Abstract
The process of gasification of biomasses of plant origin containing the additives of sulfur oil shale was studied. It was shown that the presence of sulfur oil shale in the mixtures decreased and, in some cases (at a high sulfur content of oil shale), prevented the appearance of deposits (slagging) on the equipment walls. It was established that a maximum effect was achieved with the addition of 5% oil shale containing at least 16% sulfur.
Similar content being viewed by others
The total world volume of biomass exceeds 1.8 billion tons, and its annual growth is estimated at 200 billion tons. In essence, this is an inexhaustible and, most importantly, renewable resource of raw materials and energy. It is obvious that, as the reserves of traditional sources of raw materials and energy—oil, gas, and coal—decrease, interest in the qualified use of renewable plant biomass will increase because the entire range of hydrocarbon compounds obtained from oil, gas, or coal can be produced from renewable plant biomass. It is very important that plant biomass is an environmentally friendly source of energy.
Gasification is a common process of biomass conversion.
The target product of biomass conversion processes using gasification is synthesis gas, which is a gas mixture containing large amounts of carbon monoxide and hydrogen. Synthesis gas is used for the production of methanol and hydrogen and in the Fischer–Tropsch process to obtain synthetic oil or feedstock for organic chemistry and petrochemistry.
One of the problems of the biomass gasification process is the following: As compared to coal, plant biomass contains much more alkali metals, in particular, potassium and sodium. The ash content of biomass gasification products is much lower than that of coal gasification products, and the chemical and mineral compositions of ashes are significantly different: coal ash mainly consists of SiO2, A12O3, and Fe2O3, and biomass ash contains SiO2, CaO, Na2O, and K2O. Biomass gasification ash has a lower softening point (typically, 750 to 1000°C), as compared to 1000°C or higher for coal ash. Water vapor formed as a result of raising the temperature in the gasifier converts alkalis into highly volatile hydroxides. Sodium and potassium hydroxides partially penetrate into the pores of the gasifier lining to cause its destruction. This phenomenon is known as alkaline destruction (alkaline corrosion). A portion of alkalis in a gaseous state formed on the gasification of biomass also enters the colder part of the unit after a gasifier, where gas condensation occurs and the deposition of solid alkali salts (slagging) on the internal surfaces of the equipment, in particular, heat exchangers and filters, takes place.
The well-known methods used for preventing slagging and alkaline corrosion in the gasification of biomass—the contact with a gas-absorbing ceramic material [1], the use of a gasification catalyst (accelerator), such as clay, which has catalytic functions and/or a heat carrier function [2], the use of gas-absorbing ceramics for binding alkali [3], and the preliminary gasification of biomass in a remote furnace [4]—are complex and inefficient. We suggested that the introduction of an acidic agent into the gasified mixture can neutralize and bind alkalis and thereby prevent undesirable effects of slagging and alkaline corrosion because the biomass components that cause slagging are mainly alkalis. Obviously, such an agent should meet the following requirements: it should not complicate the gasification process and not worsen its results, that is, the characteristics of the resulting synthesis gas, and not lead to an increase in ash formation. Taking into account previously published data on the use of oil shale in the processing of hydrocarbon raw materials [5–7] and the fact that, as a rule, oil shale contains sulfur, which can become an active acidic neutralizing agent during gasification in the presence of water vapor, we decided on oil shale as a neutralizing additive.
The developed variant of gasification of raw materials in the form of aqueous suspensions requires preliminary preparation of raw materials; however, its advantage is that this technology makes it possible to reduce the process temperature and decrease soot formation and the concentration of impurities in the resulting synthesis gas [8–10].
The results of studies on the selection of conditions and regimes that provide the effect of binding alkalis and preventing slag formation in the gasification of aqueous biomass suspensions of are presented below.
EXPERIMENTAL
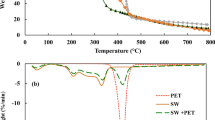
Two types of large-tonnage waste of the agro-industrial complex were used as vegetable raw materials: sunflower seed husks and corn cobs. These are highly reactive materials with the yields of volatile substances to 80% upon their processing [11]. Table 1 summarizes the characteristics of representative biomass samples, and Table 2 shows the chemical composition of the ash (mineral matter).
The following three samples of oil shale were selected as additives (neutralizing components), the physicochemical characteristics of which are presented in Table 3: oil shale with a high sulfur content (shale of the Savel’evskoe deposit in the Orenburg oblast), oil shale with a minimum sulfur content (shale of the Leningrad deposit), and oil shale with an intermediate sulfur content (shale of the Kashpir deposit in the Samara oblast). Raw biomass (corn cobs and sunflower seed husks) was crushed in a jaw mill. The obtained samples were stored in hermetically sealed containers.
The biomass was dispersed in a specially designed grinder apparatus (Fig. 1). The pre-crushed biomass samples were weighed and loaded into bunker 1. The material from the bunker was poured onto upper disk 4, where it was crushed to a particle size of no more than 8 mm, and poured through the holes in the disk onto the lower disk of the upper pair. The biomass was intensely ground in the gap between grinding blades 6 of the rotating and fixed discs. Through a hole in the fixed disk, the biomass particles fell onto lower pair 5, where additional grinding took place. The crushed biomass was poured into receiving hopper 7.
To analyze the dispersity of the biomass, a Rotap device (an AC-200U impact sieve analyzer) was used. Table 4 summarizes the granulometric composition of the biomass (a fraction of 0.2 mm) after grinding.
Oil shales were preliminarily crushed in a jaw crusher to a particle size of 10–100 µm.
When preparing the mixtures of biomass and oil shale, we used the results of studies reported previously [12]. The mixtures in which the water content (taking into account the moisture of biomass) was 27–30% were prepared; they contained at least 80% of water particles with a size of 10 μm, and the effective size of dispersed biomass particles was 5–20 μm.
The dispersion of the raw material components was carried out in an emulsifier apparatus, the design of which combines two principles of grinding, impact and abrasion at the same time, which makes it possible to shorten processing time and reduce energy costs. To obtain the desired dispersity of biomass particles, two or three cycles of processing in the emulsifier were carried out. After reaching this value, weighed portions of oil shale were added to the biomass suspension (1–6% based on the weight of the suspension), and this mixture was loaded into the emulsifier to ensure its homogenization and the penetration of oil shale particles into the biomass.
The gasification of the mixtures of biomass and oil shale was carried out on a laboratory fluidized-bed unit (Fig. 2) a under the following conditions: temperature, 1000°C; oxygen content of the blast, 21 vol %; excess air factor, 0.3–0.5; and suspension capacity, 5–10 L/h.
The gas generated upon gasification was cooled in a cooler and purified with the separation of soot and ash. The resulting ash was collected and accumulated in a bunker. The composition of the resulting gas was analyzed by gas adsorption chromatography on a Kristallyuks chromatograph; the detector was a katharometer, and helium was a carrier gas. Two chromatographic columns were used. A column packed with molecular sieves CaA (3 m × 3 mm) was used to separate CO and N2 in an isothermal regime at a temperature of 90°C. To separate CO2 and CH4, a column packed with HayeSepR (3 m × 3 mm) was used in the regime of temperature programming (50–220°C) at a heating rate of 10 K/min.
The effect of oil shale addition to the biomass was evaluated by the concentrations of sodium and potassium compounds in the resulting gasification ash. It is obvious that it is very difficult to evaluate this effect based on a decrease in the slag formation on the equipment surfaces in a laboratory unit: an examination of the internal surfaces of the gasifier, cooler, and pipelines after two months of operation in a periodic mode showed only a slight coating on the walls. It is all the more difficult to evaluate the effect of reducing alkaline corrosion for a laboratory unit with a small capacity in terms of raw materials with a gasifier and a small-capacity cooler.
Table 5 shows the results of experiments, which allowed us to draw the following conclusions:
the ash of biomass gasified without the addition of oil shale contained about 20% of potassium and sodium compounds on an initial basis in the biomass; consequently, at least 80% of the alkalis contained in the biomass were deposited on the walls of the internal surfaces of the equipment;
the presence of oil shale in the biomass significantly affected the concentrations of sodium and potassium in the gasification ash;
the higher the sulfur content of the added oil shale, the higher the concentration of sodium and potassium in the resulting ash: at a 16% sulfur content of oil shale, to 90% of sodium and potassium contained in the biomass entered the ash and, therefore, was not deposited on the walls of the equipment;
the addition of high sulfur oil shale to the biomass not only decreased slagging but also, probably, reduced the alkaline corrosion of equipment.
We proposed the following interpretation of the experimental results:
The presence of organic sulfur in oil shale in the course of gasification process makes it possible to convert the potassium and sodium compounds in the biomass into salts that are nonvolatile at the gasification temperature, in particular, sulfite and sulfate salts. This process starts at temperatures below the gasification temperature of mixed suspensions of biomass, oil shale, and water. Thus, the conversion of oil shale (for example, from the Kashpir deposit in the Volga region) during thermal gasification begins at a feedstock temperature of 450°C and ends with almost complete conversion of oil shale into gas at 750°C [13]. At this temperature, the volume of gas produced by oil shale conversion reaches its maximum. In the process of thermolysis, the almost complete decomposition of kerogen occurs, as a result of which this gas contains mainly hydrogen, carbon monoxide, carbon dioxide, methane, and hydrogen sulfide, which is formed from the organic sulfur of oil shale.
When the temperature reached 750–800°С, alkalis—the compounds of sodium and potassium contained in biomass—sublimed with the formation of volatile hydroxides. Volatile alkali hydroxides reacted with hydrogen sulfide to result in the formation of solid sodium and potassium sulfates and sulfites, which are nonvolatile at the gasification temperature. They were removed from the reaction zone with the resulting gas before the start of the main gasification process (to 800–1000°С) and, as a consequence, potassium and sodium compounds were not deposited on the walls of the equipment. The sulfates and sulfites of sodium and potassium were mixed with ash formed upon the gasification of biomass, which mainly consisted of SiO2 (70.0 wt % or higher), CaO (to 6.0 wt %) and MgO (to 8.0 wt %). In this case, the sulfur-containing additive (oil shale), which contributes to the binding of alkalis and the prevention of alkaline corrosion, should not be disposed because it was almost completely converted into a gas, which slightly increased the volume of gasification gases and the amount of ash formed.
The results of the gasification of biomasses of various origins (corn cobs and sunflower seed husks) with and without oil shale additives presented in Table 6 indicate that the addition of oil shale has a little effect on the composition of the resulting gas.
Thus, the above studies illustrate the fundamental possibility and the specific conditions of the method that makes it possible to significantly prevent the deposition of alkali metal compounds on the surfaces of equipment (slagging) in the gasification of plant biomass (and, probably, prevent the alkaline corrosion of equipment) by adding sulfur oil shale to the biomass
REFERENCES
Abrakham, R., Pavone, D., and Riger, M., RF Patent No. 2490314, 2016.
US Patent 9187704, 2001.
Khainrits-Adrian, M. Pavone, D., and Abrakham, R., RF Patent No. 2639911, 2017.
http://novostynauki.com/e-ntsiklopediya/bioenergetika/sovmestnoe-szhiganie-biomassy-s-predvaritelnoj-ee-gazifikatsiej-v-vynosnoj-topke/
Rebrov, A.I. and Gorlov, E.G., Khim. Tverd. Topl. (Moscow), 2011, no. 5, p. 349.
Krylova, A.Yu., Gorlov, E.G., Shumovskii, A.V., Yas’yan, Yu.P., and Niskovskaya, M.Yu., Chem. Technol. Fuels Oils, 2018, vol. 3, p. 3.
Gorlov, E.G., Shumovskii, A.V., Yas’yan, Yu.P., Anikushin, B.M., Svarovskaya, N.A., and Niskovskaya, M.Yu., Chem. Technol. Fuels Oils, 2018, vol. 5, p. 3.
Gorlova, E.E., Nefedov, B.K., and Gorlov, E.G., Sovremennaya nauka. Sbornik nauchnykh statei (Modern Science: Collection of Scientific Articles), 2012, p. 15.
Kotov, A.S. and Gorlov, E.G., Khim. Tverd. Topl. (Moscow), 2009, no. 3, p. 30.
Gorlova, E.E., Nefedov, B.K., Gorlov, E.G., and Ol’gin, A.A., Khim. Tverd. Topl. (Moscow), 2008, no. 2, p. 36.
Os’mak, A.A. and Seregin, A.A., Vost.-Evrop. Zh. Peredov. Tekhnol., 2014, no. 68, p. 57.
Shumovskii, A.V. and Gorlov, E.G., Solid Fuel Chem., 2021, vol. 4, p. 59.
Avakyan, T.A., Strizhakova, Yu.A., and Lapidus, A.L., Gazokhimiya, 2011, nos. 3–4 (19–20), p. 74.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by V. Makhlyarchuk
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Shumovskii, A.V., Gorlov, E.G. Prevention of Slagging in the Gasification of Plant Biomass. Solid Fuel Chem. 56, 166–170 (2022). https://doi.org/10.3103/S0361521922030090
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0361521922030090