Abstract
Stress-adaptive mechanisms enable tumour cells to overcome metabolic constraints under nutrient and oxygen shortage. Aspartate is an endogenous metabolic limitation under hypoxic conditions, but the nature of the adaptive mechanisms that contribute to aspartate availability and hypoxic tumour growth are poorly understood. Here we identify GOT2-catalysed mitochondrial aspartate synthesis as an essential metabolic dependency for the proliferation of pancreatic tumour cells under hypoxic culture conditions. In contrast, GOT2-catalysed aspartate synthesis is dispensable for pancreatic tumour formation in vivo. The dependence of pancreatic tumour cells on aspartate synthesis is bypassed in part by a hypoxia-induced potentiation of extracellular protein scavenging via macropinocytosis. This effect is mutant KRAS dependent, and is mediated by hypoxia-inducible factor 1 (HIF1A) and its canonical target carbonic anhydrase-9 (CA9). Our findings reveal high plasticity of aspartate metabolism and define an adaptive regulatory role for macropinocytosis by which mutant KRAS tumours can overcome nutrient deprivation under hypoxic conditions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data supporting the findings of this study are available from the corresponding authors on reasonable request. Source data are provided with this paper.
References
Lyssiotis, C. A. & Kimmelman, A. C. Metabolic interactions in the tumor microenvironment. Trends Cell Biol. 27, 863–875 (2017).
DeBerardinis, R. J., Lum, J. J., Hatzivassiliou, G. & Thompson, C. B. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 7, 11–20 (2008).
Prior, I. A., Hood, F. E. & Hartley, J. L. The frequency of ras mutations in cancer. Cancer Res. 80, 2969–2974 (2020).
Ying, H. et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 149, 656–670 (2012).
Son, J. et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 496, 101–105 (2013).
Guo, J. Y. et al. Autophagy suppresses progression of K-ras-induced lung tumors to oncocytomas and maintains lipid homeostasis. Genes Dev. 27, 1447–1461 (2013).
Yang, S. et al. Pancreatic cancers require autophagy for tumor growth. Genes Dev. 25, 717–729 (2011).
Commisso, C. et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature 497, 633–637 (2013).
Olive, K. P. et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324, 1457–1461 (2009).
Goldfine, H. The evolution of oxygen as a biosynthetic reagent. J. Gen. Physiol. 49, 253–274 (1965).
Garcia-Bermudez, J. et al. Aspartate is a limiting metabolite for cancer cell proliferation under hypoxia and in tumours. Nat. Cell Biol. 20, 775–781 (2018).
Sullivan, L. B. et al. Aspartate is an endogenous metabolic limitation for tumour growth. Nat. Cell Biol. 20, 782–788 (2018).
Koong, A. C. et al. Pancreatic tumors show high levels of hypoxia. Int. J. Radiat. Oncol. Biol. Phys. 48, 919–922 (2000).
Provenzano, R. et al. Oral hypoxia-inducible factor prolyl hydroxylase inhibitor roxadustat (FG-4592) for the treatment of anemia in patients with CKD. Clin. J. Am. Soc. Nephrol. 11, 982–991 (2016).
Chandel, N., Budinger, G. R., Kemp, R. A. & Schumacker, P. T. Inhibition of cytochrome-c oxidase activity during prolonged hypoxia. Am. J. Physiol. 268, L918–L925 (1995).
Birsoy, K. et al. An essential role of the mitochondrial electron transport chain in cell proliferation is to enable aspartate synthesis. Cell 162, 540–551 (2015).
Cardaci, S. et al. Pyruvate carboxylation enables growth of SDH-deficient cells by supporting aspartate biosynthesis. Nat. Cell Biol. 17, 1317–1326 (2015).
Sullivan, L. B. et al. Supporting aspartate biosynthesis is an essential function of respiration in proliferating cells. Cell 162, 552–563 (2015).
Titov, D. V. et al. Complementation of mitochondrial electron transport chain by manipulation of the NAD+/NADH ratio. Science 352, 231–235 (2016).
Bardeesy, N. et al. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev. 20, 3130–3146 (2006).
Sousa, C. M. et al. Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature 536, 479–483 (2016).
Hwang, R. F. et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 68, 918–926 (2008).
Pérez-Escuredo, J. et al. Monocarboxylate transporters in the brain and in cancer. Biochim. Biophys. Acta 1863, 2481–2497 (2016).
Cantor, J. R. et al. Physiologic medium rewires cellular metabolism and reveals uric acid as an endogenous inhibitor of UMP synthase. Cell 169, 258–272.e17 (2017).
Barretina, J. et al. The cancer cell line encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 483, 603–607 (2012).
Davidson, S. M. et al. Direct evidence for cancer-cell-autonomous extracellular protein catabolism in pancreatic tumors. Nat. Med. 23, 235–241 (2017).
Kamphorst, J. J. et al. Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer Res. 75, 544–553 (2015).
Palm, W. et al. The utilization of extracellular proteins as nutrients is suppressed by mTORC1. Cell 162, 259–270 (2015).
King, M. P. & Attardi, G. Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science 246, 500–503 (1989).
Bowman, E. J., Siebers, A. & Altendorf, K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc. Natl. Acad. Sci. USA 85, 7972–7976 (1988).
Weber, R. A. et al. Maintaining iron homeostasis is the key role of lysosomal acidity for cell proliferation. Mol. Cell 77, 645–655.e7 (2020).
Aguilera, K. Y. & Brekken, R. A. Hypoxia studies with pimonidazole in vivo. Bio Protoc. 4, e1254 (2014).
Wang, G. L., Jiang, B. H., Rue, E. A. & Semenza, G. L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 92, 5510–5514 (1995).
Majmundar, A. J., Wong, W. J. & Simon, M. C. Hypoxia-inducible factors and the response to hypoxic stress. Mol. Cell 40, 294–309 (2010).
Yan, Q., Bartz, S., Mao, M., Li, L. & Kaelin, W. G. The hypoxia-inducible factor 2alpha N-terminal and C-terminal transactivation domains cooperate to promote renal tumorigenesis in vivo. Mol. Cell. Biol. 27, 2092–2102 (2007).
Cummins, E. P. et al. Prolyl hydroxylase-1 negatively regulates IkappaB kinase-beta, giving insight into hypoxia-induced NFkappaB activity. Proc. Natl. Acad. Sci. USA 103, 18154–18159 (2006).
Wykoff, C. C. et al. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 60, 7075–7083 (2000).
Pastorek, J. et al. Cloning and characterization of MN, a human tumor-associated protein with a domain homologous to carbonic anhydrase and a putative helix-loop-helix DNA binding segment. Oncogene 9, 2877–2888 (1994).
Ramirez, C., Hauser, A. D., Vucic, E. A. & Bar-Sagi, D. Plasma membrane V-ATPase controls oncogenic RAS-induced macropinocytosis. Nature 576, 477–481 (2019).
Chen, Y. et al. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 289, 625–628 (2000).
Lee, S.-W. et al. EGFR-Pak signaling selectively regulates glutamine deprivation-induced macropinocytosis. Dev. Cell https://doi.org/10.1016/j.devcel.2019.05.043 (2019).
Ducker, G. S. et al. Reversal of cytosolic one-carbon flux compensates for loss of the mitochondrial folate pathway. Cell Metab. 23, 1140–1153 (2016).
Kamphorst, J. J. et al. Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Proc. Natl. Acad. Sci. USA 110, 8882–8887 (2013).
Jayashankar, V. & Edinger, A. L. Macropinocytosis confers resistance to therapies targeting cancer anabolism. Nat. Commun. 11, 1121 (2020).
Brugarolas, J. et al. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 18, 2893–2904 (2004).
McDonald, P. C. et al. Regulation of pH by carbonic anhydrase 9 mediates survival of pancreatic cancer cells with activated KRAS in response to hypoxia. Gastroenterology 157, 823–837 (2019).
Supuran, C. T. & Winum, J.-Y. Carbonic anhydrase IX inhibitors in cancer therapy: an update. Future Med Chem. 7, 1407–1414 (2015).
Buescher, J. M. et al. A roadmap for interpreting (13)C metabolite labeling patterns from cells. Curr. Opin. Biotechnol. 34, 189–201 (2015).
Zhu, X. G. et al. Functional genomics in vivo reveal metabolic dependencies of pancreatic cancer cells. Cell Metab. 33, 211–221.e6 (2021).
Soula, M. et al. Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers. Nat. Chem. Biol. https://doi.org/10.1038/s41589-020-0613-y (2020).
Garcia-Bermudez, J. et al. Squalene accumulation in cholesterol auxotrophic lymphomas prevents oxidative cell death. Nature 567, 118–122 (2019).
Acknowledgements
We thank all members of the Bar-Sagi and Birsoy laboratories for helpful suggestions. The KP mouse cancer cell lines are gifts from N. Bardeesy and T. Papagiannakopoulos. We thank the New York University Langone Medical Center Experimental Pathology Research Laboratory, which is partially supported by NIH/National Cancer Institute (NCI) 5370 P30CA16087. This research is supported by an NIH K99/R00 to J.G.-B. (grant no. 1K99CA248838-01/4R00CA248838-02). K.B. was supported by NCI (grant no. DP2 OD024174-01), AACR NextGen grant, Pershing Square Sohn foundation and the Breast Cancer Research Foundation, and is a Searle, Pew-Stewart and Sidney Kimmel Scholar. M.A.B was supported by an NIH T32 (grant no. T32 CA009161). This work was supported by NIH/NCI grant no. CA210263 (D.B.-S).
Author information
Authors and Affiliations
Contributions
D.B.-S., M.A.B., S.P., K.B. and J.G.-B. conceived the project and designed the experiments. J.G.-B. and S.P. performed most of the experiments with assistance of M.A.B., L.B., Y.L. and M.S. Macropinocytosis experiments and tumour immunohistochemistry were done by M.A.B. and S.P. K.L. performed computational analysis. H.A., B.R. and H.M. performed analysis of metabolite profiling experiments. R.T.W. performed real-time PCR experiments. R.F.H. provided hPSCs. E.S. provided PDAC PDXs and N.Y. performed experiments with them. D.B.-S., M.A.B., K.B. and J.G.-B wrote and edited the manuscript with help from L.J.T.
Corresponding authors
Ethics declarations
Competing interests
K.B. is scientific adviser to Nanocare Pharmaceuticals and a consultant to Barer Institute. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Marcia Haigis, Nabeel Bardeesy and Nicholas Denko for their contribution to the peer review of this work. Primary handling editors: Alfredo Giménez-Cassina and George Caputa in collaboration with the Nature Metabolism team
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Hypoxia and ETC inhibition trigger similar metabolic signatures in PDAC cells.
(a) Individual sgRNA scores for GOT2 under indicated conditions. (b) Heat map showing fold changes (log2) of polar metabolites significantly changed when comparing normoxic to hypoxic culture conditions. (c) Immunoblot analysis of GOT2. ACTB is a loading control. (d) Fold change in cell number (log2) of MIA PaCa-2 GOT2KO cell line expressing indicated constructs grown under indicated conditions for 5 days. (e) Common metabolite changes across three PDAC cell lines upon ETC inhibition. (f) Principal component analysis (PCA) of metabolic changes in indicated PDAC cell lines under indicated conditions. (g) Ranks of metabolites most significantly changed (p value < 0.01, dotted lines) across 3 PDAC cell lines under indicated conditions. Metabolites significantly accumulated (blue) or depleted (pink) under both conditions are in the lower left quadrant (right). (h) Top scoring genes and their differential gene scores in HY15549 cells treated with piericidin (Pier.) compared to untreated cells. (i) Individual sgRNA scores for GOT1 in HY15549 cells in indicated conditions. (j) Immunoblot analysis of GOT1 in indicated cell lines expressing indicated sgRNAs. ACTB is a loading control. (k) Fold change in cell number (log2) of indicated HY15549 cell lines expressing indicated constructs for 5 days in the presence or absence of Pier., 20 nM. (l) Fold change in cell number (log2) of indicated cell lines expressing indicated cDNAs grown for 5 days in the presence or absence of Pier., 20 nM. (m) Relative fold change in cell number of indicated parental PDAC cell lines transduced with indicated cDNAs grown for 5 days under indicated conditions; Pier., 20-100. (n) Fold change in cell number (log2) of indicated HY15549 cell lines transduced with indicated vectors grown for 5 days under indicated conditions; Pier. 50 nM. a, i, bars represent individual sgRNA scores; d, k, l, m, n, Bars represent mean ± s.d. d, k, l, m, n, n = 3 biologically independent samples. Statistical significance was determined by two-tailed unpaired t-test.
Extended Data Fig. 2 Mitochondrial NAD+ is limiting for PDAC cell proliferation under hypoxia.
(a) Immunoblot analysis of FLAG-tagged cytoplasmic (cyto-) and mitochondrial (mito-) LbNOX heterologous expression in the indicated cell lines. ACTB was used as a loading control. (b) Fraction of labelled aspartate (left) and citrate (right) derived from labelled glutamine in control and cyto- or mito-LbNOX expressing HY15549 cells cultured for 8 h with [U-13C]-Glutamine (500 μM) with piericidin treatment (50 nM) or under 0.5% oxygen. Colours indicate mass isotopomers. (c) Expression of LbNOX enzymes rescues proliferation of MIA PaCa-2 cells under piericidin treatment (50 nM), whereas only mito-LbNOX expression rescues proliferation under 0.5% O2. Data is shown as fold change in cell number in the indicated cell lines grown for 5 days relative to untreated cells cultured at 21% O2. (d) Fraction of 13C-labeled aspartate (m + 4) and malate (m + 4) in HY15549 parental and GOT2-knockout cells transduced with a control vector or an sgRNA-resistant GOT2 cDNA cultured for 24 hours under hypoxia in the presence of [U-13C]-L-glutamine (1 mM). (e) Relative fold change in cell number of HY15549 GOT2-knockout cells transduced with a control vector or the indicated cDNAs grown for 5 days under hypoxia (0.5% O2) to those cultured under normoxia (21% O2). b-e, Bars represent mean ± s.d. b-e, n = 3 biologically independent samples. Statistical significance was determined by two-tailed unpaired t-test.
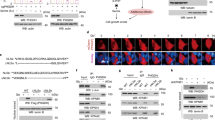
Extended Data Fig. 3 Aspartate synthesis by GOTs is essential in culture but redundant for in vivo tumor growth.
(a) Immunoblot analysis of indicated proteins and cell lines with vinculin loading control. (b) Weights of subcutaneous tumor xenografts from MIA PaCa-2 cells transduced with indicated vectors. (c) In vivo sgRNA competition assay scheme (left). Gene scores for indicated conditions (right). Each dot represents one sgRNA (n = 5 tumours). (d) Gene scores for in vivo sgRNA competition assay performed in KRAS-mutant PDAC patient-derived xenografts (PDX). Each dot represents an sgRNA (n = 5 subcutaneous tumors). (e, f) Fold change cell number of GOT2-KO HY15549 cells transduced with indicated vectors grown 5 days under indicated conditions. Pyruvate (Pyr). (g) Fold change in cell number of HY15549 cells grown 5 days in regular or conditioned media from hPSCs (hPSC CM) with or without monocarboxylate transporter inhibitor AR-C155858 (5 μM). (h) Immunoblot analysis of indicated cells under indicated conditions with vinculin as loading control. (i). Representative bright-field micrographs of indicated cells grown 6 days in the indicated media. Scale bar = 50 μm (left). Quantification graph (right); aspartate (20 mM). (j) Weights, images, and immunoblot analysis of indicated tumors with vinculin loading control (right). (k) Relative aspartate abundance normalized to lysine levels of established xenografts from indicated cells transduced with indicated vectors. (l) Immunoblot of SLC1A3 in indicated cell lines with b-actin loading control. (m) SLC1A2 and SLC1A3 mRNA expression data from the Cancer Cell Line Encyclopedia (CCLE) (log transformed) in KP PDAC cell lines and a glioma cell line (KNS-42). (n) Relative abundance of indicated metabolites in the culture media of indicated MIA PaCa-2 cells grown under indicated conditions for 24 hours in the presence of pyruvate (100 μM). c, d, e, f, g, i, k, n Bars represent mean ± s.d. b, j, boxes represent the median, first, and third quartiles; whiskers represent the minima and maxima. c, d, e, f, g, i, k, n, n = 3; b, n = 10; j, n = 5; k, n = 4. All replicates are biologically independent. Statistical significance determined by two-tailed unpaired t-test.
Extended Data Fig. 4 Macropinocytosis enables proliferation of KRAS-mutant cells under conditions where aspartate is limited.
(a) Fold change in cell number (log2) of HeLa (left) or BxPC-3 tet-KRASV12 (right) cells cultured under indicated concentrations of oxygen for 5-6 days in the presence or absence of 1% BSA. BxPC-3 tet-KRASV12 cells were cultured in the presence or absence of doxycycline (0.1μg/mL) to activate KRASV12 expression. (b) Immunoblot analysis of several members of the ETC complex in parental MIA PaCa-2 cells or Rho(0) counterparts. ACTB was used as a loading control. (c) Representative bright-field micrographs of HY15549 cells cultured for 5 days in media with or without 2% BSA. Where indicated, cells were treated with bafilomycin A1 (BafA1, 10 nM), ferric ammonium citrate (FAC, 0.1 μg/mL) and complex III inhibitor antimycin A (Anti. A, 100 nM). Scale bar = 50 μm. (d) Immunoblot analysis of GOT1 and GOT2 in GOT1/2- double knockout BxPC-3 tet-KRASV12 cells compared to parental controls. ACTB was used as a loading control. (e) Fold change in cell number (log2) of GOT1/2- double knockout BxPC-3 tet-KRASV12 cells cultured in the indicated media conditions for 7 days in the presence or absence of doxycycline (0.1 μg/mL) (top). Representative bright-field micrographs of GOT1/2- double knockout BxPC-3 tet-KRASV12 cells in media supplemented with 1% BSA in the presence and absence of doxycycline (0.1 μg/mL) (bottom). a, e, Bars represent mean ± s.d. a, e, n = 3 biologically independent samples. Statistical significance was determined by two-tailed unpaired t-test.
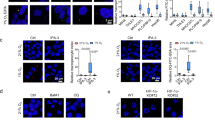
Extended Data Fig. 5 Hypoxia-induced macropinocytosis depends on oncogenic KRAS in PDACs.
(a) Immunoblot analysis of FLAG and T7, showing inducible expression of stably transduced FLAG-tagged KRASG12V BxPC-3 cells and T7-tagged KRASG12V HeLa cells after addition of doxycycline (1 μg/mL for 2 days). ACTB or vinculin were used as a loading control. (b-e) Representative images (b, d) and quantification (c, e) of DQ-BSA fluorescence (green) in PANC-1 (b, c) and MIA PaCa-2 (d, e) cells under normoxia (21% O2) and hypoxia (0.5% O2). Nuclei are labeled with DAPI (blue). Scale bar, 10 μm. In c, e, data are presented relative to normoxia. c, e, Bars represent mean ± s.e.m. At least 500 (c, d) cells were quantified in each biological replicate (n = 3). Statistical significance was determined by two-tailed unpaired t-test.
Extended Data Fig. 6 HIF1A stabilization stimulates macropinocytosis in PDAC cells and tumors.
(a) Immunoblot analysis indicated proteins in the indicated sgRNA-transduced PANC-1 cells treated with FG-4592 as shown. Vinculin is a loading control. (b, c) Representative images (b) and quantification (c) of TMR-dextran (red) uptake in control or sgHIF1A- or sgARNT-transduced PANC-1 cells cultured under indicated conditions. Nuclei are blue. Scale bar, 10 μm. In c, data are values normalized to control normoxic cells. (d) Relative mRNA levels of the HIF1A-target lactate dehydrogenase A (LDHA) in the indicated MIA PaCa-2 cell lines expressing the indicated vectors and treated with the prolyl-hydroxylase (PHD)-inhibitor FG-4592 (100 μM, 72 hrs) as shown. (e, f) Representative images (e) and quantification (f) of TMR-dextran (red) uptake in PANC-1 cells expressing indicated constructs cultured under indicated conditions. Nuclei are blue. Scale bar, 10 μm. In f, data are normalized to values for control normoxic cells. (g) Immunoblot analysis of HIF1A in MIA PaCa-2 and BxPC-3 cells treated with 0, 100, and 500 μM of the prolyl-hydroxylase inhibitor DMOG under normoxia (21% O2). Tubulin was used as a loading control. (h) Representative images of TMR-dextran (red) uptake in MIA PaCa-2 cells in the absence or presence of DMOG (500 μM) under normoxia (21% O2). Nuclei are labeled with DAPI (blue). Scale bar, 10 μm. (i) Quantification of macropinocytic uptake in MIA PaCa-2, PANC-1 and BxPC-3 cells treated in the absence or presence of DMOG (500 μM) under normoxia (21% O2). Data are presented relative to values obtained for untreated cells. (j) Representative images of TMR-dextran (red) uptake from sections of xenograft tumors arising from MIA PaCa-2 cells transduced with sgRNAs targeting HIF1A or ARNT with tumor cells immunostained with anti-CK8 (green), and pimonidazole detected with anti-pimonidazole (purple). Nuclei are labeled with DAPI (blue). Scale bar = 50 μm, inset = 10 μm c, d, f, i, Bars represent mean ± s.e.m. d, n = 3 independent, biological replicates. At least 500 (c, f, i) cells were quantified in each biological replicate (n = 3). Statistical significance for all experiments was determined by two-tailed unpaired t-test.
Extended Data Fig. 7 Bicarbonate generation by CA9 mediates the effect of HIF1A on PDAC macropinocytosis under hypoxia.
(a) Representative images of CA9 (red) from sections of indicated tumor xenografts with +CK8 tumor cells (green), and hypoxic areas (purple). Nuclei are blue. Scale bar = 10 uM. (b) Immunofluorescence of CA9 relative to CK8 in sections of MIA PaCa-2 xenograft tumors. Data are normalized to values from CK8 + /pimo- areas. (c) Immunoblot analysis of CA9 in MIA PaCa-2 cells grown under hypoxia (0.5% O2) for the indicated times (hours). (d) Immunoblot analysis of indicated proteins in the indicated cell lines expressing indicated constructs. (e) Immunoblot analysis of CA9 in the indicated cell lines transduced with the indicated sgRNAs. (f, g) Representative images (f) and quantification (g) of TMR-dextran (red) uptake in PANC-1 cells transduced with indicated constructs and cultured under the indicated conditions. Nuclei are blue. Scale bar, 10 μm. In g, data are normalized to values from normoxic control cells. (h) Immunoblot analysis of CA9 in indicated cells transduced with indicated vectors. (i) Representative images of TMR-dextran (red) uptake in indicated PANC-1 cells cultured under indicated conditions. Nuclei are blue. Scale bar = 10uM. (j) Quantification of TMR-dextran uptake in indicated PANC-1 cells under indicated conditions. Data are normalized to values from control cells. (k) Quantification of TMR-dextran uptake in the indicated cell lines transfected with indicated constructs and cultured under indicated conditions. Data are normalized to values from control normoxic cells. (l) Quantification of TMR-dextran uptake in MIA PaCa-2 cells treated with soluble adenylate cyclase inhibitor KH7 under indicated conditions. Data normalized to values obtained from control normoxic cells. (m) Quantification of TMR-dextran uptake in MIA PaCa-2 cells treated with the PKA inhibitor H89 (15 μM) as shown under indicated conditions. Data are normalized to values obtained for control normoxic cells. b, g, j, k, l, m, Bars represent mean ± s.e.m. At least 300 (b) and 500 (g, j, k, l, m) cells were quantified in each biological replicate (n = 3). Vinculin is a loading control in all immunoblots shown. Statistical significance was determined by two-tailed unpaired t-test.
Supplementary information
Supplementary Table 1
Oligos and DNA sequences.
Supplementary Table 2
Reagents/antibody information.
Source data
Source Data Fig. 1
Statistical source data Fig. 1.
Source Data Fig. 2
Statistical source data Fig. 2.
Source Data Fig. 2
Unprocessed uncropped western blots Fig. 2.
Source Data Fig. 3
Statistical source data Fig. 3.
Source Data Fig. 4
Statistical source data Fig. 4.
Source Data Fig. 5
Statistical source data Fig. 5.
Source Data Fig. 5
Unprocessed uncropped western blots Fig. 5.
Source Data Fig. 6
Statistical source data Fig. 6.
Source Data Fig. 7
Statistical source data Fig. 7.
Source Data Fig. 7
Unprocessed uncropped western blots Fig. 7.
Source Data Extended Data Fig. 1
Statistical source data Extended Data Fig. 1.
Source Data Extended Data Fig. 1
Unprocessed uncropped western blots Extended Data Fig. 1.
Source Data Extended Data Fig. 2
Statistical source data Extended Data Fig. 2.
Source Data Extended Data Fig. 2
Unprocessed uncropped western blots Extended Data Fig. 2.
Source Data Extended Data Fig. 3
Statistical source data Extended Data Fig. 3.
Source Data Extended Data Fig. 3
Unprocessed uncropped western blots Extended Data Fig. 3.
Source Data Extended Data Fig. 4
Statistical source data Extended Data Fig. 4.
Source Data Extended Data Fig. 4
Unprocessed uncropped western blots Extended Data Fig. 4.
Source Data Extended Data Fig. 5
Statistical source data Extended Data Fig. 5.
Source Data Extended Data Fig. 5
Unprocessed uncropped western blots Extended Data Fig. 5.
Source Data Extended Data Fig. 6
Statistical source data Extended Data Fig. 6.
Source Data Extended Data Fig. 6
Unprocessed uncropped western blots Extended Data Fig. 6.
Source Data Extended Data Fig. 7
Statistical source data Extended Data Fig. 7.
Source Data Extended Data Fig. 7
Unprocessed uncropped western blots Extended Data Fig. 7.
Rights and permissions
About this article
Cite this article
Garcia-Bermudez, J., Badgley, M.A., Prasad, S. et al. Adaptive stimulation of macropinocytosis overcomes aspartate limitation in cancer cells under hypoxia. Nat Metab 4, 724–738 (2022). https://doi.org/10.1038/s42255-022-00583-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-022-00583-z
This article is cited by
-
Immunosurveillance encounters cancer metabolism
EMBO Reports (2024)