Abstract
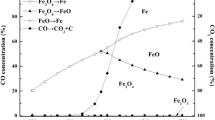
Clean energies are promising alternatives to fossil fuels in the steel industry to reduce carbon dioxide (CO2) emissions and consequently suppress global warming. The first step for achieving the goal is to partially replace carbon monoxide (CO) by hydrogen (H2) in the iron oxide reduction reactions. Therefore, the competitive interaction between the CO and H2 in gas–solid reactions should be first unraveled. The gaseous reduction experiments in this work employ CO, H2, and their mixture to reduce the wustite (FeO) when the interfacial chemical reaction is the rate-controlling step. We establish an electric circuit analogy model to clarify the gaseous components’ interaction based on experimental kinetics analysis, and a linear relation is highlighted and further validated to model gaseous reduction reaction rates under present experimental conditions. The results are expected to provide quantitative insight into the competitive interaction between gaseous components in the reduction process controlled by the interfacial reaction.





Similar content being viewed by others
References
X.W. Zhang, L. Pan, L. Wang, and J.J. Zou: Chem. Eng. Sci., 2018, vol. 180, pp. 95–125.
C. Liao, J.T. Erbaugh, A.C. Kelly, and A. Agrawal: Renew. Sustain. Energy Rev., 2021, vol. 145, 111063.
J. Wadsworth: Metall. Mater. Trans. B, 2010, vol. 41B, pp. 259–74.
Y. Liu, Z. Hu, and Y. Shen: Metall. Mater. Trans. B, 2021, vol. 52B, pp. 2971–91.
C.L. Chang, Q.C. Lin, Z.W. Liao, J.D. Wang, and Y.R. Yang: Chem. Eng. Sci., 2022, vol. 247, 117021.
T. Egeland-Eriksen, A. Hajizadeh, and S. Sartori: Int. J. Hydrog. Energy, 2021, vol. 46, pp. 31963–83.
K.H. Ma, J.Y. Deng, G. Wang, Q. Zhou, and J. Xu: Int. J. Hydrog. Energy, 2021, vol. 46, pp. 26646–64.
A. Ranzani da Costa, D. Wagner, and F. Patisson: J. Clean. Prod., 2013, vol. 46, pp. 27–35.
D. Spreitzer and J. Schenk: Steel Res. Int., 2019, vol. 90, p. 1900108.
Y.D. Wang, X.N. Hua, C.C. Zhao, T.T. Fu, W. Li, and W. Wang: Int. J. Hydrog. Energy, 2017, vol. 42, pp. 5667–75.
B.L. Hou, H.Y. Zhang, H.Z. Li, and Q.S. Zhu: Chin. J. Chem. Eng., 2015, vol. 23, pp. 974–80.
H.S. Chen, Z. Zheng, and W.Y. Shi: Procedia Eng., 2015, vol. 102, pp. 1726–35.
H.S. Chen, Z. Zheng, Z.W. Chen, and X.T. Bi: Powder Technol., 2017, vol. 316, pp. 410–20.
H.Y. Lin, Y.W. Chen, and C.P. Li: Thermochim. Acta, 2003, vol. 400, pp. 61–67.
T. Zhang, C. Lei, and Q.S. Zhu: Powder Technol., 2014, vol. 254, pp. 1–11.
G.Y. Lee, J.I. Song, and J.S. Lee: Powder Technol., 2016, vol. 302, pp. 215–21.
J. Zielinski, I. Zglinicka, L. Znak, and Z. Kaszkur: Appl. Catal. A, 2010, vol. 381, pp. 191–96.
D. Spreitzer and J. Schenk: Metall. Mater. Trans. B, 2019, vol. 50B, pp. 2471–84.
A.A. El-Geassy and V. Rajakumar: Trans. Iron Steel Inst. Jpn, 1985, vol. 25, pp. 449–58.
D. Xie and G.R. Belton: Metall. Mater. Trans. B, 2003, vol. 34B, pp. 225–34.
O. El-Sepelgy, N. Alandini, and M. Rueping: Angew. Chem. Int. Ed., 2016, vol. 55, pp. 13602–05.
B. Weiss, J. Sturn, S. Voglsam, F. Winter, and J. Schenk: Chem. Eng. Sci., 2011, vol. 66, pp. 703–08.
K. Piotrowski, K. Monda, T. Wiltowski, P. Dydo, and G. Rizeg: Chem. Eng. J., 2007, vol. 131, pp. 73–82.
M.H. Jeong, D.H. Lee, G.Y. Han, C.H. Shin, M.K. Shin, C.K. Ko, and J.W. Bae: Fuel, 2017, vol. 202, pp. 547–55.
D.D. Wang, J. Xu, K.H. Ma, Y. Xu, J. Dang, M.Y. Kou, X.W. Lv, and L.Y. Wen: Int. J. Hydrog. Energy., 2017, vol. 42, pp. 14047–57.
J. Xu and J. Deng: ACS Omega, 2020, vol. 5, pp. 4148–57.
Acknowledgments
The authors are grateful for the financial support from the Natural Science Foundation of Chongqing, China (cstc2021ycjh-bgzxm0165), the Venture and Innovation Support Program for Chongqing Overseas Returnees (cx2019138) and the National Natural Science Foundation of China (91634106).
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Deng, J., Bai, L., Ma, P. et al. An Electric Circuit Analogy Model for Analyzing the Relation Between CO and H2 in Interfacial Reduction Reactions. Metall Mater Trans B 53, 2867–2872 (2022). https://doi.org/10.1007/s11663-022-02570-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-022-02570-x