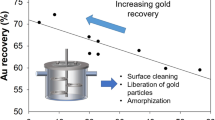
Abstract
A recent Nevada Gold Mines research program focused on pressure oxidation pre-treatment of a Carlin-type double refractory ore from the Turquoise Ridge Mine. The research team found that both arsenian pyrite and carbonaceous matter were highly oxidized within an hour at 300 °C using a bench-top autoclave reactor and that the gold recovery from the residues after cyanide/carbon-in-leach approached the technical limit set by the amount of gold encapsulated by silicate minerals. Although the 60-min pre-treatment at 300 °C yields robust performance when treating a whole ore, copper additives can catalyze the oxidation rate of carbonaceous matter and reduce the residence time needed at elevated temperature. Certain gangue minerals are susceptible to dissolution in acidic solutions at 300 °C, and this may cause poor gold recovery. This recovery challenge is mitigated by keeping free acid in the discharge solution below a key threshold.
Similar content being viewed by others
1 Introduction
Nevada Gold Mines (NGM) processes Carlin-type ores that are difficult to float due to challenging regional mineralogy and that are increasingly double refractory. Whole ore roasting is currently the preferred pre-treatment step ahead of gold recovery by carbon-in-leach (CIL). Roasting can simultaneously oxidize the ore’s gold-bearing sulfides and the naturally occurring carbonaceous matter (CM) that is often preg-robbing.
Roasting pre-treatment of the gold-bearing arsenian pyrite ores often yields dense-phase hematite grain morphologies that rarely release more than 80% of their contained gold during CIL. However, a significant amount of free leaching gold within NGM’s ores often masks this unfavorable roasting aspect and the overall gold recovery after roasting these ores is reasonable in most cases.
Conversely, the pre-treatment of the arsenian pyrite at the lower temperature afforded by pressure oxidation (POx) typically yields hematite morphologies that are more amenable to CIL particularly when the pyrite grains have higher concentrations of arsenic in solid solution. Although POx treatment enjoys this advantage, the challenge in treating double refractory ore by POx is that NGM’s existing facilities operate at 225 °C which is too low of a temperature for oxidizing the CM.
With help from FLSmidth’s Minerals Testing and Research Center (FLS) based in Salt Lake City, Utah, NGM embarked on a program to find the POx conditions needed for the rapid oxidation of the CM within double refractory whole ores. Phase 1 of this program focused specifically on acidic POx conditions. NGM selected Turquoise Ridge (TR) Stockpile O ore (the ore) as a reasonable proxy for double refractory ores having a S2−/CO3 ratio compatible with acidic POx pre-treatment. This long-term stockpile holds ore mined from the Twin Creeks MegaPit and has elevated levels of preg-robbing CM.
The specific goal of Phase 1 was to find treatment conditions and methods that would consistently yield greater than 80% CM oxidation in the ore within 20 min. NGM considered 20 min to be the practical limit for high-temperature, high-pressure plug flow reactor designs. Optimization of the treatment temperature to the lowest possible, yet still compatible with the targeted residence time, was a key focus because these aspects are drivers for both operating cost and reactor capital cost.
The authors discuss the advantages of using both catalysts and a dual-stage oxidation flowsheet when oxidizing CM under subcritical water conditions (< 374 °C and < 22.1 MPa). When CM oxidation is greater than 80%, gold recovery from the POx residues after CIL can approach the technical limit set by the gold encapsulated in silicates.
1.1 Supercritical Water Oxidation of CM
At least two groups have found that supercritical water oxidation (SCWO) of double refractory ore was very effective [1, 2]. General Atomics performed pilot testing on the ore and found that complete oxidation of sulfides and CM was possible in as little as 15 s [2]. However, SCWO’s harsh conditions and high energy requirements caused NGM to opt for treatment of the ore under subcritical water conditions. Nevertheless, NGM may find that SCWO is the only effective POx pre-treatment for the alkaline, double refractory ore types that are the focus of Phase 2 of the program.
1.2 Subcritical Water Oxidation of CM
During 1993, air products and chemicals patented a plug flow reactor concept for wet oxidation of organic aqueous streams. The target temperature for the reactor ranged between 325 and 350 °C, with pressures ranging from 22 to 35 MPa to ensure a single-phase reaction. Oxidation of aqueous organics was complete within 5 min [3].
Santa Fe Pacific Gold Corporation (SFPG) studied CM oxidation by POx for a suite of Twin Creeks ores over an 8-year period beginning in 1987. SFPG found that CM oxidation was as high as 40% when treating a 20-micron feed at 225 °C for 120 min. Gold recovery averaged 86% on the representative composite sample evaluated under these conditions [4].
SFPG also investigated the use of copper sulfate as a catalyst for enhancing CM oxidation and found that additions up to 0.5 kg CuSO4/tonne of ore increased gold recovery by as much as 4%. This was due to more complete CM oxidation [4].
Hundreds of sites worldwide use the Catalyzed Wet Air Oxidation (CWAO) technology for treating a variety of organic waste streams. Reaction temperatures typically range between 230 and 260 °C when catalysts are employed. The CWAO industry prefers using iron and copper salts as homogeneous catalysts although solutions having other soluble, multivalent cations are also effective [5]. The industry considers ferric cation the best for catalytic oxidation of solid organics and cupric cation the best for oxidation of soluble organics [6].
PolyMetal is presently constructing a commercial POx facility in Russia for the treatment of double refractory gold flotation concentrates in a stirred autoclave reactor at 240 °C and a residence time of less than 360 min [7]. Mass reduction by flotation was key in allowing PolyMetal to keep the capital cost of the reactor circuit within reason. PolyMetal expects first gold from the facility during Q3 2023 [8].
2 Ore Preparation
TR site personnel collected a large bulk sample of the ore and shipped it to FLS for sample preparation. FLS dried the ore, crushed it to − 10 mesh, and thoroughly blended the crushed material to ensure homogeneity.
After a series of scoping tests at 75 microns, NGM decided to conduct the rest of the Phase 1 program at 20 microns to enhance oxidation kinetics and because this P80 is compatible with the design of TR’s Sage autoclave facility. One thousand kilograms of the ore was dry ground to P80 of 20 microns in a pilot scale ball mill, blended, and rotary split into 450-g charges for bench-top autoclave testing.
3 Ore Characterization
FLS submitted ore charges for the following analytical analyses to confirm sample homogeneity and to characterize the ore [9]:
-
Gold concentration by fire assay with atomic adsorption finish
-
29 element concentrations by inductively coupled plasma–optical emission spectrometry (ICP-OES) using 4-acid digestion
-
Mercury (Hg) concentration by Milestone Direct Mercury Analyzer
-
Sulfur and carbon concentrations by LECO
-
Preg-Rob value by cyanide gold spike
-
Mineral concentration by quantitative X-ray diffraction (XRD)
-
Gold deportment by diagnostic leach
Table 1 gives the concentration of select elements in the ore. Deportment of arsenic was primarily within orpiment. However, arsenic was also present in solid solution in the gold-bearing pyrite and was enriched near the surface of these grains, along with gold.
Table 2 provides the concentration of CM, CO32−, and sulfide sulfur concentrations of the ore by the LECO HCL digest method. The S2−/CO32− ratio of 0.75 is below the preferred operational range of 1.0–1.5 normally treated by TR’s Sage POx facility. Typically, NGM would blend this ore with other ores higher in sulfide so that the blend would fall within the range and produce free acidity greater than 20 g per liter (gpl) of solution.
Table 3 offers an estimate of the preg-rob value of the ore using a gold spike solution concentration that is standard for NGM’s mines. The gold spike would add another 3.43 g Au/tonne to the solids assay if fully retained by the CM.
Table 4 shows the major minerals within the ore as found by XRD. Quartz and muscovite are the primary gangue minerals. Dissolution of muscovite may be a critical issue during high-temperature acidic POx treatment because of the possible impact on gold recovery.
Table 5 presents a view on the ore’s gold deportment from FLS’s proprietary diagnostic leaching procedure. The procedure uses an acetonitrile leach following each of the early extraction steps with a goal of minimizing preg-robbing interference by CM. The method implies that there was a considerable amount of gold-bearing minerals that are intimately associated with CM and difficult to extract without a CM oxidation step. Full oxidation of most of the CM to CO2 would be required to recover most of the gold from this category. Based on gold loss due to silica encapsulation at a grind P80 of 20 microns, NGM viewed 95% as the technical limit for gold recovery after full sulfide and CM oxidation.
Considering that oxidation of CM was the primary focus for the program, NGM and FLS (the team) sent a sample of the ore to the University of Western Ontario – Surface Science Western (SSW) for detailed characterization of the CM in terms of its distribution, morphology, maturity, and preg-robbing capacity [10].
SSW used a scanning electron microscope coupled with an energy dispersive X-ray analyzer (SEM/EDX) for identifying the CM morphologies along with their relative distribution and compositional variation. The SEM/EDX evaluation of the carbon grains in the ore showed two morphological and compositional varieties of CM, disseminated carbonaceous matter (DCM) and massive carbonaceous matter (MCM). Approximately 80% of the CM examined by SSW was DCM particles with the rest being MCM grains.
DCM particles were compositionally variable gangue mineral particles having finely disseminated CM. The CM was in the form of irregular lenses and stringers within various gangue minerals, such as quartz and mica. The average sulfur concentration of the CM matrix was highly variable ranging from 0 to 5 wt. % from particle to particle but having a uniform distribution throughout the individual particles. Figure 1 shows a typical DCM particle along with an EDX elemental signature of a zone of CM enrichment (Spectrum 8). The units for the y-axis and x-axis for the EDX spectrum graphs are cps/eV and keV respectively.
SSW found that MCM grains consist of greater than 80 wt. % C and show no significant incorporation of gangue phases inside the CM matrix but may have very small inclusions or surface-attached gangue particles. The average sulfur concentration of the grain matrix was variable but averaged 5 wt. % and had a uniform distribution within each grain. Figure 2 shows a pure MCM grain along with its EDX elemental signature (Spectrum 28).
SSW examined 8 MCM grains along with the zones of CM enrichment within 60 DCM particles using Dynamic Secondary Ion Mass Spectrometry (D-SIMS) scans of polished surface mounts. The average sub-microscopic gold concentration of all the CM irrespective of morphology was 45 ppm Au and was positively correlated with Fe, As, and S concentrations in the CM. This correlation suggests that the gold carrier was micro-fine arsenian pyrite encapsulated within the CM. Consequently, there was a need to oxidize the CM to liberate the encapsulated gold and achieve the highest gold recovery.
SSW analyzed the MCM grains and DCM particles using Raman spectroscopy (RS) and calculated the degree of disorder of each by Raman ratio (RR). The RR, the ratio of the D2/G, and D1 parameters determined from RS spectra data allowed benchmarking of the ore’s CM against known ratios for crystalline graphite and activated carbon.
In general, crystalline graphite is highly ordered, while activated carbon is highly disordered. The preg-robbing capacity (PRC) of an ore is most often positively correlated with an increasing degree of disorder within the CM [11].
Figure 3 shows the structural variability of the ore’s CM ranging between crystalline graphite and activated carbon, with a sizable proportion of the RRs plotting close to activated carbon. This distribution in RRs supports an overall view that the CM in the ore was strongly preg-robbing.
Figure 4 shows the PRC for a range of gold doping solution strengths which established the maximum loading capacity of the ore at 13.9 g Au/tonne of ore.
4 High-Temp POx Program—Phase 1
4.1 Developmental Test Work
Based on the SFPG’s early work, the team recognized that rapid and complete oxidation of the CM in the ore would require a reactor temperature well above 225 °C. FLS upgraded their existing PARR bench-top reactor to support testing up to 300 °C. This equipment constraint sets the range of subcritical water conditions evaluated during the Phase 1 program.
Figure 5 shows that a more finely ground material yielded a 5% higher gold recovery by POx at 300 °C. POx treatment at 300 °C also yields appreciably higher gold recovery as compared to roasting the ore. NGM selected a grind P80 of 20 microns for the Phase 1 program based on these results.
Early testing of the ore followed the standard NGM protocol of acidulating the ore to achieve S2−/CO32− ratio greater than 1. However, NGM and FLS found that muscovite dissolution at 300 °C was significant when the free acid concentration was more than about 10 g/liter (gpl) in the POx discharge solution. Aluminum from the dissolution of muscovite deported primarily to the POx solids as an alunite precipitate as measured by XRD, and to the POx solutions as measured by ICP-OES. Gold recovery was also highly variable and appeared to be correlated to the extent of the pre-acidulation prior to POx.
The team sent POX/CIL residues from Tests 2, 12, and 26, to SSW for detailed examination to help explain the impact of acidulation of the POx feed on gold recovery. Although the sulfide and CM oxidations were > 99.5% and > 80% respectively for the three residues, the gold recovery was vastly different. Table 6 provides key metrics for these residues. The amount of sulfuric acid used to pre-acidulate the POx feed was 133, 68, and 0 kg per ore tonne when generating POx residues for Tests 2, 12, and 26 respectively. The amount of alunite generated in the POx residue increased and gold recovery decreased when more acid was used during pre-acidulation.
SSW found that the morphology of the gold-bearing hematite changed with thin layers forming on gangue minerals during the POx of the two acidulated feeds, while coarse-grained hematite formed during POx of the non-acidulated feed. The average gold concentration measured by D-SIMS for the layered hematite after CIL treatment was as much as 15 times higher than the concentration measured for the coarse-grained hematite. SEM–EDX also found 5 times more aluminum incorporated into the matrix of the layered hematite as compared to the coarse-grained hematite [12].
Although CM oxidation was high for all three of the POx/CIL residues, the average surface gold concentration of the residual CM as measured by D-SIMS was as much as 18 times higher for the acidulated feeds as compared to the non-acidulated feed. SSW found more gold colloids within the residual CM from the acidulated feeds suggesting that gold metal may have precipitated on the CM due to the gold-halide effect [12]. The chloride concentration was below 10 ppm in the POx discharge solutions for all the tests performed during the Phase 1 program but the threshold concentration triggering the gold-halide effect is unexplored at 300 °C.
Based on these findings, pre-acidulation of the ore was stopped and the ore’s natural S2−/CO32− established the POx discharge-free acid concentration just below 10 gpl. Table 7 gives the standard conditions used for all of the focused test work at 300 °C.
4.2 Focused Test Work
Under the standard conditions, the team found that a POx temperature as high as 300 °C might not meet NGM’s residence time and CM oxidation targets for the ore. Figure 6 illustrates the gold recovery versus CM oxidation at 300 °C for non-acidulated feeds. The CM oxidation was greater with increasing residence time which ranged between 20 and 60 min for this series of tests. Sulfide oxidation was above 99% in all cases reinforcing that CM oxidation was the primary challenge for the ore’s pre-treatment by POx.
SSW further analyzed the residual CM in the POx/CIL residue from Test 26 (the data point having 88% CM oxidation and 96% gold recovery in Fig. 6) by SEM/EDX to understand why it did not oxidize. The residual CM was found to be encapsulated or only partially liberated within the largest silicate gangue grains. Figure 7 is a polished section mount of a large quartz grain having CM partially liberated after mount preparation (red arrow). The bright phases are residual sulfides [12].
Although 12% of the CM survived the aggressive POx conditions due to partial encapsulation, the activated carbon used during the post-POx CIL treatment was able to compete effectively for gold during cyanidation yielding an average surface gold concentration for the residual CM of only 1 ppm. This low average value may suggest modification of the surfaces during POx to make them less preg-robbing. SSW examined the residual CM using RS to determine the RR. Figure 8 shows a subtle modification of the maturity of the residual CM having at least some surface exposure to the oxidizing solutions when compared against the larger CM population for the untreated ore [12].
4.3 Catalyzed Oxidation of CM
The developmental test work at 300 °C was not achieving the program goal with the residence time being too long. The team considered the purchase of a new reactor compatible with temperatures as high as 350 °C. However, the team opted to begin investigating copper sulfate addition for potential enhancement of the single-stage POx performance, as well as the dual-stage POx concept for maximizing the kinetics of a high-temperature CM oxidation stage following a 225 °C first stage.
4.4 CM Oxidation by Dual-Catalyst POx
The team conducted a series of dual-catalyst (DC) POx tests to evaluate the synergy of having both ferric and cupric ions available to catalyze oxidation of the CM [9]. Although a range of copper concentration was evaluated, the team opted to focus the test series using a copper concentration of 1 g/l Cu2+ because higher copper concentrations did not appear to enhance either CM oxidation or gold recovery. Also, the copper catalyst would be from copper sulfide concentrate in an industrial setting and a balance between copper concentration and free acidity in the POx discharge would be required to maximize both CM oxidation and gold recovery at 300 °C.
The DC POx test series used a copper sulfate addition to the feed slurry to establish a cupric concentration of 1 g/l Cu2+ in solution along with the ferric cation solubilized from the ore during POx treatment. When compared against using the ore’s ferric cation concentration alone, Fig. 9 shows that the DC treatment reduced the residence time at 300 °C by half for equivalent amounts of CM oxidation. CM oxidation was 60% after 20 min of treatment at 300 °C.
4.5 CM Oxidation by Dual-Stage POx
The team conducted a series of dual-stage (DS) POx tests to evaluate the advantage of having higher levels of ferric ion is present at time zero of the CM oxidation stage [9]. The DS POx concept first treats the ore in an existing 225 °C autoclave for 60 min to oxidize greater than 95% of the sulfides, followed by a CM oxidation stage at 300 °C.
When compared against single-stage treatment, Fig. 10 shows that DS treatment reduced the residence time needed at 300 °C by half for equivalent amounts of CM oxidation. The DS 20-min test, corresponding to 60 min of POx at 225 °C, with an extra 20 min of POx at 300 °C achieved 60% CM oxidation.
4.6 CM Oxidation by DC/DS POx
Based on these encouraging results, the team investigated a coupling of DC and DS concepts to see if a joint synergy might further reduce the residence time for the CM oxidation step [9]. The DC/DS POx achieved more than 90% CM oxidation after 60 min of sulfide oxidation at 225 °C and 10 min of CM oxidation at 300 °C with 1 g/l Cu2+ added as a catalyst. Gold recovery was also close to the technical limit.
5 Conceptual Flowsheets
5.1 DS Flowsheet
Conceptually, NGM would grind the ore to a P80 of 20 microns, and then POx the ore at 225 °C for 45–60 min within TR’s existing Sage autoclave facility, achieving nearly complete sulfide oxidation and maximizing the concentration of ferric ion in solution. The subsequent CM oxidation stage would occur in plug flow reactors having a maximum residence time of 20 min and operating as high as 350 °C. The control of the discharge slurry-free acidity during the CM oxidation stage may be vital to minimize gangue mineral dissolution and to maximize gold recovery.
The plug flow reactor circuits placed after the Sage autoclaves would accommodate the elevated temperature and pressure needed for the CM oxidation stage. Oxygen may be dissolved in supercritical water prior to its injection to enhance kinetics and eliminate the need for troublesome inline mixers. The supercritical water would supply extra heat for the overall energy balance.
5.2 DC/DS Flowsheet
In addition to concepts discussed for the DS flowsheet, the DC/DS flowsheet may require a separate copper autoclave for TR’s Juniper mill. This facility would treat Phoenix copper concentrates, supplying the copper sulfate required for the treatment of the ore at the TR Sage POx facility. Copper recovery from the slurry after POx would require integration of counter-current decantation, solvent extraction, and electrowinning circuits before gold recovery by Sage’s existing CIL circuit. The CM oxidation stage may require a temperature as high as 300 °C when catalyzed.
6 Conclusions
NGM’s Phase 1 program proved that acidic POx treatment of the ore at temperatures as low as 300 °C could oxidize more than 80% of the CM within 20 min. Gold recovery can approach the ore’s technical limit of 95% when CM oxidation was more than 80%. NGM may need to deploy a DC/DS flowsheet to achieve this level of CM oxidation when using a plug flow reactor operating at 300 °C. The ore held considerable amounts of gold within the CM and required fine grinding to liberate the CM from gangue phases prior to oxidation to achieve the highest gold recovery.
NGM must conduct more testing to understand the primary mechanism for gold loss when the ore was acidulated ahead of POx. If the gold loss can be mitigated, pre-acidulation can create a more favorable environment for ferric iron, enhancing the oxidation kinetics of CM, potentially making the copper catalyzation unnecessary.
The Phase 1 program found that soluble catalysts are essential for appreciable CM oxidation at 300 °C and within an acceptable POx residence time. POx treatment of alkaline double refractory ores will most likely require SWCO conditions due to the low concentrations of catalysts when operating at a lower free acidity.
References
Ma C et al (2004) Treatment of refractory gold ore by supercritical water oxidation. Chin J Process Eng 4(5):420–423
Atomics G (2021) Use of industrial supercritical water oxidation for the processing of NGM ore. Project Report 20547:1–18
Sawicki JE et al (1993) “Wet oxidation of aqueous streams”, United States Patent 5,250,193, October, pp 3-4
Simmons GL (1996) “Pressure oxidation process development for treating carbonaceous ores at Twin Creeks”, Randol Gold Forum ’96, pp. 199-208
Levec J, Pintar A (2007) Catalytic wet-air oxidation processes: a review. Catal Today 124:172–184
Zeng X et al (2018) Highly efficient degradation of pharmaceutical sludge by catalytic wet oxidation using CuO-CeO2/AL2O3 as a catalyst. PLoS One 13(10):e0199520
POLYMETAL International PLC (2019) “POX workshop”, February, pp. 18
POLYMETAL International PLC (2020) Analyst and investor day presentation. November, p 23
Dyson D et al (2021) “High-temperature POx R&D testing – phase 1”, FLSmidth Project Report, pp. 1-25
Dimov SS, Hart BR (2021) TR Stockpile O head analysis report”, SSW Analysis Report: 19621SD.NGM, pp 1–65
Hart BR et al (2011) Procedure for characterization of carbonaceous matter in an ore sample with estimation towards its preg-robbing capacity. World Gold 2011:35–50
Dimov SS, Hart BR (2021) “TR POx residues analysis report”, SSW Analysis Report: 19521SD.NGM, pp 1–89
Acknowledgements
The authors wish to recognize Gary L Simmons and Larry A. Enloe for their peer review of this manuscript.
Funding
Nevada Gold Mines funded the Phase 1 program exclusively with the goal of developing technology that may become part of its strategic Life-of-Mine plan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Nevada Gold Mines (NGM) is a joint venture having two parent companies, Barrick Gold Corporation (61.5% ownership) and Newmont Corporation (38.5% ownership). NGM may benefit from this research should it be deployed commercially. NGM has filed two provisional patent applications as the assignee based on discoveries related to this research. Authors Devy Dyson, Steve Yopps, and John Langhans are the inventors.
Authors Steve Yopps and John Langhans are employed by NGM and own stock and have been assigned restricted shares in Barrick Gold Corporation.
Author Devy Dyson is employed by FLSmidth (FLS). Devy Dyson certifies that he has no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
NGM hired FLS as a consultant for the research. FLS is not an assignee for the provisional patent applications. However, FLS may enjoy enhanced reputation enhancement due to their participation in the research which may lead to more consulting work.
Authors Stamen Dimov and Brian Hart are employed by the University of Ontario – Surface Science Western (SSW). These authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
NGM hired SSW as a consultant for the research. SSW is not an assignee for the provisional patent applications. However, SSW may enjoy enhanced reputation due to their participation in the research which may lead to more consulting work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dyson, D., Yopps, S., Langhans, J. et al. Near-Technical Limit Gold Recovery from a Double Refractory Carlin-Type Ore After Pre-treatment by High-Temperature Pressure Oxidation. Mining, Metallurgy & Exploration 39, 1563–1570 (2022). https://doi.org/10.1007/s42461-022-00638-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42461-022-00638-5