth rates are low (Bell, 1992; Johnson and Koehl, 1994). Nonetheless, one study of the material properties of kelp blades did show that tissues under encrusting bryozoans had similar stiffness to, but lower breaking strength, breaking extension, and toughness than unfouled tissue, and that cells in the outer layers of blades under bryozoans were degraded (Krumhansl et al., 2011). Effects of epibionts on the material properties of other types of macrophytes and during seasons of high algal growth are not yet documented.
Macrophytes have defenses against epibionts. Many produce antifouling chemicals that interfere with the recruitment or survival of epibionts (Wahl, 1989; Paul, 1992; Steinberg and de Nys, 2002; Pereira et al., 2003; Walters et al., 2003; Nylund and Pavia, 2005; Matson et al., 2010; da Gama et al., 2014). Some macrophytes have hydrophobic surfaces, mucilage coatings, hairs, spicules, or non-stick textures that interfere with the attachment of epibionts (Walters et al., 2003). If epibionts are weaker than their host, they can be washed off the host by ambient water flow (Anderson and Martone, 2014). Very flexible macrophytes can shed rigid epibionts, such as worms with calcareous tubes, as the hosts are bent and twisted by moving water (Walters et al., 2003). Some macrophytes rid themselves of epibionts by shedding their cuticle or outermost cell layers, or by harboring animals that eat their epibionts. while others produce unfouled surfaces by outgrowing their epibionts (Wahl, 1989; Wahl et al., 1998; Walters et al., 2003; Saunders and Metaxas, 2008a; Harder, 2009).
1.3 Research System
We studied the physical interactions between the red macroalga, Mazaella splendens, and the epibiotic bryozoan, Membranipora membranacea, comparing their performance at sites exposed to different ambient water flow conditions.
M. splendens (formerly Iridaea chordata), a red alga (Rhodophyta) that has a short (1-2 cm), narrow stipe supporting one wide blade (typically 20 - 40 cm long), is abundant in low intertidal and high subtidal habitats of rocky shores along the Pacific coast of North America that are subjected to low or intermediate wave exposure (Abbott and Hollenberg, 1992; Shaughnessy et al., 1996). M. splendens are also common on man-made structures such as docks, pilings, and breakwaters (Figures 1A, B). This species has a complex life cycle with sexual stages (haploid male or female gametophytes) and an asexual stage (diploid tetrasporophyte), all of which have the same thallus morphology and co-occur at the same sites (Dyck and de Wreede, 2006a). The density of M. splendens blades is generally higher in the summer (Dyck and de Wreede, 2006b) and larger individuals are dislodged during winter storms (Bell, 1999), but they have a perennial, fleshy holdfast out of which new thalli can grow (Abbott and Hollenberg, 1992; Bell, 1999).
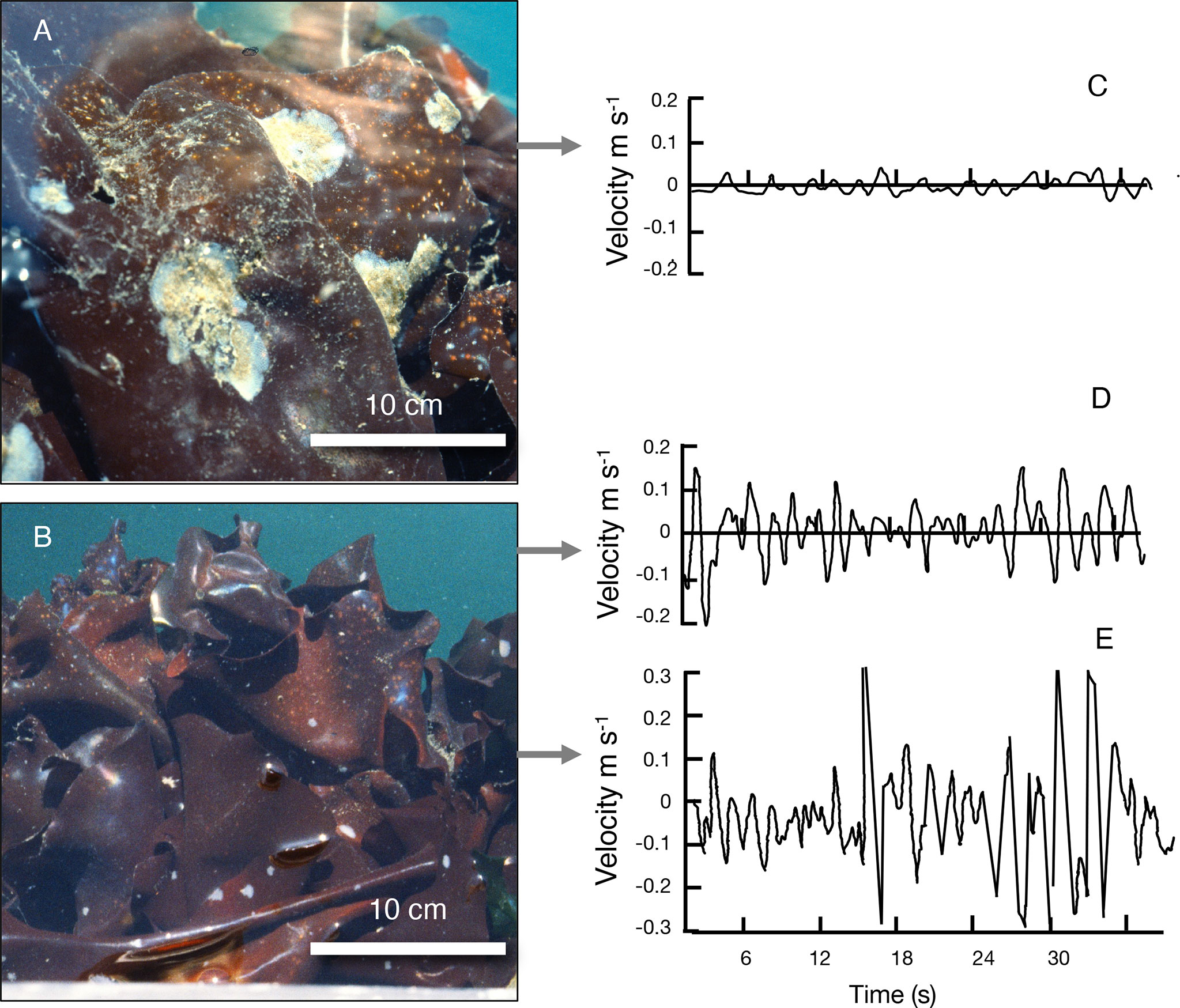
Figure 1 Study sites on protected and exposed sides of the floating breakwater protecting the dock at Friday Harbor Laboratories (map of sites in Figure 2A). (A) A canopy of M. splendens at the protected site, where white colonies of M. membranacea were living on the algal blades. (B) A canopy of M. splendens at the exposed site, where the algal blades were not covered with M. membranacea. For both (A, B), the tire bumper on which the algae were growing is along the bottom of the picture (tire bumpers on the breakwater are shown in Figure 2B). (C) Examples of water velocities recorded just upstream of M. splendens at the protected site, (D) at the exposed site, and (E) at the exposed site as the wake of a large ferry hit the dock. The flow oscillations in (C, D) were due to small waves (wind chop), and in (E) were due to larger waves in the ferry wake.
M. membranacea is an abundant cheilostome bryozoan that forms flat colonies that encrust macrophytes (Figure 1A) in the Pacific and Atlantic Oceans (Bernstein and Jung, 1979; Seed and O’Connor, 1981; Manriquez and Cancino, 1996; Saunders and Metaxas, 2008; Caines and Gagnon, 2012; Arkema and Samhouri, 2019). They are often the competitive dominant among the epibionts on a host (Bernstein and Jung, 1979), and they are associated with canopy loss in kelp beds (e.g. Lambert et al., 1992; Scheibling et al., 1999; Levin et al., 2002; Saunders and Metaxas, 2008).
1.4 Objectives of This Study
The complex and dynamic effects of epibionts and macrophytes on each other depend on the hydrodynamic habitats in which they live. In this study we explore the fluid-structure interactions of these epibionts and hosts, using a combination of field and laboratory experiments to address three questions:
(1) How does a flexible host affect the water flow environment experienced by encrusting epibionts attached to its surface?
(2) How do encrusting epibionts affect the hydrodynamic forces on a flexible host?
(3) How do encrusting epibionts and deformable hosts affect each other’s material properties and biomechanical performance?
2 Methods
2.1 Water Flow at Exposed and Protected Field Sites
We studied Mazaella splendens and the epibiotic bryozoan, Membranipora membranacea living on the floating breakwater protecting the dock at Friday Harbor Laboratories, San Juan Island, Washington. Our “exposed site” was on the seaward side of the breakwater that was exposed to currents, waves, and ferry wakes, and our “protected site” was on the shoreward side of the same section of breakwater, so it experienced much calmer flow conditions (Figure 2A). The floating breakwater was 5 m wide, so both sites were exposed to the same light, temperature, water chemistry, and ferry traffic. Dense beds of M. splendens (Figures 1A, B) grew on the submerged portions of tire bumpers (Figure 2B) along both sides of the breakwater.
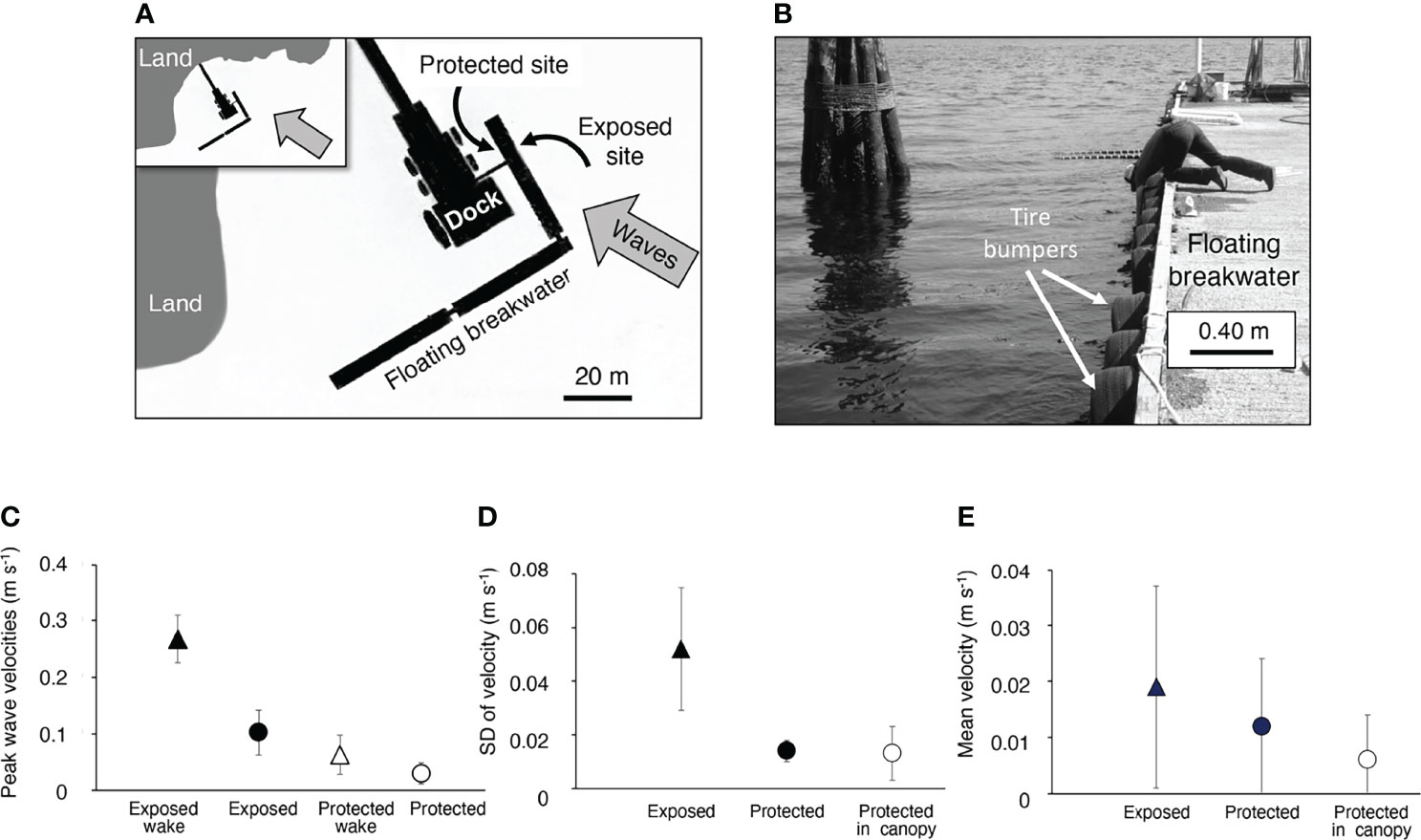
Figure 2 Water flow measured at the protected and exposed study sites. (A) Map of the location of the exposed and protected sites on the floating breakwater at Friday Harbor Laboratories (inset shows a wider view of the area). Open water is to the right of the region shown in the maps. The direction of oncoming waves (both wind chop and ferry wakes) is indicated by the grey arrows. Measurements on a satellite image of the breakwater that maps the ferry paths (https://www.google.com/maps) show that ferries come within 450 to 530 meters of the breakwater. (B) Photograph of the tire bumpers along the exposed side on the floating breakwater, which is 5 m wide. (C) Peak flow velocities measured at a depth of 20 cm below the air-water interface at positions just upstream from or within M. splendens canopies on different tires. The mean of the peaks of each flow record (1 minute duration, except for the case of the ferry wakes, which lasted about 10 to 15 s) were calculated. The grand mean of all the replicate measurements is plotted (number of replicate measurements, n, at different locations along the dock for each condition are: exposed site, n = 10; exposed site in ferry wake, n = 3; protected site, n = 10; protected site in ferry wake, n = 2; protected site within the algal canopy, n = 2). The peak velocities on the exposed side of the dock were significantly higher than those on the protected side of the dock (p < 0.01), and the peak velocities in the ferry wakes hitting the exposed side of the dock were significantly higher than all the other flow conditions (p < 0.01) (one-way ANOVA followed by Tukey HSD test, F = 214,24). (D) Variation in flow velocities measured during 1-minute flow records as described in (A) Because ferry wake records were shorter, they were not included in this analysis. Fluctuations, represented by the standard deviation of the velocities measured at each location, were due to turbulence and waves. The variation in flow velocity was significantly greater at the exposed site than at the protected site both outside the algal canopy (p < 0.05) and inside the algal canopy (p < 0.01). There was no significant difference between flow variation outside vs. inside the canopy at the protected site (p > 0.05) (one-way ANOVA followed by Tukey HSD test, F = 15.862,18). (E) Mean flow velocity, a measure of the net transport of water through a site, measured during the 1-minute flow records described in (A, B). There was no significant difference between the mean flow velocities at the exposed and protected sites, nor between mean velocities outside vs. within an algal canopy at the protected site (p > 0.05, one-way ANOVA followed by Tukey HSD test, F = 0.872,18). In all graphs, error bars represent one standard deviation.
Water flow velocities at each site were measured with an electromagnetic EPCO Water Current Meter (Model 6130). At each recording location, the flow probe was supported by an adjustable scaffolding on the breakwater and was positioned 20 cm below the water surface just outside the canopy of M. splendens (distance of the probe from the algae waving in the flow was 10 to 20 cm). The measuring electrodes of the cylindrical flow probe (diameter = 1cm, length = 15 cm) were 1 cm from the tip of the probe. Flow was recorded near the algae on different tire bumpers at the exposed and protected sites. In addition, to assess what we expected to be the slowest water velocities encountered by M. splendens, we placed the flow probe within canopies of M. splendens on two different tires on the protected side of the breakwater. We oriented the flow probe to record water moving in the direction of most rapid flow, which was perpendicular to the breakwater in every case. Output from the flow meter was recorded on a field portable, battery-operated chart recorder (Omega Engineering Model 142) and we sampled flow velocity every 0.6 s from these records. The breakwater was subjected to ambient wave-driven flow and to the wakes of large ferries passing nearby (Figure 2A). Each ferry produced a wake that hit the breakwater as it entered the harbor, and another when it departed from the harbor, and there were usually more than 10 ferries per day. Ambient flow was wavy, so water moved back and forth (i.e. velocities oscillated between positive and negative with each wave). Mean flow velocity is a measure of the net transport of water through a site, so we calculated the mean velocity for each ambient flow record (duration 1 min). In wavy flow, peak water velocities are a better indication than mean velocity of a benthic organism’s exposure to hydrodynamic forces. Therefore, for each ambient flow record (duration of 1 minute) and each record of a ferry wake (duration 10 to 15 s), we chose the flow direction that had the largest peaks (towards the floating breakwater for the exposed site, and away from the breakwater at the protected site) and calculated the mean of the peak velocities in each wave that occurred during the record.
2.2 Distribution of Bryozoan Cover and Structure of Algal Canopies
2.2.1 Determination of Percent Cover of Bryozoans on Algal Blades
M. splendens blades are ruffled, so blade area could not be measured using images. Instead we weighed each thallus and then converted the mass to area. Squares 1 x 1 cm were cut from the middle of blades of six M. spendens. These squares were blotted on paper towels and their mass was measured to the nearest 0.01 g using a Mettler Model P136 Balance. The mean of those masses per cm2 of blade was used to convert the masses of blotted algal thalli (measured using the same balance) into areas. We also weighed M. membranacea to determine their area. Bryozoan colonies were gently peeled off algal blades and squares 1 x 1 cm were cut from each of six colonies. Those squares were placed in a dying oven at 80°C for 24 hours and then weighed to the nearest 0.01 g. The mean dry mass per cm2 was used to convert dry weight of bryozoans removed from an alga to area of bryozoan cover. The percent cover of bryozoans on an algal thallus was determined by peeling all the bryozoans off an algal thallus, measuring the wet mass of the cleaned algal thallus to determine its area, drying the removed bryozoans and converting their total dry weight to area, and then calculating percent cover = 100 (area of bryozoans)/(blade area).
2.2.2 Determination of the Distribution of Bryozoans Along the Length of Algal Thalli
We observed that M. splendens at the exposed site were free of bryozoan epibionts (Figure 1B), so we only measured the spatial distribution of M. membranacea cover along the length of 12 thalli collected from different tires at the protected site. The distance between holdfast and blade tip was measured to the nearest cm. A transect from blade base to tip was marked at intervals that were one tenth of the total blade length, and then the blade was cut transversely into strips at these intervals. All the bryozoans were peeled off each strip of blade and the % cover of bryozoans on each strip was determined as described in 2.2.1.
2.2.3 Description of Canopies of M. splendens
M. splendens in the field often occur in aggregations (Figures 1A, B). Although the vertical distribution of macrophyte structures in canopies can be determined for beds of aquatic macrophytes in steady unidirectional flow (reviewed in Koehl, 2022), such canopy geometry changes rapidly for flexible macroalgae in waves as they flap back and forth. Therefore, to describe the canopies of M. splendens at our field sites, we instead measured lengths and widths of their thalli and determined their density (number of algae per area of substratum). At both the exposed and protected sites, we used a random-number table to pick a tire, determined the submerged area (to the nearest 0.001 m2) of the tire, counted the number of M. splendens, and measured the lengths and widths (to the nearest mm). We then moved to neighboring tires to the South and repeated the counts (n = 3 tires) and measurements (for the first 17 M. splendens encountered).
2.3 Effects of Flexible Host Algae on Local Flows Near Bryozoan Epibionts
2.3.1 Local Flow Encountered by Epibionts on Algal Blade Surfaces
The fine-scale local water flow relative to epibionts riding on the surface of a flexible host determines both the forces on the epibionts and the transport rates of dissolved substances between the moving water and the organisms. In turbulent flow, boundary shear velocity (u*) is a measure of momentum flux in the boundary layer and is a correlate of the flux of material between the water and a surface. We used calibrated dissolution modules (DM’s; Pep-o-Mint Life Savers) sewn onto three different positions on blades of M. splendens to get an average measure at both field sites of the local mass and momentum exchange experienced by algal blade surfaces and the M. membranacea living on them (Koehl and Alberte, 1988; Shaughnessy et al., 1996; Anderson and Martone, 2014). We calibrated the rate of weight loss of the DM’s at a range of u*’s that we measured in steady flow over the smooth, rigid floor of a flume. Although the instantaneous flow along the surface of a flapping algal blade in waves is more complex than the steady boundary layer flow in the flume, the weight loss rate of a DM on an algal blade tells us that the time-averaged exchange at a defined position on the blade surface is equivalent to the exchange produced in steady flow over a rigid, flat, smooth surface at a given u*.
We calibrated the dissolution modules (DM’s) in a laboratory flume (design described in Vogel and LaBarbera 1978) following principles outlined by Nowell and Jumars (1984; 1987) to develop a turbulent benthic boundary layer. The flume had a flat, smooth, rigid floor and a working section 1 m long, 0.32 m wide, and a water depth of 0.30 m. Free stream velocities were measured with a Marsh-McBirney Electromagnetic Flow Meter (model 511). Water temperature in the flume was the same as field water temperature (11°C). We measured water velocities at heights of 1, 2, 3, and 4 cm above the flume floor by measuring the time for neutrally buoyant particles to travel 40 cm along the midline of the flume. Time was measured to the nearest 0.01 s using a stopwatch. Four replicate measurements were made at each height. We repeated this process for three free-stream flume velocities: 0.08m/s, 0.26m/s, and 0.40m/s. For each flume stetting, we calculated u* using the turbulent boundary layer equation (Campbell, 2000):
where is the mean velocity at height z above the substratum, k is von Karman’s constant (0.4), and zo is the roughness height of the substratum. Therefore, u*/k is the slope the regression of mean velocity plotted as a function of the log transformed height (z), and the value of z where the regression crosses the y axis is zo. We used our measurements of to determine the slope (u*/k) for each flow setting in the flume, and then multiplied the slope by k (k = 0.4) to determine u*. For calibration, we measured the mass of a DM to the nearest 0.01 g using a Mettler model P136 Balance, sewed the DM to the middle of an overhead transparency (27 cm x 19.5 cm) that was attached flush with the flat, smooth, rigid floor of the flume, and then exposed the DM for 10 minutes to water flowing at one of the speeds at which u* had been determined. We then removed the DM from the water and the transparency, allowed the DM to air dry for 24 hours, and then measured its mass to the nearest 0.01 g. Three such replicates were done at each flow setting. We calculated the linear regression of DM mass loss as a function of u* (R2 = 0.83, F(1,7) = 34.02, p = 0.0006419), and thus determined the calibration factor for DM’s used to measure a time-averaged u* in the field.
M. splendens were collected from different tires and kept in sea water until deployed in the field. Right before deployment, an alga was removed from the water and blotted dry, and then pre-weighed DM’s were sewn onto the alga in three locations: at the base of the blade near the stipe, in the middle of the blade 8 cm distal from the base, and at the distal tip of the blade (Figure 4A). Duct tape attached to the stipe and holdfast (Figure 4A) of each alga was clamped to the rim of a tire on the dock so that the alga was within a canopy of M. splendens, or alone on a tire that had been cleared of algae. After 10 min, the alga was removed from the water and the DM’s were removed from the alga, air dried, and weighed as described above. The rate of mass loss by each DM was converted to a time-averaged u* using our calibration described above. Four replicate algae were deployed for each treatment (exposed site in canopy, exposed alone, protected site in canopy, protected alone)
2.3.2 Water Exchange
We measured the rate at which water in the vicinity of M. splendens blades was replaced using the dye technique described in (Koehl et al., 1997). Our goal was to get a measure of the rate of replacement of water by advection and turbulent diffusion on the spatial scale of the water encountered by a whole algal blade as it flapped in the wavy flow (a scale of roughly 20 to 30 cm). A 0.5 ml puff of fluorescein dye dissolved in sea water was released by a syringe through a brass tube (40cm long, inner diameter 0.1 cm) into water next to the midpoint of an algal blade. A digital stopwatch was used to time (to the nearest 0.5 s) how long it took for the dye to disappear (either to be carried away by the current beyond the volume of water through which the alga moved, or to be so diluted by mixing with the surrounding water that it was no longer visible). Nine replicate dye puffs were timed for each alga. To focus just on the effects of flexibility on water exchange, measurements were made for algae (20 cm long) after all their neighbors on a tire bumper had been cleared away. Immediately after the dye measurements were made for an alga, it was removed from the tire and replaced by a stiff wooden ruler (3 cm wide) clamped at the same position so that it was normal to the surface of the tire bumper and was 20 cm long. The times for dye puffs released next to the ruler midpoint to disappear were measured as described above. We also compared an alga with the ruler within the canopy of other algae attached to a tire bumper at the protected site. Water exchange in algal canopies at the exposed site, where the water surface was very uneven due waves, could not be measured reliably because the dye-disappearance times were very short and transient light reflections from the waves blocked our view of the dye.
2.4 Effects of Bryozoan Epibionts on Host Algae in Moving Water
2.4.1 Growth Rates
The effect of fouling by M. membranacea on the growth rates of M. splendens was measured in the field during August, a period of high M. splendens growth (Dyck and de Wreede, 2006a). Algae at the protected site that were heavily fouled were selected on different tires, and their % cover of bryozoans was determined (see Determination of Percent Cover of Bryozoans on Algal Blades) after they were collected at the end of the growth study (mean % cover of bryozoans on these blades was 40%, SD = 25, n = 7). No heavily fouled algae were found at the exposed site. In addition, lightly fouled algae (Figure 1B) that were free of bryozoans or that bore only a few very small bryozoan colonies (diameter < 5 mm) were selected on different tires at both the protected and the exposed sites. The algae were left in place and all measurements were made in situ, with the algae underwater and surrounded by a canopy of neighboring M. spendens. Algae used in the growth study were selected to be of similar size (at the start of the growth study, mean blade length = 20 cm, SD = 4, n = 17, and mean width = 14 cm, SD = 3, n = 17).
The meristematic region where most of the growth occurs in blades of M. splendens is around the periphery (Krumhansl et al., 2015), so we measured the increase in length and in width of each blade. A hollow brass rod (2 mm diameter) was used to punch marker holes in each blade, one ~1 cm from the blade base, and another ~1cm the edge of the distal tip. Then a ruler was laid on each blade, lined up with these holes, and the distance was measured (to the nearest mm) between the junction of the blade with the stipe and the distal edge of the blade. Similarly, holes were punched ~1cm from the right and left edges of the widest part of each blade, the ruler was lined up with the holes, and the width of the blade from the right edge to the left edge was measured. The algae were left in the field for 17 days and then the same length and width measurements were repeated using the ruler lined up with the marker holes. The increases in length and width were determined for each blade, and the % change in length and in width were calculated.
2.4.2 Hydrodynamic Forces
2.4.2.1 Drag in Unidirectional Flow
Drag on algal blades with and without bryozoan epibionts was measured in unidirectional flow. These experiments were done in the flume described in Section 2.3.1 above. We measured F (to the nearest 0.001 N) using a force transducer that consisted of two strain gauges (Micro Measurements 120 W) mounted on both sides (for temperature compensation) of a plexiglass beam (5 cm long, 1 cm wide, 0.3 cm thick). The change in resistance of the strain gauges when the transducer was bent was measured by a Gould Bridge Amplifier (model 13-4615) in half bridge configuration and recorded on a Gould Brush Strip Chart recorder (model 220). The holdfast of an alga was gripped by an alligator clip glued to a metal rod (length = 5cm, diameter = 2 mm) extending from the transducer. Therefore, the transducer was calibrated by mounting it horizontally and bending it with weights hung on the clip. Each alga fouled with M. membrancea was attached to the force transducer and mounted so that it hung into the middle of the flume working section where the flow velocity was uniform. The mean of the drag measured for 30 s was calculated for each of four water velocities: 0.08, 0.26, 0.40, and 0.55 m s-1. Then the epibionts were gently peeled off the blades and the drag measurements were repeated. The % cover of bryozoans on each alga was determined as described in Section 2.2.1. (mean % cover = 13, SD = 5, n = 5 algae). For each alga at each velocity, we calculated the ratio of the drag when it was fouled to the drag when it was unfouled.
2.4.2.2. Hydrodynamic Forces in Oscillatory Flow
Organisms exposed to the oscillatory water flow in waves experience time-varying drag and acceleration reaction forces (Koehl, 1977; Denny et al., 1985). To measure the net hydrodynamic forces on algal blades in oscillatory flow, we used the same force transducer system described in Section 2.4.2.1. Force measurements were made in the oscillating-flow tank described by Hunter (1988). The tank was 30 cm wide, 3 m long, had a water depth of 22 cm, and produced uniform flow over the cross section (Hunter, 1989). The water motion in the tank mimicked the flow experienced by algae at the exposed site during a ferry wake (frequency 0.4 Hz, peak velocity 0.3 m s-1). Each algal blade fouled with M. membrancea was mounted so that it hung into the middle of the tank as described in Section 2.4.2.1 and the forces were recorded for 1 min. Then the epibionts were gently peeled off the blades and the force measurements were repeated. The % cover of bryozoans on each alga was determined as described in Section 2.2.1. (mean % cover = 8, SD = 3, n = 5 algae). For each alga we calculated the ratio of the mean of the peak forces when it was fouled to the mean of the peak forces when it was unfouled.
2.4.2.3 Flexural Stiffness
The magnitude of the hydrodynamic force that a flexible macroalga experiences depends on how it is bent over and folded by the flow, so we measured the effects of encrusting bryozoans on the resistance to bending by algal blades. The flexural stiffness (EI) was determined for strips of blade tissue that were free of epibionts, had 100% cover of a bryozoan colony on one side, or had 100% cover on both sides. Algae were collected from different tires on the protected side of the breakwater and kept in running seawater in a sea table until a strip of blade was cut for immediate testing. Only one specimen was taken per alga, so all specimens were independent.
We measured EI by cantilever bending tests, where
where F is a point force applied to the free end of the cantilever, L is the distance of the point of force application from the fixed end of the cantilever, and δ is the deflection of the free end of the cantilever. We measured F (to the nearest 0.1 mN) using a force transducer that consisted of two strain gauges (Micro Measurements 120 W) mounted on both sides (for temperature compensation) of a thin (0.005 cm thick) strip of precision steel shim stock 5 cm in length. The transducer was calibrated and the forces were recorded as described in section 2.4.2.1. For measurements of flexural stiffness, the transducer was mounted vertically. Strips (1 cm wide x 1.5 cm long) of clean or encrusted algal blade were cut from the proximal regions of blades, and pieces of cardboard (1 cm wide x 0.5 cm long) were attached with cyanoacrylate glue to one end of the blade strip. The cardboard was gripped by the alligator clip of the force transducer so that the length (L) of the algal strip to be bent was 1 cm. A metal rod (1.6 cm diameter) attached to vernier calipers clamped horizontally was used to deflect the distal tip of the specimen a distance (δ) of 1.0 mm, and the force (F) was measured and used to calculate EI. For specimens with bryozoan cover on only one side, the bryozoan was on the compression side of the bending specimen.
2.4.2.4 Tenacity
To determine if hydrodynamic forces on hosts were likely to lead to their dislodgement by ambient flow, we measured the force required to dislodge (“tenacity”) M. splendens from the substratum in the field. The peak force to pull the alga off the tire was measured to the nearest 0.25N using an Ohaus 20N dial spring scale (Model #3011NO). We attached each individual M. splendens to the force transducer with soft nylon cord that was wrapped three times around the basal part of the blade at its junction with the stipe and then tied. If the thallus broke near the cord attachment, we did not use the data. For each tenacity measurement, we noted whether the stipe broke or the holdfast detached. The diameter of the stipe at the break point or of the holdfast was measured with vernier calipers to the nearest 0.1 mm and was used to calculate the tissue area where failure occurred. At both the exposed and protected sites, we used a random-number table to pick a tire, measured the tenacity of all the M. spendens on that tire, and then moved to the neighboring tire to the South until we had measured the tenacity of 18 individuals.
2.5 Mechanical Interactions Between Algae and Bryozoans
2.5.1 Young’s Modulus, Breaking Stress, and Extension Ratio
The mechanical responses of algae and epibiotic bryozoans to the hydrodynamic forces they experience depend on the mechanical properties of their tissues (“material properties”). We conducted stress-strain tests following previously established methods for measuring algal material properties (Koehl and Wainwright, 1985). M. splendens fouled with M. membranacea were collected from the protected site and kept in running seawater at 11°C until tested. Strips (length = 61 mm) parallel to the longitudinal axis of an alga were cut from the blades. Strips used for measuring stiffness and extensibility were rectangular (11 mm wide) and strips used to measure strength were cut into hourglass shapes (6 mm wide at the narrowest point) using a cork borer with a radius of curvature of 27 mm). Pieces of paper towel (10 x 10 mm) were glued with cyanoacrylate to both sides of each end of a specimen to protect the tissue from damage by the grips of the material-testing machine, a Hounsfield tensometer (Type-W). Samples were prepared for testing that had different degrees of fouling: unfouled blade tissue, blade tissue bearing a small bryozoan colony (diameter ≤ 5mm), blade tissue with 100% cover of a bryozoan colony on one side that extended into the Tensometer grips so that both algal and bryozoan tissues were being pulled, and blade tissue with 100% bryozoan cover on both sides that extended into the grips. The thickness, width, and length between the grips of each specimen were measured to the nearest 0.1 mm using vernier calipers
During a stress-strain test, the specimen is pulled and the force with which it resists that stretching is measured. Strain gauges (Micro Measurements 120 W) were attached to both sides of the Tensometer’s steel force beam (D513, 62.5 lb maximum) providing temperature compensation. The force signal was amplified by a Gould Bridge Amplifier (model 13-4615) in half bridge configuration. Length changes (ΔL) of a specimen were measured by a linear variable differential transformer (LVDT, Schaevitz Engineering 500HR-DC) with the shaft mounted to one grip and the coil mounted to the other grip. The output of the bridge amplifier (force) was recorded as a function of the output of the LVDT (ΔL) on an X-Y analog plotter (Houston Instrument Omnigraphic 2000). The force beam was calibrated using the mercury force scale on the Tensometer. The LVDT was calibrated using known displacements measured with vernier calipers. Specimens were extended at a strain rate of 0.18 s-1 (strain rate = ΔL/(Lo t), where Lo is the length of the specimen between the grips before the pulling started and t is the time, measured by a stopwatch to the nearest 1 s, to extend the specimen to that ΔL). Tensile stress (σ, force per the cross-sectional area of a specimen) was plotted as a function of the extension ratio (λ = [ΔL+Lo]/Lo). We used the slope of the initial linear portion of the plot of stress as a function of extension ratio as a measure of tissue stiffness (elastic modulus, E1) (Figure 10A). The strength (breaking stress, σmax) and extensibility (breaking extension ratio, λmax) were also determined for each sample (Figure 10A).
2.5.2 Transplant Experiments and the Fates of Bryozoans in Different Flow Habitats
We assessed the fates of M. membranipora colonies living on M. splendens blades when exposed to different water flow habitats. We collected 12 algae fouled with bryozoans from different tires at the protected site. Each alga was spread out in a pan of seawater and photographs were taken of the bryozoans on each side of a blade. Six of the algae were reattached to tires at the protected site, while six other algae were transplanted to the exposed site. After 24 hours in the field, the algae were collected and both sides were photographed again. The fate of each bryozoan colony was determined by comparing its images in the before and after photographs. Some colonies were missing in the after photo (“lost”), some were smaller because part of the colony had broken off (“broken”), and some were intact or grew.
2.6 Statistical Analyses
Data used in parametric statistical tests met the assumption of normality (Shapiro-Wilke test) and homogeneity of variance (Levene’s test). All data that were percentages were arcsin transformed (Sokal and Rohlf, 1995) to meet the assumptions of normality and homogeneity of variance. Shapiro-Wilke tests, Levene’s tests, linear regressions, and paired-T tests were done using Statistics Kingdom Online Calculators (Statistics Kingdom 2017; https://www.statskingdom.com). One-way ANOVA with post hoc Tukey HSD analyses, and Kendall’s tau rank correlation tests were done using the Astasa Online Statistical Calculator (Navendu Vasavada, 2016; https://astatsa.com). ANCOVA tests were done using the VassarStats Website for Statistical Computation (©Richard Lowry 1998-2021; http://vassarstats.net).
For measurements of hydrodynamic forces, both in unidirectional flow and in oscillatory flow, we calculated the ratio of force on each alga when fouled with bryozoans relative to the force on that alga when cleared of bryozoans. We used a bootstrap method (Efron and Tibshirani, 1986) to determine if the ratio of forces on fouled algal blades relative to cleared algal blades was greater than 1.0 (i.e. hydrodynamic force was greater on fouled than on unfouled algae). To do so, we used the observed mean ratio and standard deviation from our data (n = 5 algae for each type of flow) to establish a range (mean ± standard deviation) from which 100,000 samples of 5 random numbers were chosen within that range. We then calculated the probability that the mean ratio was greater than 1.
3 Results
3.1 Water Velocities Encountered by Algae in the Field
Water flow near Mazaella splendens at both the exposed and protected sites was characterized by small waves, with water moving back and forth (Figures 1C–E). Peak velocities (and thus hydrodynamic forces on organisms) were brief, while mean velocities (and thus net transport of water across a site) were low at both sites. Peak velocities were greater at the exposed site than at the protected site, both in ambient flow and in ferry wakes (Figure 2C). Turbulence intensity, a measure of the variation in velocity in a turbulent current, is mathematically the same as the standard deviation (SD) (Koehl and Alberte, 1988). Because our velocity fluctuations were due to both turbulence and waves, we report the variability of flow velocities as the SD (Figure 2D). Although the SD was much greater at the exposed site, the mean water velocity was the same at both sites (Figure 2E). Flow within canopies of algae was not different from flow just outside the canopies at the protected site (statistics reported in the legend of Figure 2).
3.2 Distribution of Bryozoan Cover and Structure of Algal Canopies
We observed that M. splendens were nearly free of Membranipora membranacea epibionts at the exposed site (Figure 1B), so we only measured the spatial distribution of M. membranacea, on blades of M. splendens at the protected site. There was greater cover of bryozoans on the older basal region of algal blades than on younger distal tissue (Figure 3).
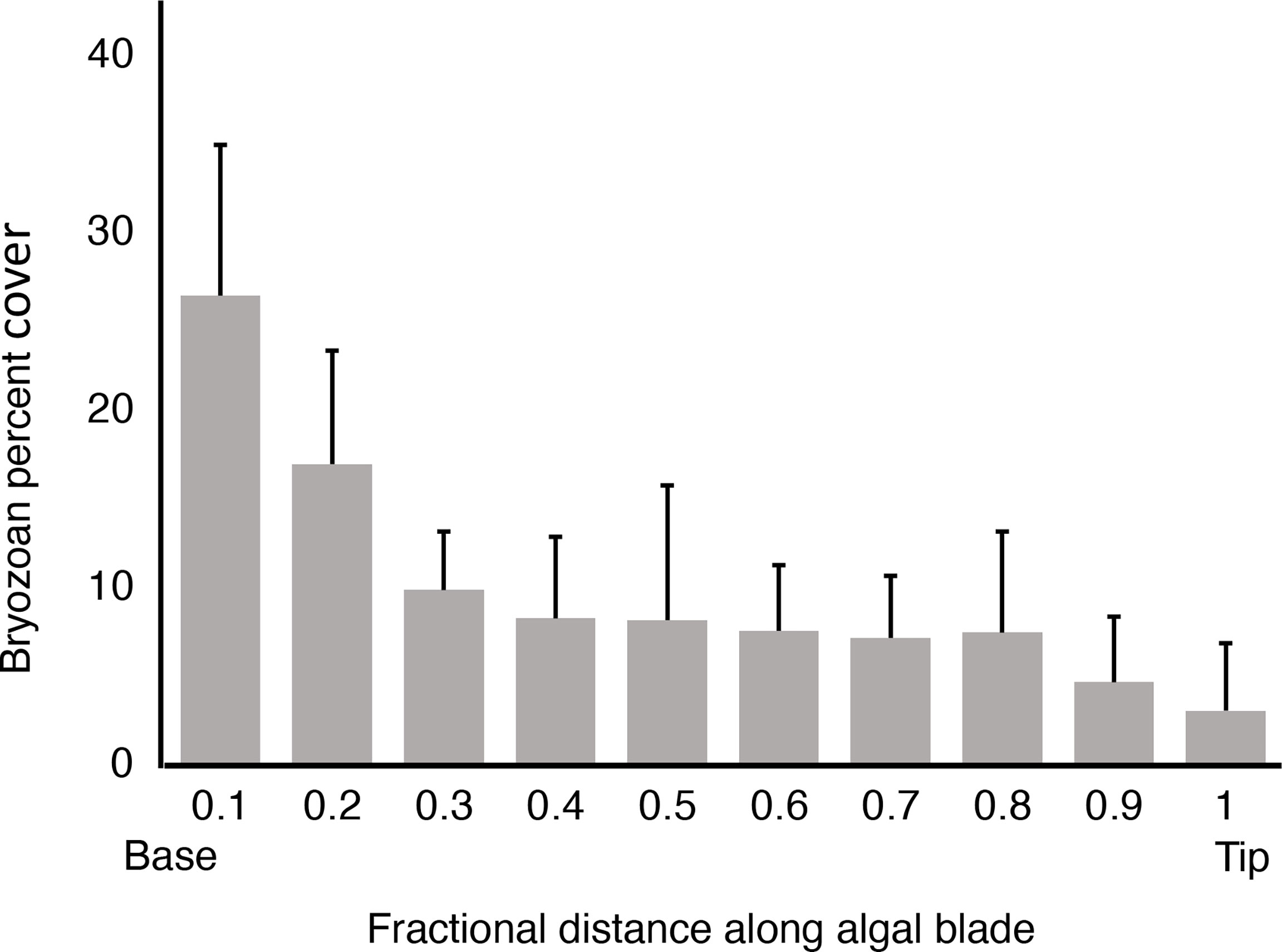
Figure 3 Percent of the area of an algal blade covered by M. membranacea, plotted as a function of fractional distance along an algal blade ([distance from base of blade]/[total blade length]). Means for ten M. splendens from the protected site are plotted, and error bars represent one standard deviation. There is a significant negative association between % cover of bryozoans and distance from the base of an algal blade (p < 0.01, one-tailed Kendall’s tau rank correlation test).
The density of M. splendens in canopies at both sites ranged from 180 to 260 individuals/m2. There was no difference between the dimensions of the algae at the exposed and protected sites (p > 0.05, ANOVA), so data were pooled to calculate the mean thallus length of 23.2 cm (SD = 7.2, n = 34 thalli) and mean thallus width of 16.3 cm (SD = 5.0, n = 34 thalli).
3.3 Effects of Flexible Host Algae on Local Flows Near Bryozoan Epibionts
3.3.1 Time-Averaged Local Boundary Shear Velocity
We used dissolution modules (DM’s) at different positions on blades of M. splendens in the field to assess the fine-scale local mass and momentum exchange at the surface of the flexible algae and the epibionts living at those positions (Figure 4A). The weight loss rates of the DM’s were calibrated to be equivalent to the exchange produced in steady unidirectional currents over a flat, rigid surface at measured boundary shear velocities (u*). We found that the time-averaged u* was lower for algae and bryozoans in a canopy than for algae without neighbors at the exposed site, except at the distal tips of blades (Figure 4B). However, at the protected site, canopies had no effect on time-averaged u* (Figure 4C). There was no correlation between position along an algal thallus and time-averaged u* at the exposed site (Figure 4B). In contrast, at the protected site the time-averaged u* was higher near the base than near the tip of algal blades in canopies, but did not vary with position for isolated algae (Figure 4C). There was no significant difference between the time-averaged u*’s at the exposed site and at the protected site for algae that were alone or for algae in canopies (p > 0.05, ANOVA using pooled u*’s for all positions along the blade of each alga: for algae that were alone, F = 3.461,20, and for algae in a canopy, F = 0.521,20).
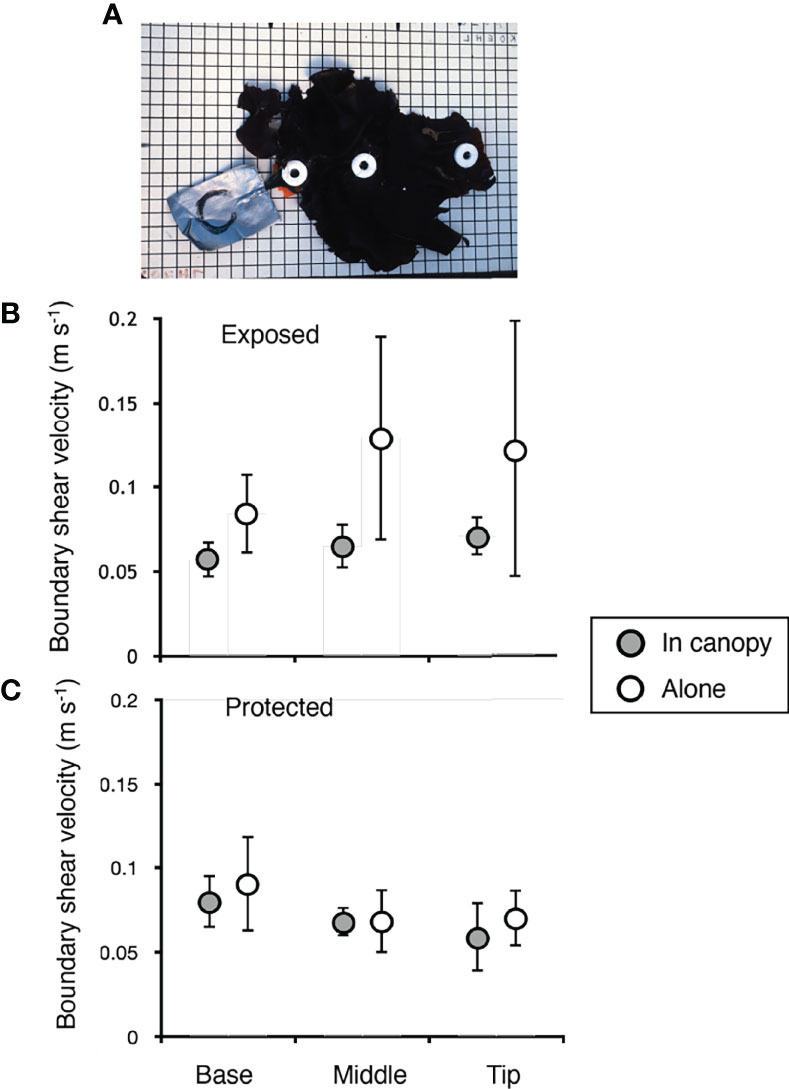
Figure 4 Boundary shear velocity (u*) along blades of M. splendens, measured using calibrated dissolution modules. (A) Photograph of a M. splendens showing the positions at which the dissolution modules were sewn onto the thallus: base (proximal end of blade next to stipe), middle (8 cm from base), tip (distal end of blade). Duct tape attached to the stipe and holdfast of each alga was clamped to the rim of a tire on the dock so that the alga was within the canopy of M. splendens (grey circles in B and C). A second measurement of u* was done for the same individual on the same tire, but with the surrounding canopy removed (open circles in B, C). (B) u*’s measured at the exposed site. At the base and at the middle of the algal blades, u* is significantly higher for algae that are alone than for algae in canopies (p < 0.05), whereas at the tip the u*’s of algae that are alone are not significantly higher than the u*’s of algae in canopies (right-tailed paired-T test, df = 3 for each position). There was no association between u* and distance from the stipe for algae that were alone or for algae in canopies (p > 0.05, two-tailed Kendall’s tau rank correlation test). (C) u*’s measured at the protected site. The u*’s of algae that were alone were not significantly higher than the u*’s of algae in canopies at any position along the blade (right-tailed paired-T test, df = 3 for each position). For algae in a canopy, there was a significant negative association between u* and distance from the base of the alga, but there was no association between u* and position along a blade for algae that were alone (p < 0.05 for significance, two-tailed Kendall’s tau rank correlation test). In all graphs, error bars represent one standard deviation.
3.3.2 Water Exchange
Dye studies in wave-driven flow in the field showed how the flapping by flexible M. spendens affects the exchange of water near the algae and their epibionts. In the rapid, fluctuating flow at the exposed site, the flapping of flexible algae did not affect the rate at which water was replaced near the algae (Figure 5A). In contrast, at the protected site, flapping by algae increased water exchange, both for algae in canopies and for solitary algae (Figures 5B, C). Moreover, water was replaced in flapping canopies of flexible algae more rapidly than water was exchanged near isolated individuals (Figures 5B, C).
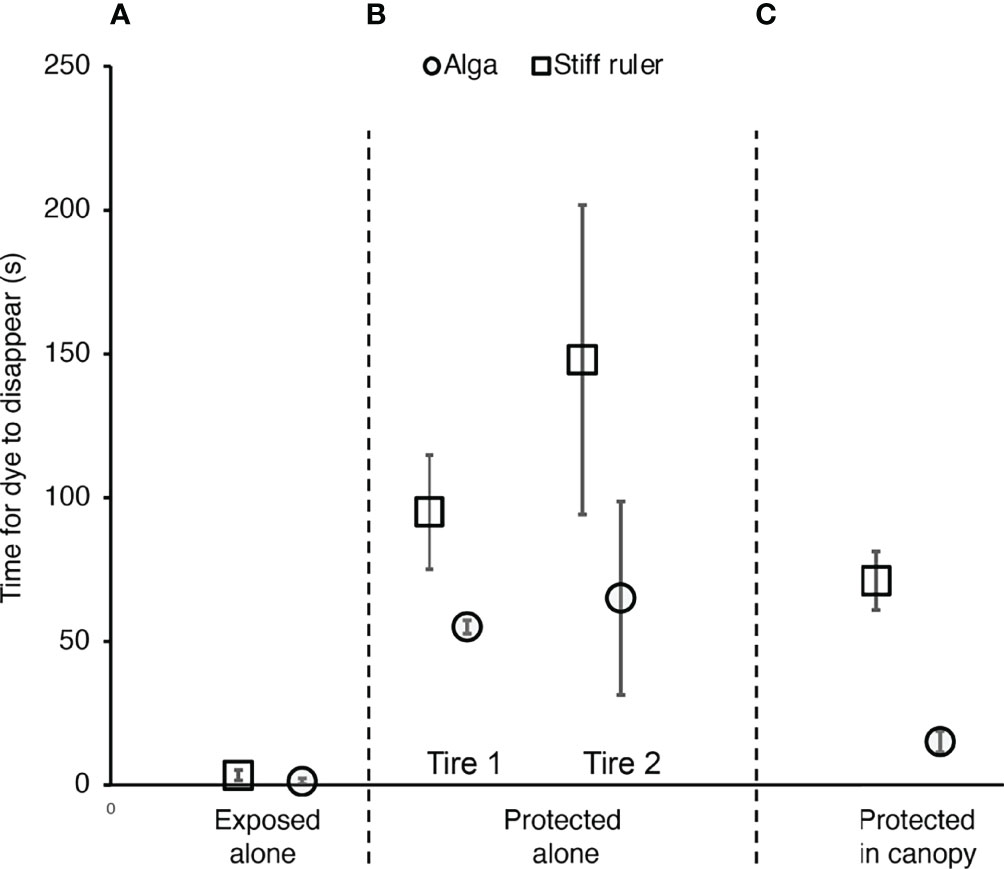
Figure 5 Effect of flexibility and neighbors on water exchange, as measured by the time for dye to disappear that had been released mid-blade next to a flexible M. splendens (circles) or a stiff wooden ruler (squares) of the same length (20 cm) at the same position in the field. (A) Exposed site, where an alga or ruler was alone, with all neighboring algae on the tire removed. There was no significant difference between the time for dye to leave the flexible alga and to leave the stiff ruler (p > 0.05, one-way ANOVA, F = 2.491,14). (B) Protected site, where all the algae but one were cleared from two different tires, and each solitary alga was then replaced by a solitary stiff ruler at the same location. Dye left each flexible alga significantly faster than it left the rigid ruler (p < 0.05, one-way ANOVA, F = 11.271,8 for one location, F = 6.851,6 for the other location). (C) At a third location at the protected site, within a canopy of M. splendens. Dye left the flexible alga significantly faster than it left the rigid ruler (p > 0.05, one-way ANOVA, F = 227.991,11). If all data for algae without neighbors are pooled for the protected site, dye leaves algae in a canopy significantly faster than dye leaves solitary algae (p < 0.05, one-way ANOVA, F = 9.951,8). In all graphs, error bars represent one standard deviation.
3.4 Effects of Bryozoan Epibionts on the Host Algae in Moving Water
3.4.1 Growth Rates
The summer growth rates of M. splendens were not affected by being encrusted with M. membranacea, or by the flow exposure of their habitats (Figure 6).
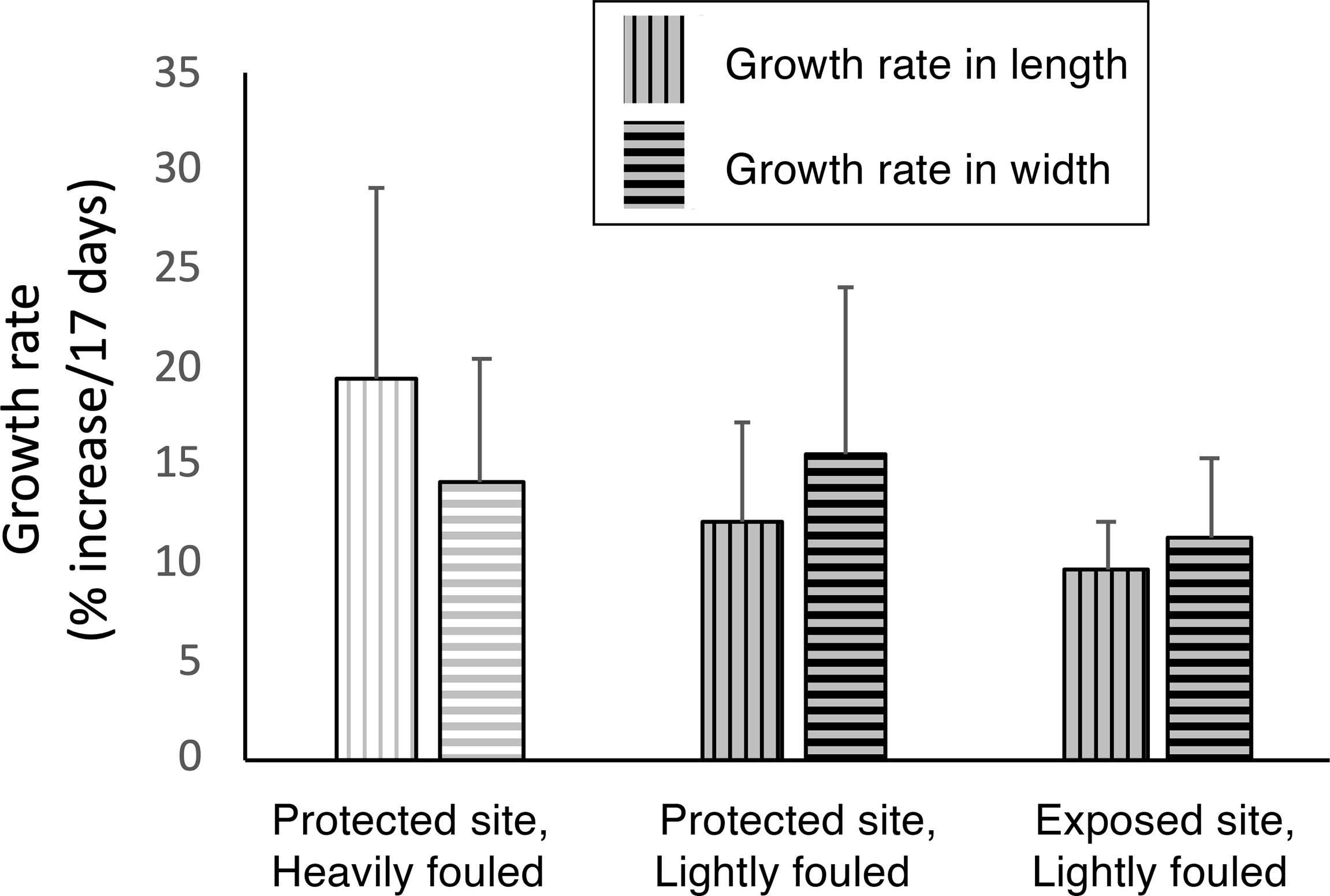
Figure 6 Effect of fouling by M. membranacea on linear growth (% increase per 17 days) of M. splendens in length (vertical hatch marks) and width (horizontal hatch marks). Bars with white backgrounds indicate algae heavily fouled with bryozoans, and bars with grey backgrounds indicate lightly fouled algae. Neither flow habitat nor degree of fouling had a significant effect on algal growth rates in length (p > 0.05, one-way ANOVA followed by Tukey HSD test, F = 3.052,13) or in width (p > 0.05, one-way ANOVA followed by Tukey HSD test, F = 1.142,13).
3.4.2 Hydrodynamic Forces
3.4.2.1 Drag in Unidirectional Flow
Encrusting bryozoan epibionts increased the drag on algae, except at very slow water velocities (Figure 7). The ratio of the drag when fouled to the drag when unfouled (DF/DU) for ten M. splendens was calculated for each flow velocity tested. The mean DF/DU for 10 algae was greater than 1.0 for each velocity, indicating that the bryozoans increased the drag on the algae (at 0.08 m s-1, mean DF/DU = 2.2, SD = 4.6; at 0.26 m s-1, mean DF/DU = 1.7, SD = 1.1; at 0.40 m s-1, mean DF/DU = 1.9, SD = 0.7; at 0.55 m s-1, mean DF/DU = 1.9, SD = 0.5). We did bootstrap calculations of the probability (p) that a mean ratio greater than 1.0 was due to chance and found that p < 0.01 for all but slowest of the flow velocities tested (i.e. the increase in drag due to bryozoan epibionts was significant). At the slowest flow: at 0.08 m s-1, p = 0.07, so bryozoan fouling did not have a significant effect on drag.
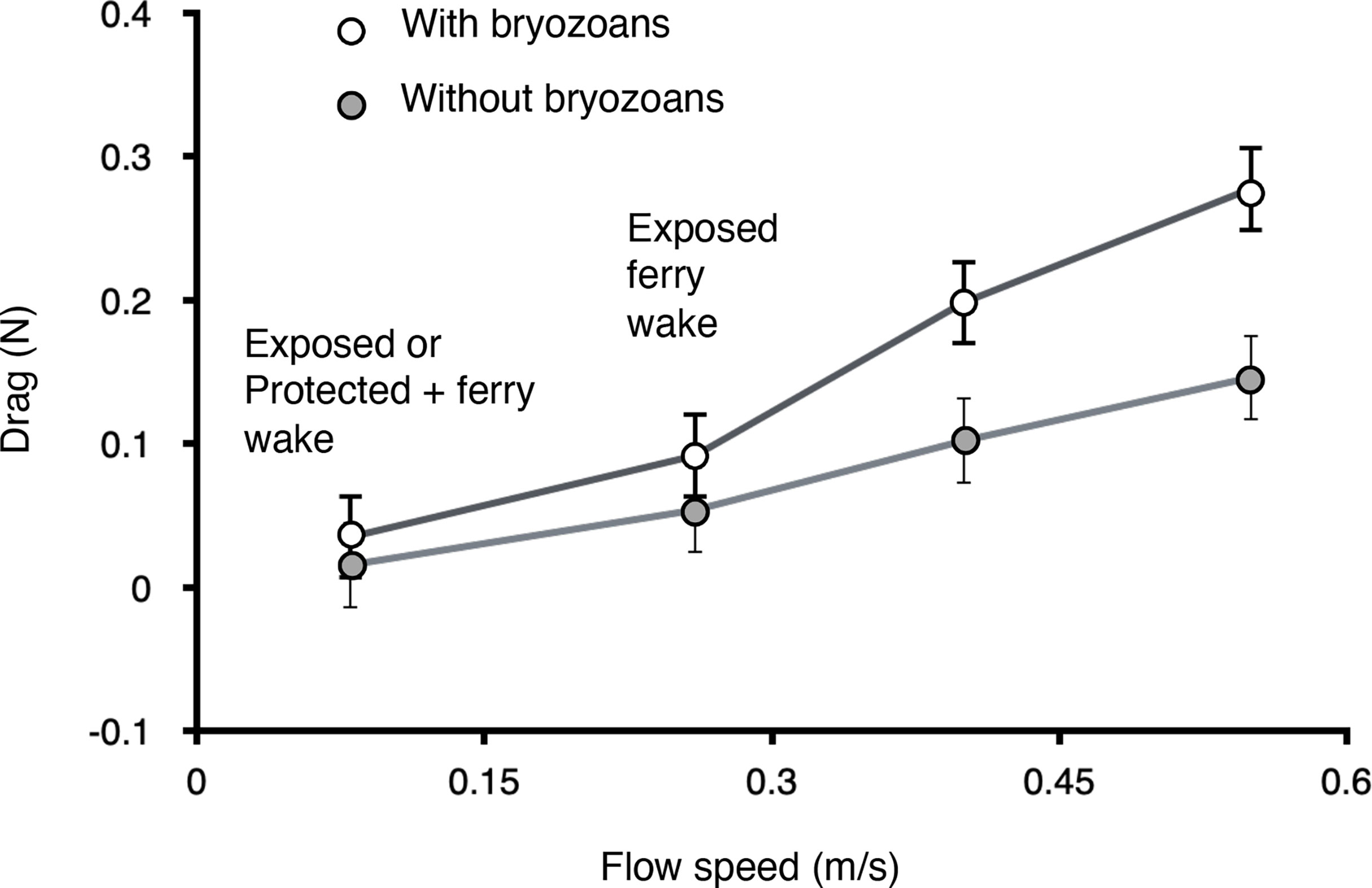
Figure 7 Drag in unidirectional flow on M. splendens fouled by M. membranacea (white circles; mean cover of bryozoans = 12%, SD = 5.0, n = 5) and on the same algae after the bryozoans were removed (grey circles), measured at a range of flow velocities in a flume. The 0.8 m s-2 flow represents the peak velocities measured in wind chop at the exposed site, and also the peak velocities measured in ferry wakes at the protected site. The 0.26 m s-2 flow represents the peak velocities measured in ferry wakes at the exposed site. Error bars represent one standard deviation.
Flexible organisms can be bent over and reconfigured into more streamlined shapes by ambient water flow. As water velocities were increased, both clean and fouled M. splendens were bent over parallel to the flow direction, but clean blades were rolled up into compact streamlined shapes, while blades encrusted with bryozoans were folded over like flat greeting cards. A measure of the drag reduction caused by such flow-induced reconfiguration is the Vogel number, which is the slope of a plot of [log (drag/velocity2)] as a function of [log (velocity)] (Vogel, 1989; Albayrak et al., 2012; Nepf, 2012). We used drag measured at different velocities to calculate the regressions of these plots for clean M. splendens and for those fouled with M. membranacea. The larger the absolute values of the slopes of these regressions (Vogel numbers), the greater the drag reduction due to blade reconfiguration. For clean thalli, the Vogel number was -0.84 (R2 = 0.99, p < 0.01), and for fouled thalli, the Vogel number was -0.93 (R2 = 0.96, p < 0.05). A one-way ANCOVA for two independent samples showed that the slopes of these plots for clean and for fouled thalli were not significantly different from each other (p = 0.57), but that values of log(drag/velocity2) for fouled algae were significantly higher than for clean algae (p < 0.01). Thus, although the drag at a given velocity was greater for fouled thalli than for clean ones, their two different modes of passive reconfiguration as flow velocities increased were equally effective at reducing drag.
3.4.2.2 Hydrodynamic Forces in Oscillatory Flow
Bryozoan epibionts increased the peak hydrodynamic forces on M. splendens in oscillating flow mimicking water motion encountered at the exposed site when hit by waves from a ferry wake. The mean of the ratios of peak wave force when fouled to peak wave force when unfouled was 1.2 (SD = 0.3, n = 10). Bootstrap calculation of the probability (p) that a mean ratio greater than 1.0 was due to chance yielded p = 0.003, so the increase in hydrodynamic force due to fouling by bryozoans was significant.
The peak hydrodynamic forces on M. splendens in waves that mimicked those due to a ferry wake were an order of magnitude lower than the mean drag forces they experienced in unidirectional flow at the same velocity as the peaks in the ferry wake (Figure 7). The mean of the peak forces on fouled algae in waves was 0.012 N (SD = 0.004, n = 10), and on unfouled algae was 0.011 N (SD = 0.005, n = 10). By watching the motion of neutrally-buoyant particles in the water relative to an alga flapping back and forth in the wave tank, we observed that the distal parts of the thallus moving with the flow experienced no water motion relative to their surfaces during the parts of the wave cycle when water accelerations and velocities were highest. Since hydrodynamic forces depend on water velocity and acceleration relative to a body, the ability of a flexible alga to move with the water flowing back and forth in waves can reduce the forces it experiences.
3.4.2.3 Flexural Stiffness
Encrusting M. membranacea grew on both surfaces of M. splendens blades. Regions of blades with bryozoans on both sides had a higher flexural stiffness than clean blades. (Figure 8)
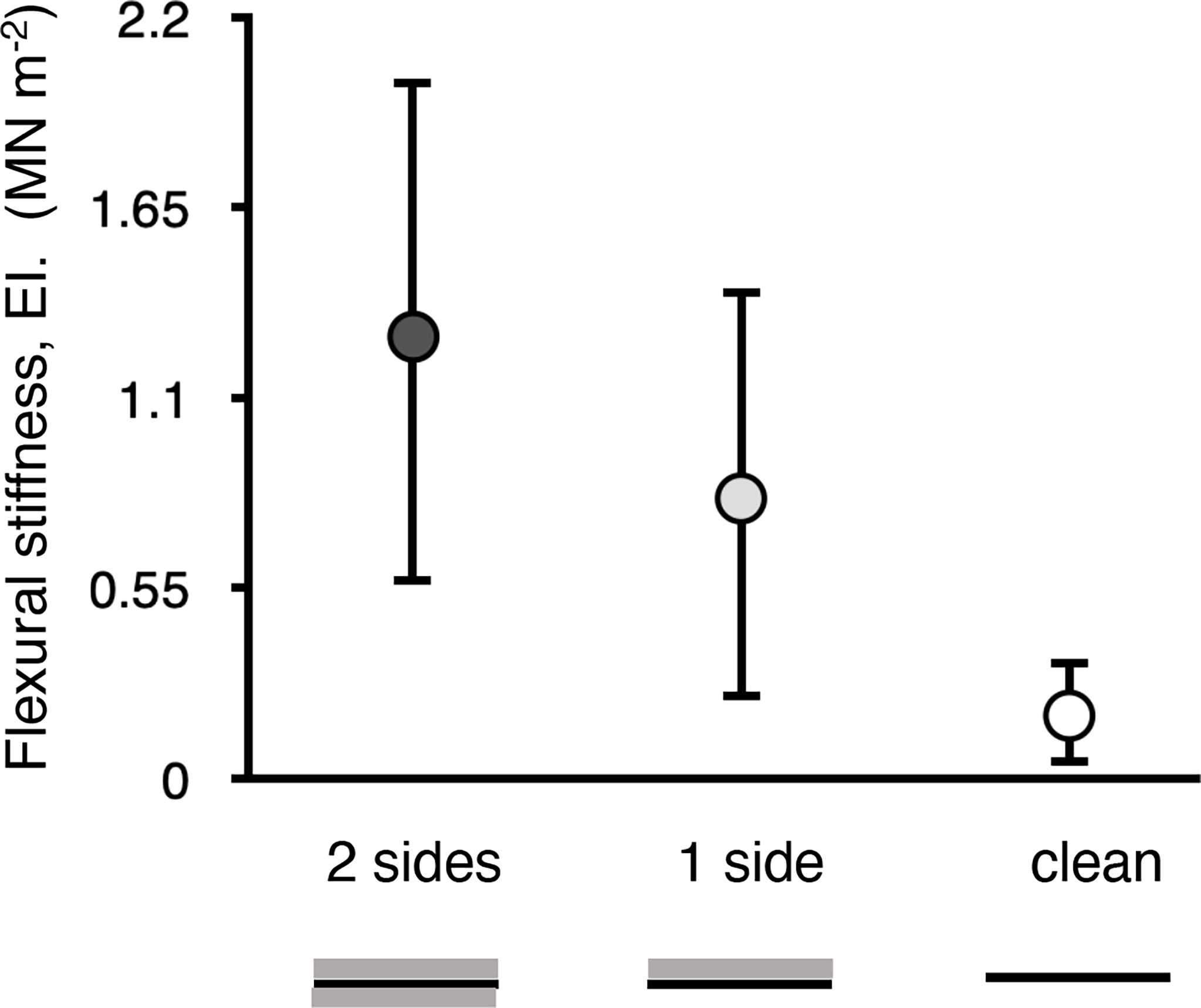
Figure 8 Flexural stiffness (EI) of samples of blades M. splendens with 100% cover of M. membranacea on both the top and bottom surfaces of the blade (2 sides) or on just the top surface (1 side), and of blades with no fouling (clean). Error bars represent one standard deviation (n = 7). The EI of a region of an algal blade with bryozoans growing on both sides was significantly greater than that of a clean blade (p < 0.01, one-way ANOVA followed by Tukey HSD test, F = 8.452,18), whereas the EI of a region of blade with bryozoans growing on only one side was not significantly different from 2 sides or clean (p > 0.05).
3.4.2.4 Tenacity and Environmental Stress Factor
When dislodged from surfaces at both of our field sites, some M. splendens failed when their stipes broke, while other failed when their holdfasts detached. The force to dislodge an alga (“tenacity”) was the same for stipe breakage and for holdfast failure (Figure 9A). The breaking stress of stipe tissue was higher than the failure stress of the holdfasts for algae at both the exposed and protected sites (Figure 9B). The tenacities of holdfasts and stipes at the exposed site were greater than at the protected site (Figure 9A), but there was no difference in stipe breaking stress or holdfast failure stress between the two sites (Figure 9B). This indicates that differences in tenacity were due to differences in stipe cross-sectional areas or holdfast attachment areas rather than in stipe tissue strength or in holdfast glue strength.
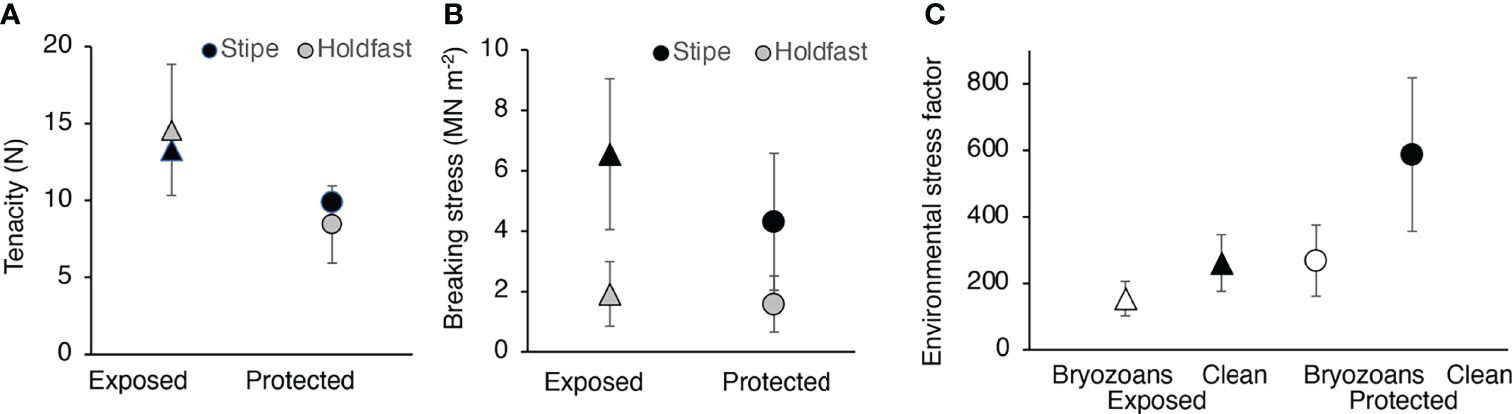
Figure 9 Dislodgement of M. splendens. (A) Tenacity (force required to dislodge an alga) of individuals that broke at the stipe (black symbols) or failed at the holdfast (grey symbols) at the exposed site (triangles) and at the protected site (circles). Although there was no significant difference (p > 0.05) between the tenacity of stipes and holdfasts at each site, the tenacity of the holdfasts at the exposed site was significantly higher than that of the holdfasts and the stipes at the protected site (p < 0.05, one-way ANOVA followed by Tukey HSD test, F = 4.743,38), but there was no significant difference between the stipe tenacity of algae at the two sites (p > 0.05). (B) Breaking stress of the stipe (force per cross-sectional area, black symbols) and failure stress of the holdfast (force per area of attachment, grey symbols) of algae at the exposed site (triangles) and at the protected site (circles). The breaking stress of the stipes was significantly higher than the failure stress of the holdfasts for algae at the exposed site (p < 0.01, one-way ANOVA followed by Tukey HSD test, F = 12.973,38) and at the protected site (p < 0.01), but there was no difference between sites of stipe breaking stress (p > 0.05) or of holdfast failure stress (p > 0.05). This indicates that differences in tenacity in A were due to differences in stipe cross-sectional areas or holdfast attachment areas rather than in stipe tissue strength or in holdfast glue strength. (C) Environmental stress factor (ESF) for M. splendens at the exposed site (triangles) and at the protected site (circles). The stipe-breaking or holdfast-failure stress of each alga at the exposed site was divided by the stress due to the mean drag on an alga fouled by bryozoans (white triangles) or on a clean, unfouled alga (black triangles) at the exposed site hit by a ferry wake (Figure 7). At the exposed site, the ESF of clean algae was significantly greater than the ESF of algae fouled with bryozoans (p < 0.01, one-way ANOVA followed by Tukey HSD test, F= 20.671,34). The stipe-breaking or holdfast-failure stress of each alga at the protected site was divided by the stress due to the mean drag on an alga fouled by bryozoans (white circles) or on a clean, unfouled alga (black circles) at the protected site hit by a ferry wake (Figure 7). At the protected site, the ESF of clean algae was significantly greater than the ESF of algae fouled with bryozoans (p < 0.01, one-way ANOVA followed by Tukey HSD test, F= 37.721,46).
Although bryozoan epibionts increase the hydrodynamic forces on M. splendens, they made little difference to the likelihood of the algae being ripped away by ambient water flow in the summer. Safety factor, the ratio of the strength of a structure to the maximum load it will bear during its lifetime, is a measure of how likely that structure is to break. Unlike man-made structures, living algae change in size, shape, and tenacity as they grow and age. Members of the same species can differ between sites, and the hydrodynamic conditions they experience depend on their habitat and can change with season. Therefore, we calculated the environmental stress factor (ESF, sensu Johnson and Koehl, 1994) to describe the likelihood that M. splendens would be washed away during the summer, when blades are growing and big storms do not occur. The ESF is the ratio of the stress in the stipe or holdfast required to rip an alga off a substratum to the maximum stress it is likely to experience in is habitat during the season when its tenacity was measured. For each of our field sites during the summer, we assumed that the largest forces occurred during ferry wakes, so we used the mean drag on fouled and on unfouled M. splendens at the peak velocity experienced in ferry wakes at each site (Figure 7) to calculate an estimate of the maximum stress in the stipe or holdfast. We estimated the failure danger imposed by bryozoan fouling by calculating the ESF for each alga, dividing its stipe-breaking or holdfast-failure stress by the peak stress it would experience at its site if the alga was fouled and if it was unfouled (Figure 9C). The ESF was lower for algae bearing M. membrancea than for unfouled algae at both the exposed and protected sites (Figure 9C), but this summertime ESF was so high that bryozoan fouling makes little difference to the likelihood of M. splendens being washed away by ferry wakes in a harbor.
3.5 Mechanical Interactions Between Algae and Bryozoans
3.5.1 Elastic Modulus, Breaking Stress, and Extension Ratio
M. splendens has a life cycle with gametophyte and sporophyte stages. The thalli of these stages are similar in size and shape, but can be distinguished by the morphology of the small reproductive bodies that form on their blades. We compared the material properties of unfouled male and female gametophytes, tetrasporophytes, and non-reproductive thalli. There were no significant differences between these life stages in their the elastic moduli, EI (p > 0.05, one-way ANOVA followed by Tukey HSD test, F = 2.053,46), their extensibilities, λmax (p > 0.05, F= 2.773,33), or their breaking stresses, σbrk (p > 0.05, F = 1.993.34). Therefore, the results of stress-strain tests done to determine the effects of epibiotic bryozoans on the material properties of algal blades were pooled for all life stages.
M. membrinacea epibionts did not affect the material properties of their host M. splendens. The elastic modulus (E1) for clean blade tissue was not different from the E1 of blade tissue with a small bryozoan colony attached to it (Figure 10B), and the E1 of clean blade tissue was the same as the E1 of a composite specimen of algal blade tissue plus a bryozoan colony on one side or on both sides (Figure 10B). Furthermore, bryozoans did not affect the breaking extension (λmax, Figure 10C), or breaking strength (σbrk, Figure 10D) of algal blade tissue.
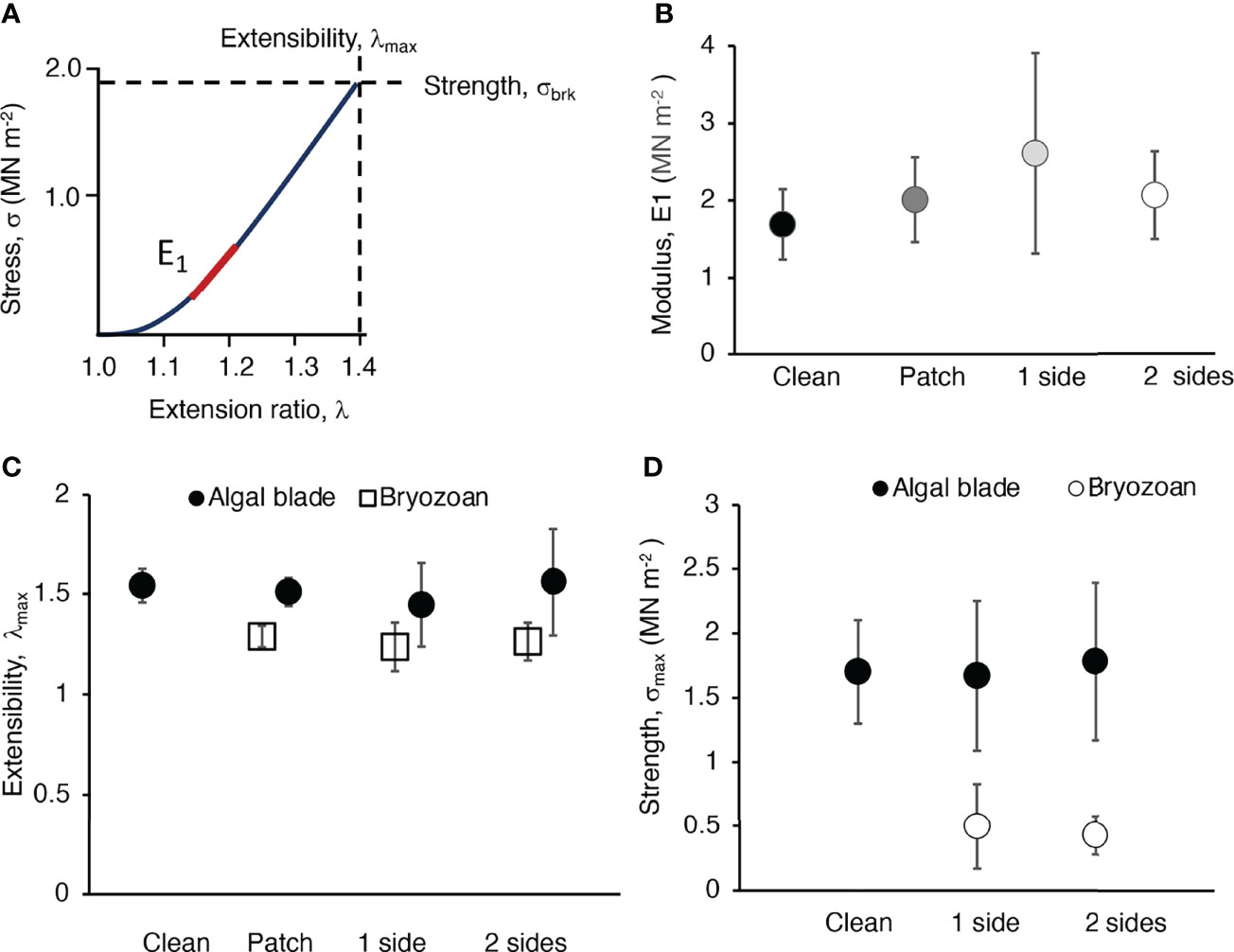
Figure 10 Material properties of blade tissue of M. splendens and of colonies of M. membranacea. (A) Stress (σ) plotted as a function of extension ratio (λ) for a strip of blade of a M. splendens. The stiffness of the blade tissue (elastic modulus, E) is the slope of this curve. Because the slope changes with λ, we used the slope of the first linear part of the curve (indicated by the red line) to determine the elastic modulus (E1). The strength of the blade tissue is the stress at which it breaks (σbrk), and the extensibility of the tissue is the extension ratio at which it breaks (λmax). (B) Stiffness (E1) of blade tissue without epibionts (clean, black circle), blade tissue with a small bryozoan colony that did not extend into the grips of the Tensometer (patch, dark grey circle), blade tissue with 100% cover of a bryozoan colony on one side that extended into the Tensometer grips so that both algal and bryozoan tissues were being pulled (1 side, light grey circle), and blade tissue with 100% bryozoan cover on both sides that extended into the grips (2 sides, white circle). There was no significant difference between the E1’s of any of these treatments (p > 0.05, one-way ANOVA followed by Tukey HSD test, F= 1.703,21). (C) Extensibility (λmax) of algal blade tissue (black circles) when clean, and when fouled by bryozoans (as described in B). White squares show the λ’s at which the bryozoans popped off the blade (patch) or broke (1 side and 2 sides). The λmax of clean algae and algae with a small bryozoan colony were not significantly different (p > 0.05), but the λ at which small bryozoan colonies popped off algal blades was significantly lower than the λmax of the algal tissues (p < 0.01, one-way ANOVA followed by Tukey HSD test, F = 18.302,10). The λmax of bryozoan colonies on one side of and algal blade was significantly lower than the λmax of the algal tissue (p < 0.05, one-way ANOVA followed by Tukey HSD test, F = 5.061,12). Similarly, the λmax of bryozoan colonies on both sides of an algal blade was significantly lower than the λmax of the algal tissue (p < 0.01, one-way ANOVA followed by Tukey HSD test, F = 10.831,16). (D) Strength (σbrk) of algal blade tissue (black circles) and bryozoan colonies (white circles) when clean and when fouled as described in (B). A one-way ANOVA for the five treatments shown in the graph was followed by pairwise comparison using a Tukey HSD test (F = 21.424,33). The σbrk of algal blades (when clean or with bryozoans on one or two sides) was significantly higher than that of bryozoan colonies (when on one side of the host blade or when on both sides of the blade (p < 0.01 for all pairwise comparisons of algal tissue with bryozoans). The σbrk of clean algal blade tissue was not significantly different from that of algal tissue with bryozoans growing on one side (p > 0.05) or on both sides (p > 0.05). The σbrk of bryozoans covering just one side of an algal blade was not significantly different from the σbrk of bryozoans growing on both sides of a blade (p > 0.05).
The blade tissue of M. splendens was more extensible (had higher λmax) and stronger (had higher σbrk) than sheet-like bryozoan colonies attached to their surfaces (Figures 10C, D). We observed that small bryozoan colonies popped off algal blades at λ’s that were lower than the λmax’s of the blades. We also discovered that large bryozoan colonies broke at λmax’s and σbrk’s that were lower than those of the blade tissue. The λmax and σbrk of a bryozoan was not affected by having another bryozoan growing on the opposite side of a section of an algal blade.
3.5.2 Transplants and the Fate of Bryozoans
The stress-strain test results (Figure 10) led us to hypothesize that the flexing and stretching of algal blades by moving water might cause the breakage or loss of epibiotic bryozoans from those blades. We tested that idea by transplanting algae encrusted with bryozoans to the exposed site or back to the protected site. We found that a greater percentage of bryozoans were lost or broken if they were growing on algal blades exposed to rapid flow than if they were on blades exposed to slow water motion (Figure 11).
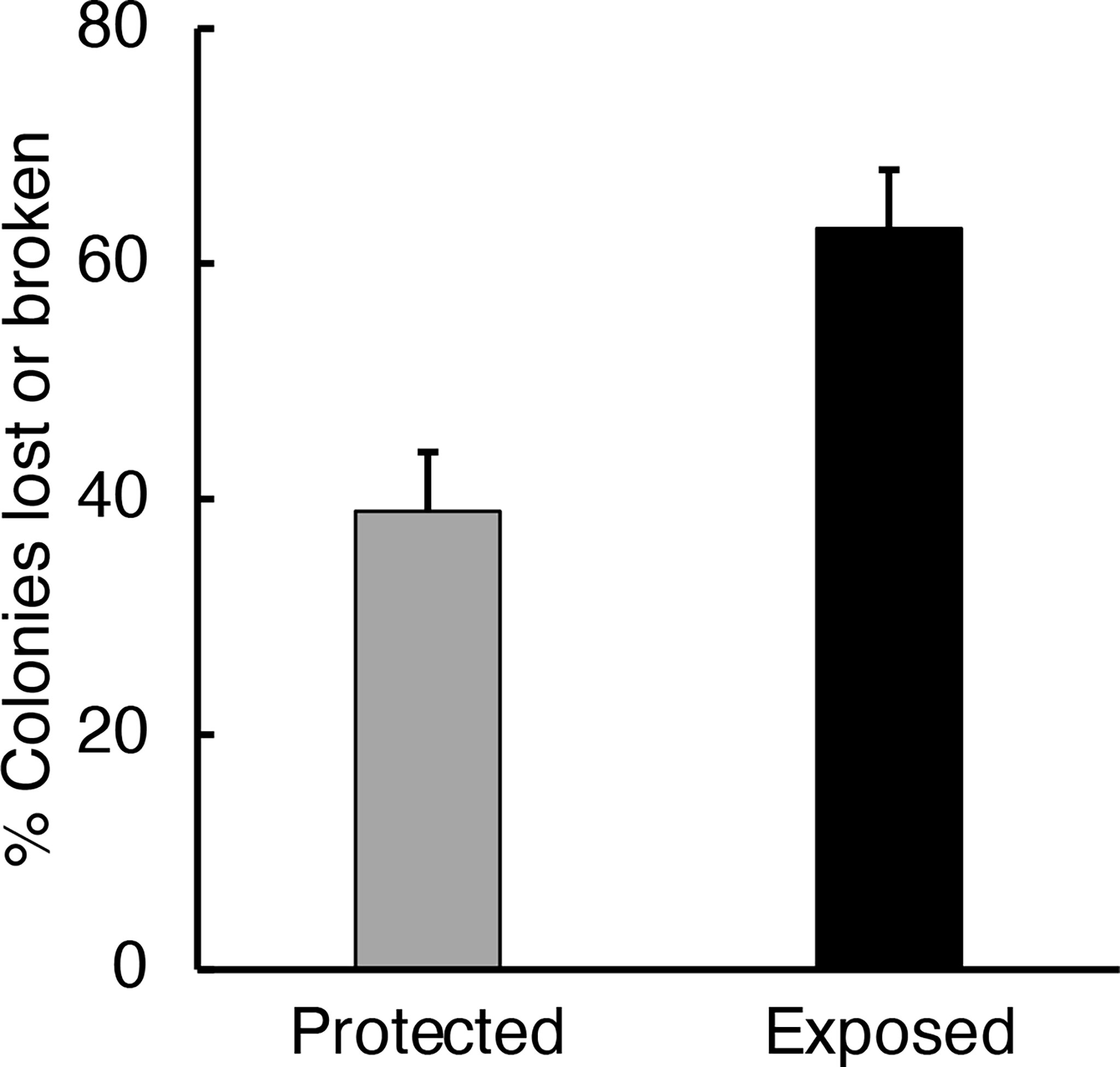
Figure 11 Percent of the colonies of M. membranacea on M. splendens (collected from the protected site) that were lost or broken over a 24-hour period after the algae were attached to tires at the protected site (grey bar) or at the exposed site (black bar). A significantly greater percentage of the bryozoan colonies were damaged or lost at the exposed site than at the protected site (p < 0.05, one-way ANOVA followed by Tukey HSD test, F= 6.251,9).
4 Discussion
Many organisms in benthic habitats live on the surfaces of flexible macrophytes, so an important aspect of understanding how hydrodynamics affects benthic communities is to discover how the epibionts and host macrophytes affect each other’s performance in different types of ambient water flow. We used blade-like red algae, Mazzaella splendens, and encrusting bryozoans, Membranipora membranacea, to investigate physical consequences for bryozoans of living on flexible algae, and for macroalgae of bearing crustose epibionts. Unlike earlier studies that focused on the effects of epibionts on hosts in unidirectional currents, we also studied host effects on epibionts, the hydrodynamic consequences of wave-driven flow and surrounding algal canopies, and the ramifications for host and epibiont of the differences in their material properties. We complemented earlier research on effects of M. membrancea on breakage of kelp blades in winter storms by instead examining the effects of this bryozoan on leafy red algae during late summer, when blade growth rates are high and storms are rare.
4.1 Effects of a Flexible Host on the Water Flow Environment Experienced by Epibionts
The local water flow experienced by epibionts affects the flux of materials between the organisms and the surrounding water as well as the hydrodynamic forces they experience. That local flow depends on the location of an epibiont on the host, the flexibility of the host, the exposure of the habitat to waves or unidirectional water currents, and whether or not the host is surrounded by a canopy of flexible macrophytes. M. membranacea cover is greater on the older regions of macroalgal blades, which is the basal region of the red alga M. splendens (Figure 3), but is near the distal blade tips of kelp (Seed and O’Connor, 1981; Durante and Chia, 1991). In small waves at protected sites, local boundary shear velocity (u*) encountered by epibionts near the base of a flexible alga surrounded by a canopy of other algae is greater than near the blade tip (Figure 4C). The low u* at blade tips occurs because, in wavy flow, the distal end of a flexible blade moves back-and-forth with the ambient water and experiences little water motion relative to its surface for most of the wave cycle (Koehl, 1999; Koehl, 2022). Another mechanism can contribute to lower u* at the distal ends of blades within canopies of macrophytes like M. splendens with slender basal regions and broad, leafy distal regions: the gaps where water flows between algae are smaller near the top of the canopy where the wide, ruffled regions of blades are pushed together as they are bent over by the flow (akin to the canopy compression that slows flow in upper regions of seagrass beds; Koch et al., 2006; Koehl, 2022). In contrast, the local u* encountered by epibionts at sites exposed to bigger waves is independent of position along a flexible host (Figure 4B). In waves with higher peak velocities, water flows a greater distance before reversing direction, and thus a flexible host experiences flow relative to its surfaces once it reaches the end of its tether (Koehl, 1999; Koehl, 2022). However, if macrophytes live in habitats exposed to unidirectional currents rather than waves, it has been suggested that epibionts on the distal parts of macroalgae encounter faster flow than organisms on the substratum (Keough, 1986; Wahl, 1989; Harder, 2009), but the host must be stiff or buoyant enough to hold the epibionts up into the faster flow (Koehl, 2022). Conversely, it has been argued that in unidirectional flow, if a flexible host bends over, epibionts on blade tips are sheltered from rapid flow by the upstream proximal parts of the alga (Anderson and Martone, 2014).
When flexible hosts flutter like flags or flap back and forth in ambient water flow, they increase the rate at which old, depleted water is replaced by new water near their surfaces and the epibionts sitting on them (Koehl and Alberte, 1988; Hunter, 1989; Koehl, 1999; Koch et al., 2006; Jabbari et al., 2021). Water exchange near fluttering M. splendens is faster than near rigid structures of the same length at a protected site where net water transport is slow (Figures 5B, C). Water exchange rates are slower in canopies than for solitary macroalgae (e.g. Arkema and Samhouri, 2019; Zhu et al., 2021; Koehl, 2022), but flapping helps to stir things up within aggregations of macroalgae (Figure 5C). In contrast, at sites exposed to bigger waves, water transport can be so rapid that flexible flapping does not make a difference to exchange rates (Figure 5).
4.2 Effects of Encrusting Epibionts on the Hydrodynamics of a Flexible Host
Epibionts can reduce the growth rates of their hosts by several mechanisms (see Effects of Epibionts on Their Macrophyte Hosts). However, growth of M. splendens, which occurs around the blade periphery, is not affected during the summer by encrusting bryozoans, which are most abundant on older regions of blades (Figure 6).
Many authors have suggested that epibionts increase the hydrodynamic forces that a host must bear in flowing water, and thus can lead to the dislodgement of the host by ambient currents or waves (see Effects of Epibionts on Their Macrophyte Hosts above). In unidirectional flow, bulbous (Anderson and Martone, 2014) and filamentous (Ruesink, 1998) algal epibionts have been shown to increase the drag on flexible arborescent algae. We complemented that work by measuring how crustose epibionts on blade-like algae affect drag in unidirectional flow and hydrodynamic forces in waves. The drag coefficients (CD = 2 D/[ρ u2 S], where D is drag, ρ is the density of the fluid, which is 999.7 kg/m3 for seawater at 11° C, and S is the planform area of the algal blade) that we measured for unfouled M. splendens in unidirectional flow ranged from 0.03 to 0.12, which falls within the range of drag coefficients reported for this species exposed to the water velocities we tested (Carrington, 1990; Mach, 2009). It has been proposed that encrusting epibionts could increase hydrodynamic forces on a macrophyte host by stiffening it, thereby hindering its ability to be reconfigured by ambient flow into a streamlined shape (see Effects of Epibionts on Their Macrophyte Hosts above). Encrusting M. membranacea increase the flexural stiffness of M. splendens blades (Figure 8), and increase both drag forces in unidirectional flow (Figure 7) and peak hydrodynamic forces in waves. Both unfouled and fouled blades reconfigure in water currents in ways that reduce drag as velocity increases, but unfouled blades are rolled into compact, streamlined shapes while those encrusted with bryozoans fold into flat shapes.
Whether or not increased hydrodynamic forces on a host due to its epibionts leads to breakage or dislodgement of the host depends on the ambient flow in the host’s habitat, and on how that flow as well as the tenacity of the host vary with season. M. splendens can be dislodged by stipe breakage or holdfast detachment (Carrington, 1990; Shaughnessy et al., 1996; Bell, 1999). The tenacities of stipes and holdfasts of M. splendens that we measured (Figure 9A) match the range of values reported for M. splendens (Shaughnessy et al., 1996). We found that tenacity is greater at sites exposed to rapid water flow than at protected habitats (Figure 9A), but the environmental stress factor for both fouled and unfouled algae is so high during the summer at our field sites (Figure 9C) that the increased forces due to fouling by M. membranacea have no impact on survival of the host M. splendens. In contrast, during the winter when growth rates are low, kelp encrusted with bryozoans are defoliated or wash away during winter storms (see Effects of Epibionts on Their Macrophyte Hosts above), and red arborescent algae fouled with filamentous epibionts lose many shoots (Ruesink, 1998). M splendens are ripped away during winter storms, especially in habitats exposed to heavy wave action, but leave behind a perennial holdfast from which a new thallus can grow during the next season (Bell, 1999; Shaughnessy and DeWreede, 2001; Dyck and de Wreede, 2006a). Even if epibionts increase the breakage of M. splendens or other macrophyte hosts during the winter, they might not impact the success of species that complete their growth and reproduction before seasonal storms hit (Johnson and Koehl, 1994; Ruesink, 1998; Koehl, 1999). Therefore, we need to know the life history and reproductive strategies of the hosts to determine whether epibiont-induced breakage in a particular season will impact the population dynamics of the hosts.
4.3 Effects of Encrusting Epibionts and Stretchy Hosts on Each Other’s Mechanical Performance
Encrusting M. membrancea epibionts do not affect the material properties of the blade tissue of their M. splendens hosts during the summer (Figure 10). The elastic modulus (E1), extensibility (λmax) and strength (σbrk) of unfouled blade tissue was the same as of blade tissue with attached bryozoans. The range of λmax’s and σbrk’s we measured overlap with published values for M. splendens blade tissue (Hale, 2001; Mach, 2009). Our study of the effects of M. membrancea on the blade tissue material properties of a red alga were made during the summer when blade growth rates are high (Dyck and de Wreede, 2006b). In contrast, reports abound about how infestations of M. membrancea make kelp blades brittle and lead to defoliation during winter storms (see Effects of Epibionts on Their Macrophyte Hosts above). In the late fall and winter when growth rates are low, measurements made of the effects of M. membrancea on the material properties of kelp blades showed that kelp tissue under the bryozoans had surface lesions and broke at lower extensions (λmax) and lower stresses (σbrk) than did unfouled blade tissue, although the bryozoans did not affect the elastic modulus of kelp tissue (Krumhansl et al., 2011). Furthermore, during the summer, we found that there was no difference between the σbrk’s of the blade tissues of male and female gametophytes, tetrasporophytes, and non-reproductive individuals, whereas during the autumn Mach (2009) found that tissue fracture started at the edges of reproductive bodies on blades such that the σbrk’s of reproductive blades were lower than of non-reproductive blades, and that female blades were weaker than tetrasporphyte blades. Furthermore, in the field, increased tattering of blades of M. splendens occurs at the time when blades become reproductive (Dyck and de Wreede 2006a). We only tested basal and mid regions of blades without reproductive bodies because those regions were where bryozoan fouling occurred, so the difference between our results and those of (Mach, 2009) could be due to seasonal changes in material properties and/or to spatial differences along blades in material properties. M. splendens blade tissues break at lower stresses if subjected to cyclical loading, as they would be in waves, than the σbrk’s measured in stress-strain tests (Mach, 2009), so future studies of effects of epibionts on the material properties of their hosts should be designed to mimic the time course of loading that the hosts experience in their natural habitats.
The blade tissue of M. splendens is stronger (higher σbrk) and more extensible (higher λmax) than the M.membrancea colonies attached to their surfaces, so small colonies pop off blades and large colonies fracture as blades are stretched (Figures 10C, D). Similarly, rigid epibionts, such as worms that build calcareous tubes, pop off flexible algae or break when the algae are bent (Walters et al., 2003). Thus, being extensible and flexible can be added to the list of defenses that macrophytes use to rid themselves of epibionts (see Effects of Epibionts on Their Macrophyte Hosts above). Our field study and another by Pratt (2008) showed that when M. splendens blades flail around in waves at exposed sites, more epibiotic bryozoans are broken or lost than in slow-flow habitats, so the effectiveness of this defense mechanism depends on the flow environment of the host. Likewise, stiff encrusting bryozoans fracture when host kelp are exposed to heavy wave action (Seed and O’Connor 1981). The cover of encrusting bryozoans on algal blades is greater in slow flow habitats than at sites exposed to more rapid water motion (Figures 1A, B) (Seed and O’Connor, 1981; Pratt, 2008), but this could be due to the settlement behavior and recruitment patterns of the larvae of epibionts (Bernstein and Jung, 1979; Seed and O’Connor, 1981; Durante and Chia, 1991; Steinberg and de Nys, 2002; Harder, 2009; Matson et al., 2010; Arkema and Samhouri, 2019), as well as to mechanical dislodgement of epibionts.
4.4 Conclusions
Flexible macrophyte hosts and epibionts affect each other’s flow environments, and thus the flux of water-borne substances and hydrodynamic forces they experience, and they also can alter each other’s mechanical responses to the water motion they encounter.
(1) How does a flexible host affect the water flow environment experienced by encrusting epibionts attached to its surface? We found that the flapping of a flexible host in waves enhances the rate of renewal of water near its epibionts, but the positions along a flapping algal blade where epibionts encounter the highest time-averaged boundary shear velocities depends both on the wave exposure of the habitat and on whether the host is surrounded by other algae.
(2) How do encrusting epibionts affect the hydrodynamic forces on a flexible host? We discovered that hydrodynamic forces on flexible algae moving back-and-forth with the water can be lower in waves than in unidirectional flow. Encrusting bryozoans stiffen host algae and alter their passive reconfiguration in flowing water, thereby increasing hydrodynamic forces on the host in both unidirectional currents and waves. However, during the summer growing season, the environmental stress factor for M. splendens is so high that bryozoan encrustation should not lead to breakage of the host algae.
(3) How do encrusting epibionts and deformable hosts affect each other’s material properties and biomechanical performance? During the summer when macroalgae are growing rapidly, we found that bryozoan encrustation did not affect the material properties of host M. splendens. If algal blades are more extensible and stronger than encrusting bryozoans, the bryozoans can be fractured or popped off algae as they are stretched and bent by ambient flow.
Thus, our study reveals that the complex fluid-structure interactions of flexible macrophyte hosts and their epibionts depend on their life history strategies (e.g. seasonal changes in morphology, mechanical properties, growth patterns, and reproduction), as well as on the flow habitat (e.g. local exposure to turbulent currents and waves, and seasonal patterns of storms), and on the structure and mechanical behavior of the surrounding community of other organisms.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
MK and TD designed and conducted the laboratory experiments and field studies, MK was responsible for data analysis and statistics, and MK and TD wrote the paper together. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by National Science Foundation grant OCE9217338 to MK. TD gratefully acknowledges support from the Joan and Richard Komen Endowed Chair at the University of Washington.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Friday Harbor Laboratories, University of Washington, for use of facilities. We are grateful to C.J. Baron, F. Dardis, S. Distefano, K.V. Etch, S. Kahane, K. Lynn, F.L. Mingo, and N.D. Pentcheff for help with field experiments and image analysis.
References
Abbott I. A., Hollenberg G. J. (1992). Marine Algae of California (Stanford, CA:Stanford University Press).
Albayrak I., Nikora V., Miler O., O’Hare M. (2012). Flow-Plant Interactions at a Leaf Scale: Effects of Leaf Shape, Serration, Roughness and Flexural Rigidity. Aquat. Sci. 74, 267–286. doi: 10.1007/s00027-011-0220-9
Anderson L. M., Martone P. T. (2014). Biomechanical Consequences of Epiphytism in Intertidal Macroalgae. J. Exp. Biol. 217, 1167–1174. doi: 10.1242/jeb.088955
Arkema K. K., Samhouri J. F. (2019). Living on the Edge: Variation in the Abundance and Demography of a Kelp Forest Epibiont. Diversity 11, 120. doi: 10.3390/d11080120
Avila C., Angulo-Preckler C., Martín-Martín R. P., Figuerola B., Griffiths H. J., Waller C. L. (2020). Invasive Marine Species Discovered on Non–Native Kelp Rafts in the Warmest Antarctic Island. Sci. Rep. 10, 1–9. doi: 10.1038/s41598-020-58561-y
Bell E. C. (1992). Consequences of Morphological Variation in an Intertidal Macroalga: Physical Constraints on Growth and Survival of Mastocarpus Papillatus Kützing. Ph. D. Dissertation. Stanford:Stanford University
Bell E. C. (1999). Applying Flow Tank Measurements to the Surf Zone: Predicting Dislodgment of the Gigartinaceae. Phycol. Res. 47, 159–166. doi: 10.1046/j.1440-1835.1999.00169.x
Bernstein B. B., Jung N. (1979). Selective Pressures and Coevolution in a Kelp Canopy Community in Southern California. Ecol. Monogr. 49, 335–355. doi: 10.2307/1942488
Black R. (1976). The Effects of Grazing by the Limpet, Acmaea Insessa, on the Kelp, Egregia laevigata, in the Intertidal Zone. Ecology 57, 265–277. doi: 10.2307/1934815
Brush M. J., Nixon S. W. (2002). Direct Measurements of Light Attenuation by Epiphytes on Eelgrass Zostera marina. Mar. Ecol. Prog. Ser. 238, 73–79. doi: 10.3354/meps238073
Bueno M., Dena-Silva S. A., Flores A., Leite F. P. P. (2016). Effects of Wave Exposure on the Abundance and Composition of Amphipod and Tanaidacean Assemblages Inhabiting Intertidal Coralline Algae. J. Mar. Biol. Assoc. Unit. Kingd. 96, 761–767. doi: 10.1017/S002531541500123X
Burnett N. P., Armstrong E. J., Romero R., Runzel C. C., Tanner R. L. (2021). Kelp Morphology and Herbivory are Maintained Across Latitude Despite Geographic Shift in Lelp-Wounding Herbivores. Biol. Bull. 241, 0. doi: 10.1086/715039
Burnett N. P., Koehl M. A. R. (2018). Knots and Tangles Weaken Kelp Fronds While Increasing Drag Forces and Epifauna on the Kelp. J. Exp. Mar. Biol. Ecol. 508, 13–20. doi: 10.1016/j.jembe.2018.08.003
Burnett N. P., Koehl M. A. R. (2019). Mechanical Properties of the Wave-Swept Kelp Egregia menziesii Change With Season, Growth Rate and Herbivore Wounds. J. Exp. Biol. 222, jeb190595. doi: 10.1242/jeb.190595
Burnett N. P., Koehl M. A. R. (2020). Thallus Pruning Does Not Enhance Survival or Growth of a Wave-Swept Kelp. Mar. Biol. 167, 1–12. doi: 10.1007/s00227-020-3663-5
Caines S., Gagnon P. (2012). Population Dynamics of the Invasive Bryozoan Membranipora membranacea Along a 450-Km Latitudinal Range in the Subarctic Northwestern Atlantic. Mar. Biol. 159, 1817–1832. doi: 10.1007/s00227-012-1972-z
Campbell G. S. (2000). An Introduction to Environmental Biophysics (Springer: Science and Business Media).
Carrington E. (1990). Drag and Dislodgment of an Intertidal Macroalga: Consequences of Morphological Variation in Mastocarpus papillatus Kützing. J. Exp. Mar. Biol. Ecol. 139, 185–200. doi: 10.1016/0022-0981(90)90146-4
Christie H., Norderhaug K. M., Fredriksen S. (2009). Macrophytes as Habitat for Fauna. Mar. Ecol. Prog. Ser. 396, 221–233. doi: 10.3354/meps08351
da Gama B. A. P., de A. Santos R. P., Pereira R. C. (2008). The Effect of Epibionts on the Susceptibility of the Red Seaweed Cryptonemia seminervis to Herbivory and Fouling. Biofouling 24, 209–218. doi: 10.1080/08927010802041253
da Gama B. A. P., Plouguerné E., Pereira R. C. (2014). The Antifouling Defence Mechanisms of Marine Macroalgae. Adv. Botanic. Res. 71, 413–440. doi: 10.1016/B978-0-12-408062-1.00014-7
de Bettignies T., Wernberg T., Lavery P. S. (2013). Size, Not Morphology, Determines Hydrodynamic Performance of a Kelp During Peak Flow. Mar. Biol. 160, 843–851. doi: 10.1007/s00227-012-2138-8
de Burgh M. E., Fankboner P. v (1978). A Nutritional Association Between the Bull Kelp Nereocystis luetkeana and its Epizooic Bryozoan Membranipora membranacea. Oikos 31, 69–72. doi: 10.2307/3543385
Denny M. W., Daniel T. L., Koehl M. A. R. (1985). Mechanical Limits to Size in Wave-Swept Organisms. Ecol. Monogr. 55, 69–102. doi: 10.2307/1942526
Dixon J., Schroeter S. C., Kastendiek J. (1981). Effects of the Encrusting Bryozoan, Membranipora membranacea, on the Loss of Blades and Fronds by the Giant Kelp, Macrocystis pyrifera (Laminariales). J. Phycol. 17, 341–345. doi: 10.1111/j.1529-8817.1981.tb00860.x
Durante K. M., Chia F.-S. (1991). Epiphytism on Agarum fimbriatum: Can Herbivore Preferences Explain Distributions of Epiphytic Bryozoans? Mar. Ecol. Prog. Series. Oldendorf. 77, 279–287. doi: 10.3354/meps077279
Dyck L. J., de Wreede R. E. (2006a). Reproduction and Survival in Mazzaella splendens (Gigartinales, Rhodophyta). Phycologia 45, 302–310. doi: 10.2216/04-80.1
Dyck L. J., de Wreede R. E. (2006b). Seasonal and Spatial Patterns of Population Density in the Marine Macroalga Mazzaella splendens (Gigartinales, Rhodophyta). Phycol. Res. 54, 21–31. doi: 10.1111/j.1440-1835.2006.00405.x
Efron B., Tibshirani R. (1986). Bootstrap Methods for Standard Errors, Confidence Intervals, and Other Measures of Statistical Accuracy. Stat. Sci. 1, 54–75.
Fenwick G. D. (1976). The Effect of Wave Exposure on the Amphipod Fauna of the Alga Caulerpa brownii. J. Exp. Mar. Biol. Ecol. 25, 1–18. doi: 10.1016/0022-0981(76)90072-1
Fletcher R. L. (1995). Epiphytism and Fouling in Gracilaria Cultivation: An Overview. J. Appl. Phycol. 7, 325–333. doi: 10.1007/BF00004006
Forde H., Forbord S., Handaa A., Fossberg J., Arff J., Johnsen G., et al. (2016). Development of Bryozoan Fouling on Cultivated Kelp (Saccharina latissima) in Norway. J. Appl. Phycol. 28, 1225–1234. doi: 10.1007/s10811-015-0606-5
Hale B. B. (2001). Macroalgal Materials: Foiling Fracture and Fatigue From Fluid Forces Ph.D. (Dissertation. Stanford: Stanford University).
Harder T. (2009). “Marine Epibiosis: Concepts, Ecological Consequences and Host Defence,” in Marine and Industrial Biofouling ed. Flemming H.-C., Murthy P. S., Venkatesan R., Cooksey K. E. (Berlin: Springer), 219–231.
Heck K. L., Valentine J. F. (2006). Plant–herbivore Interactions in Seagrass Meadows. J. Exp. Mar. Biol. Ecol. 330, 420–436. doi: 10.1016/j.jembe.2005.12.044
Hepburn C., Frew R., Hurd C. (2012). Uptake and Transport of Nitrogen Derived From Sessile Epifauna in the Giant Kelp Macrocystis pyrifera. Aquat. Biol. 14, 121–128. doi: 10.3354/ab00382
Hepburn C. D., Hurd C. L., Frew R. D. (2006). Colony Structure and Seasonal Differences in Light and Nitrogen Modify the Impact of Sessile Epifauna on the Giant Kelp Macrocystis pyrifera (L.) C Agardh. Hydrobiologia 560, 373–384. doi: 10.1007/s10750-005-1573-7
Hughes A., Bando K., Rodriguez L., Williams S. (2004). Relative Effects of Grazers and Nutrients on Seagrasses: A Meta-Analysis Approach. Mar. Ecol. Prog. Ser. 282, 87–99. doi: 10.3354/meps282087
Hunter T. (1988). Mechanical Design of Hydroids: Flexibility, Flow Forces and Feeding in Obelia longissima. Ph.D. Dissertation. Berkeley, CA: University of California, Berkeley
Hunter T. (1989). Suspension Feeding in Oscillating Flow: The Effect of Colony Morphology and Flow Regime on Plankton Capture by the Hydroid Obelia longissima. Biol. Bull. 176, 41–49. doi: 10.2307/1541887
Hurd C. L., Durante K. M., Harrison P. J. (2000). Influence of Bryozoan Colonization on the Physiology of the Kelp Macrocystis integrifolia (Laminariales, Phaeophyta) From Nitrogen-Rich and-Poor Sites in Barkley Sound, British Columbia, Canada. Phycologia 39, 435–440. doi: 10.2216/i0031-8884-39-5-435.1
Jabbari A., Yanase K., Ackerman J. D. (2021). A Spanwise Oscillating Plate in a Crossflow: Implication for Mass Transfer and Locomotion. Limnol. Oceanogr. 66, 3393–3407. doi: 10.1002/lno.11886
Johnson A., Koehl M. (1994). Maintenance of Dynamic Strain Similarity and Environmental Stress Factor in Different Flow Habitats: Thallus Allometry and Material Properties of Giant Kelp. J. Exp. Biol. 195, 381–410. doi: 10.1242/jeb.195.1.381
Jones C. G., Lawton J. H., Shachak M. (1994). “Organisms as Ecosystem Engineers” in Ecosystem Management (New York, NY: Springer New York), 130–147. doi: 10.1007/978-1-4612-4018-1_14
Karez R., Engelbert S., Sommer U. (2000). Co-Consumption and Protective Coating: Two New Proposed Effects of Epiphytes on Their Macroalgal Hosts in Mesograzer-Epiphyte-Host Interactions. Mar. Ecol. Prog. Ser. 205, 85–93. doi: 10.3354/meps205085
Keough M. J. (1986). The Distribution of a Bryozoan on Seagrass Blades: Settlement, Growth, and Mortality. Ecology 67, 846–857. doi: 10.2307/1939807
Koch E. W., Ackerman J. D., Verduin J., Keulen M.v. (2006). “Fluid Dynamics in Seagrass Ecology—from Molecules to Ecosystems,” in Seagrasses: Biology, Ecology and Conservation (Dordrecht: Springer Netherlands), 193–225. doi: 10.1007/978-1-4020-2983-7_8
Koehl M. A. R. (1977). Effects of Sea Anemones on the Flow Forces They Encounter. J. Exp. Biol. 69, 87–105. doi: 10.1242/jeb.69.1.87
Koehl M. A. R. (1986). “Seaweeds in Moving Water: From and Mechanical Function,” in On the Economy of Plant Form and Function, Givnish T. J.(New York: Cambridge University Press), 603–634.
Koehl M. A. (1999). Ecological Biomechanics of Benthic Organisms: Life History, Mechanical Design and Temporal Patterns of Mechanical Stress. J. Exp. Biol. 202, 3469–3476. doi: 10.1242/jeb.202.23.3469
Koehl M. A. R. (2022). Ecological Biomechanics of Marine Macrophytes. J. Exp. Bot. 73, 1103–1121. doi: 10.1093/jxb/erab536
Koehl M. A. R., Alberte R. S. (1988). Flow, Flapping, and Photosynthesis of Nereocystis leutkeana: A Functional Comparison of Undulate and Flat Blade Morphologies. Mar. Biol. 99, 435–444. doi: 10.1007/BF02112137
Koehl M. A. R., Powell T. M. (1994). “Turbulent Transport of Larvae Near Wave-Swept Rocky Shores: Does Water Motion Overwhelm Larval Sinking” in Reproduction and Development of Marine Invertebrates (Baltimore: ohns Hopkins University Press), 261–274.
Koehl M. A. R., Powell T. M., Dairiki G. (1993). “Measuring the Fate of Patches in the Water: Larval Dispersal,”. Patch Dynamics: Lecture Notes in Biomathematics Eds. Levin S. A., Powell T. M., Steel J. W. (Berlin, DE: Springer), 50–60.
Koehl M. A. R., Powell T. M., Dobbins E. L. (1997). Effects of Algal Turf on Mass Transport and Flow Microhabitat of Ascidians in a Coral Reef Lagoon. Proc. 8th. Int. Coral. Reef. Symp. 2, 1087–1092.
Koehl M. A. R., Wainwright S. A. (1977). Mechanical Adaptations of a Giant Kelp. Limnol. Oceanogr. 22, 1067–1071. doi: 10.4319/lo.1977.22.6.1067
Koehl M. A. R., Wainwright S. A. (1985). “Biomechanics,” in Handbook of Phycological Methods: Ecological Field Methods for Macroalgae. Eds. Littler M. M., Littler D. S. (Cambridge, UK:Cambridge University Press), 292–313.
Krumhansel K. A., Demes K. W., Carrington E., Harley C. D. G. (2015). Divergent Growth Strategies Between Red Algae and Kelps Influence Bomechanical Properties. Am. J. Botany 102, 1938–1944. doi: 10.3732/ajb.1500289
Krumhansl K. A., Lee J. M., Scheibling R. E. (2011). Grazing Damage and Encrustation by an Invasive Bryozoan Reduce the Ability of Kelps to Withstand Breakage by Waves. J. Exp. Mar. Biol. Ecol. 407, 12–18. doi: 10.1016/j.jembe.2011.06.033
Krumhansl K. A., Scheibling R. E. (2011). Detrital Production in Nova Scotian Kelp Beds: Patterns and Processes. Mar. Ecol. Prog. Ser. 421, 67–82. doi: 10.3354/meps08905
Lambert W. J., Levin P. S., Berman J. (1992). Changes in the Structure of a New England (USA) Kelp Bed: The Effects of an Introduced Species? Mar. Ecol. Prog. Series. Oldendorf. 88, 303–307. doi: 10.3354/meps088303
Levin P. S., Coyer J. A., Petrik R., Good T. P. (2002). Community-Wide Effects of Nonindigenous Species on Temperate Rocky Reefs. Ecology 83, 3182–3193. doi: 10.1890/0012-9658(2002)083[3182:CWEONS]2.0.CO;2
Lobban C. S. (1978). Growth of Macrocystis integrifolia in Barkley Sound, Vancouver Island, BC. Can. J. Bot. 56, 2707–2711. doi: 10.1139/b78-323
Mach K. J. (2009). Mechanical and Biological Consequences of Repetitive Loading: Crack Initiation and Fatigue Failure in the Red Macroalga Mazzaella. J. Exp. Biol. 212, 961–976. doi: 10.1242/jeb.026989
Manriquez P. H., Cancino J. M. (1996). Bryozoan-Macroalgal Interactions: Do Epibionts Benefit? Mar. Ecol. Prog. Ser. 138, 189–197. doi: 10.3354/meps138189
Martone P. T., Kost L., Boller M. (2012). Drag Reduction in Wave-Swept Macroalgae: Alternative Strategies and New Predictions. Am. J. Bot. 99, 806–815. doi: 10.3732/ajb.1100541
Matson P. G., Steffen B. T., Allen R. M. (2010). Settlement Behavior of Cyphonautes Larvae of the Bryozoan Membranipora membranacea in Response to Two Algal Substrata. Invertebr. Biol. 129, 277–283. doi: 10.1111/j.1744-7410.2010.00203.x
Nepf H. M. (2012). Hydrodynamics of Vegetated Channels. J. Hydraulic. Res. 50, 262–279. doi: 10.1080/00221686.2012.696559
Neushul M., Haxo F. T. (1963). Studies on the Giant Kelp, Macrocystis. I. Growth of Young Plants. Am. J. Bot. 50, 349–353. doi: 10.1002/j.1537-2197.1963.tb07202.x
Norton T. A. (1973). Orientated Growth of Membranipora membranacea (L.) on the Thallus of Saccorhiza polyschides (Lightf.) Batt. J. Exp. Mar. Biol. Ecol. 13, 91–95. doi: 10.1016/0022-0981(73)90072-5
Nowell A. R. M., Jumars P. A. (1984). Flow Environments of Aquatic Benthos. Annu. Rev. Ecol. Systemat. 15, 303–328. doi: 10.1146/annurev.es.15.110184.001511
Nowell A. R., Jumars P. A. (1987). Flumes: Theoretical and Experimental Considerations for Simulation of Benthic Environments. Oceanogr. Mar. Biol. 25, 91–112.
Nylund G. M., Pavia H. (2005). Chemical Versus Mechanical Inhibition of Fouling in the Red Alga Dilsea carnosa. Mar. Ecol. Prog. Ser. 299, 111–121. doi: 10.3354/meps299111
Okamura B. (1985). The Effects of Ambient Flow Velocity, Colony Size, and Upstream Colonies on the Feeding Success of Bryozoa. II. Conopeum reticulum (Linnaeus), an Encrusting Species. J. Exp. Mar. Biol. Ecol. 89, 69–80. doi: 10.1016/0022-0981(85)90082-6
Okamura B. (1990). Particle Size, Flow Velocity, and Suspension-Feeding by the Erect Bryozoans Bugula neritina and B. stolonifera. Mar. Biol. 105, 33–38. doi: 10.1007/BF01344268
Okamura B. (1992). Microhabitat Variation and Patterns of Colony Growth and Feeding in a Marine Bryozoan. Ecology 73, 1502–1513. doi: 10.2307/1940693
Oswald R. C., Seed R. (1986). Organisation and Seasonal Progression Within the Epifaunal Communities of Coastal Macroalgae. Cahier. Biol. Mar. 27, 29–40.
Oswald R. C., Telford N., Seed R., Happey-Wood C. M. (1984). The Effect of Encrusting Bryozoans on the Photosynthetic Activity of Fucus serratus L. Estuar. Coast. Shelf. Sci. 19, 697–702. doi: 10.1016/0272-7714(84)90024-6
Paul V. J. (1992). “Seaweed Chemical Defenses on Coral Reefs,” in Ecological Roles of Marine Natural Products (Ithaca, NY:Cornell University Press), 24–50.
Pereira R. C., da Gama B. A. P., Teixeira V. L., Yoneshigue-Valentin Y. (2003). Ecological Roles of Natural Products of the Brazilian Red Seaweed Laurencia obtusa. Braz. J. Biol. 63, 665–672. doi: 10.1590/S1519-69842003000400013
Peteiro C., Freire ‘Oscar (2013). Epiphytism on Blades of the Edible Kelps Undaria Pinnatifida and Saccharina latissima Farmed Under Different Abiotic Conditions. J. World Aquacult. Soc. 44, 706–715. doi: 10.1111/jwas.12065
Pratt M. C. (2008). Living Where the Flow is Right: How Flow Affects Feeding in Bryozoans. Integr. Comp. Biol. 48, 808–822. doi: 10.1093/icb/icn052
Ruesink J. L. (1998). Diatom Epiphytes on Odonthalia floccosa: The Importance and Extent of Timing. J. Phycol. 34, 29–38. doi: 10.1046/j.1529-8817.1998.340029.x
Saier B., Chapman A. S. (2004). Crusts of the Alien Bryozoan Membranipora membranacea Can Negatively Impact Spore Output From Native Kelps (Laminaria longicruris). Botanica Mar. 47, 265–271. doi: 10.1515/BOT.2004.031
Saunders M., Metaxas A. (2008). High Recruitment of the Introduced Bryozoan Membranipora membranacea Is Associated With Kelp Bed Defoliation in Nova Scotia, Canada. Mar. Ecol. Prog. Ser. 369, 139–151. doi: 10.3354/meps07669
Scheibling R., Gagnon P. (2009). Temperature-Mediated Outbreak Dynamics of the Invasive Bryozoan Membranipora membranacea in Nova Scotian Kelp Beds. Mar. Ecol. Prog. Ser. 390, 1–13. doi: 10.3354/meps08207
Scheibling R. E., Hennigar A. W., Balch T. (1999). Destructive Grazing, Epiphytism, and Disease: The Dynamics of Sea Urchin-Kelp Interactions in Nova Scotia. Can. J. Fish. Aquat. Sci. 56, 2300–2314. doi: 10.1139/f99-163
Schultze K., Janke K., Kr"uss A., Weidemann W. (1990). The Macrofauna and Macroflora Associated With Laminaria digitata and L. hyperborea at the Island of Helgoland (German Bight, North Sea). Helgol"ander. Meeresuntersuch. 44, 39–51. doi: 10.1007/BF02365430
Seed R., O’Connor R. J. (1981). Community Organization in Marine Algal Epifaunas. Annu. Rev. Ecol. Systemat. 12, 49–74. doi: 10.1146/annurev.es.12.110181.000405
Shaughnessy F. J., DeWreede R. E. (2001). Size, Survival and the Potential for Reproduction in Transplants of Mazzaella splendens and M. linearis (Rhodophyta). Mar. Ecol. Prog. Ser. 222, 109–118. doi: 10.3354/meps222109
Shaughnessy F. J., de Wreede R. E., Bell E. C. (1996). Consequences of Morphology and Tissue Strength to Blade Survivorship of Two Closely Related Rhodophyta Species. Mar. Ecol. Prog. Ser. 136, 257–266. doi: 10.3354/meps136257
Sokal R. R., Rohlf F. J. (1995). Biometry: The Principles and Practice of Statistics in Biological Research. 3rd Edition, New York: W.H. Freeman and Co.
Steinberg P. D., de Nys R. (2002). Chemical Mediation of Colonization of Seaweed Surfaces. J. Phycol. 38, 621–629. doi: 10.1046/j.1529-8817.2002.02042.x
Stewart J. G. (1982). Anchor Species and Epiphytes in Intertidal Algal Turf. Pacific. Sci. 367, 45–59. doi: 10.2307/1307394
Vogel S. (1989). Drag and Reconfiguration of Broad Leaves in High Winds. J. Exp. Bot. 40, 941–948. doi: 10.1093/jxb/40.8.941
Wahl M. (1989). Marine Epibiosis. I. Fouling and Antifouling: Some Basic Aspects. Mar. Ecol. Prog. Ser. 58, 175–189. doi: 10.3354/meps058175
Wahl M. (2008). Ecological Lever and Interface Ecology: Epibiosis Modulates the Interactions Between Host and Environment. Biofouling 24, 427–438. doi: 10.1080/08927010802339772
Wahl M., Hay M. E. (1995). Associational Resistance and Shared Doom: Effects of Epibiosis on Herbivory. Oecologia 102, 329–340. doi: 10.1007/BF00329800
Wahl M., Kröger K., Lenz M. (1998). Non-Toxic Protection Against Epibiosis. Biofouling 12, 205–226. doi: 10.1080/08927019809378355
Walters L. J., Smith C. M., Hadfield M. G. (2003). Recruitment of Sessile Marine Invertebrates on Hawaiian Macrophytes: Do Pre-Settlement or Post-Settlement Processes Keep Plants Free From Fouling? Bull. Mar. Sci. 72, 813–839.
Watanabe S., Scheibling R. E., Metaxas A. (2010). Contrasting Patterns of Spread in Interacting Invasive Species: Membranipora membranacea and Codium fragile Off Nova Scotia. Biol. Invas. 12, 2329–2342. doi: 10.1007/s10530-009-9647-5
Williams G. A., Seed R. (1992). “Interactions Between Macrofaunal Epiphytes and Their Host Algae,” in Plant-Animal Interactions in the Marine Benthos (Oxford, UK: Claredon Press), 189–211.
Wing B. L., Clendenning K. A. (1971). Kelp Surfaces and Associated Invertebrates. Nova. Hedwigia. Beihefte. 32, 319–341
Wong M. C., Vercaemer B. (2012). Effects of Invasive Colonial Tunicates and a Native Sponge on the Growth, Survival, and Light Attenuation of Eelgrass (Zostera marina). Aquat. Invas. 7, 315–326. doi: 10.3391/ai.2012.7.3.003
Worcester S. E. (1994). Adult Rafting Versus Larval Swimming: Dispersal and Recruitment of a Botryllid Ascidian on Eelgrass. Mar. Biol. 121, 309–317. doi: 10.1007/BF00346739
Keywords: macroalgae, Bryozoans, biomechanics, fouling, Epibionts, hydrodynamics, Membranipora membranacea, Mazzaella splendens
Citation: Koehl MAR and Daniel TL (2022) Hydrodynamic Interactions Between Macroalgae and Their Epibionts. Front. Mar. Sci. 9:872960. doi: 10.3389/fmars.2022.872960
Received: 10 February 2022; Accepted: 05 May 2022;
Published: 22 June 2022.
Edited by:
Laura Ann Miller, University of Arizona, United StatesReviewed by:
Uri Shavit, Technion Israel Institute of Technology, IsraelChristina Hamlet, Bucknell University, United States
Copyright © 2022 Koehl and Daniel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: M.A.R. Koehl, cnidaria@berkeley.edu
 M.A.R. Koehl
M.A.R. Koehl Thomas L. Daniel2
Thomas L. Daniel2