Abstract
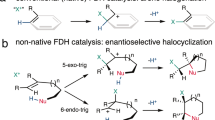
Although flavin-dependent halogenases (FDHs) are attractive for C–H bond activation, their applications are limited due to low turnover and stability. We have previously shown that leakage of a halogenating intermediate, hypohalous acid (HOX), causes FDHs to be inefficient by lessening halogenation yield. Here we employed a mechanism-guided semi-rational approach to engineer the intermediate transfer tunnel connecting two active sites of tryptophan 6-halogenase (Thal). This Thal-V82I variant generates less HOX leakage and possesses multiple catalytic improvements such as faster halogenation, broader substrate utilization, and greater thermostability and pH tolerance compared with the wildtype Thal. Stopped-flow and rapid quench kinetics analyses indicated that rate constants of halogenation and flavin oxidation are faster for Thal-V82I. Molecular dynamics simulations revealed that the V82I substitution introduces hydrophobic interactions which regulate tunnel dynamics to accommodate HOX and cause rearrangement of water networks, allowing better use of various substrates than the wildtype. Our approach demonstrates that an in-depth understanding of reaction mechanisms is valuable for improving efficiency of FDHs.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The initial structures and snapshots of molecular dynamics simulations are given as Supplementary Data and available at https://github.com/N-Lawan/Flavin-dependent-halogenase.git. The data supporting the findings of this study are available within the article and its Supplementary Information or can be obtained from the corresponding author on reasonable request.
References
Weichold, V., Milbredt, D. & van Pée, K.-H. Specific enzymatic halogenation—from the discovery of halogenated enzymes to their applications in vitro and in vivo. Angew. Chem. Int. Ed. 55, 6374–6389 (2016).
Gkotsi, D. S. et al. A marine viral halogenase that iodinates diverse substrates. Nat. Chem. 11, 1091–1097 (2019).
Durak, L. J., Payne, J. T. & Lewis, J. C. Late-stage diversification of biologically active molecules via chemoenzymatic C–H functionalization. ACS Catal. 6, 1451–1454 (2016).
Latham, J. et al. Integrated catalysis opens new arylation pathways via regiodivergent enzymatic C–H activation. Nat. Commun. 7, 11873 (2016).
Craven, E. J. et al. Programmable late-stage C−H bond functionalization enabled by integration of enzymes with chemocatalysis. Nat. Catal. 4, 385–394 (2021).
Phintha, A., Prakinee, K. & Chaiyen, P. in The Enzymes Vol. 47 (eds Chaiyen, P. & Tamanoi, F.) 327–364 (Academic, 2020).
Latham, J., Brandenburger, E., Shepherd, S. A., Menon, B. R. K. & Micklefield, J. Development of halogenase enzymes for use in synthesis. Chem. Rev. 118, 232–269 (2018).
Andorfer, M. C. & Lewis, J. C. Understanding and improving the activity of flavin-dependent halogenases via random and targeted mutagenesis. Annu. Rev. Biochem. 87, 159–185 (2018).
Payne, J. T., Poor, C. B. & Lewis, J. C. Directed evolution of RebH for site-selective halogenation of large biologically active molecules. Angew. Chem. Int. Ed. 54, 4226–4230 (2015).
Shepherd, S. A. et al. Extending the biocatalytic scope of regiocomplementary flavin-dependent halogenase enzymes. Chem. Sci. 6, 3454–3460 (2015).
Poor, C. B., Andorfer, M. C. & Lewis, J. C. Improving the stability and catalyst lifetime of the halogenase RebH by directed evolution. ChemBioChem 15, 1286–1289 (2014).
Minges, H. et al. Targeted enzyme engineering unveiled unexpected patterns of halogenase stabilization. ChemCatChem 12, 818–831 (2020).
Kokkonen, P., Bednar, D., Pinto, G., Prokop, Z. & Damborsky, J. Engineering enzyme access tunnels. Biotechnol. Adv. 37, 107386 (2019).
Büchler, J., Papadopoulou, A. & Buller, R. Recent advances in flavin-dependent halogenase biocatalysis: sourcing, engineering, and application. Catalysts 9, 1030 (2019).
Phintha, A. et al. Dissecting the low catalytic capability of flavin-dependent halogenases. J. Biol. Chem. 296, 100068 (2021).
Mondal, D., Fisher, B. F., Jiang, Y. & Lewis, J. C. Flavin-dependent halogenases catalyze enantioselective olefin halocyclization. Nat. Commun. 12, 3268 (2021).
Banerjee, R. & Lipscomb, J. D. Small-molecule tunnels in metalloenzymes viewed as extensions of the active site. Acc. Chem. Res. 54, 2185–2195 (2021).
Dong, C. et al. Tryptophan 7-halogenase (PrnA) structure suggests a mechanism for regioselective chlorination. Science 309, 2216 (2005).
Flecks, S. et al. New insights into the mechanism of enzymatic chlorination of tryptophan. Angew. Chem. Int. Ed. 47, 9533–9536 (2008).
Stourac, J. et al. Caver Web 1.0: identification of tunnels and channels in proteins and analysis of ligand transport. Nucleic Acids Res. 47, W414–W422 (2019).
Yeh, E., Blasiak, L. C., Koglin, A., Drennan, C. L. & Walsh, C. T. Chlorination by a long-lived intermediate in the mechanism of flavin-dependent halogenases. Biochemistry 46, 1284–1292 (2007).
Ainsley, J. et al. Structural insights from molecular dynamics simulations of tryptophan 7-halogenase and tryptophan 5-halogenase. ACS Omega 3, 4847–4859 (2018).
Kaushik, S. et al. Impact of the access tunnel engineering on catalysis is strictly ligand-specific. FEBS J. 285, 1456–1476 (2018).
Ridder, L., Mulholland, A. J., Rietjens, I. M. C. M. & Vervoort, J. A quantum mechanical/molecular mechanical study of the hydroxylation of phenol and halogenated derivatives by phenol hydroxylase. J. Am. Chem. Soc. 122, 8728–8738 (2000).
Karabencheva-Christova, T. G., Torras, J., Mulholland, A. J., Lodola, A. & Christov, C. Z. Mechanistic insights into the reaction of chlorination of tryptophan catalyzed by tryptophan 7-halogenase. Sci. Rep. 7, 17395 (2017).
Moritzer, A.-C. et al. Structure-based switch of regioselectivity in the flavin-dependent tryptophan 6-halogenase Thal. J. Biol. Chem. 294, 2529–2542 (2019).
Luhavaya, H., Sigrist, R., Chekan, J. R., McKinnie, S. M. K. & Moore, B. S. Biosynthesis of l-4-chlorokynurenine, an antidepressant prodrug and a non-proteinogenic amino acid found in lipopeptide antibiotics. Angew. Chem. Int. Ed. 58, 8394–8399 (2019).
Menon, B. R. K. et al. Structure and biocatalytic scope of thermophilic flavin-dependent halogenase and flavin reductase enzymes. Org. Biomol. Chem. 14, 9354–9361 (2016).
Lingkon, K. & Bellizzi Iii, J. J. Structure and activity of the thermophilic tryptophan-6 halogenase BorH. ChemBioChem 21, 1121–1128 (2020).
Zehner, S. et al. A regioselective tryptophan 5-halogenase is involved in pyrroindomycin biosynthesis in Streptomyces rugosporus LL-42D005. Chem. Biol. 12, 445–452 (2005).
Seibold, C. et al. A flavin-dependent tryptophan 6-halogenase and its use in modification of pyrrolnitrin biosynthesis. Biocatal. Biotransform. 24, 401–408 (2006).
Yeh, E. et al. Flavin redox chemistry precedes substrate chlorination during the reaction of the flavin-dependent halogenase RebH. Biochemistry 45, 7904–7912 (2006).
Minges, H. & Sewald, N. Recent advances in synthetic application and engineering of halogenases. ChemCatChem 12, 4450–4470 (2020).
Pimviriyakul, P. & Chaiyen, P. in The Enzymes Vol. 47 (eds Chaiyen, P. & Tamanoi, F.) 1–36 (Academic, 2020).
Sucharitakul, J., Chaiyen, P., Entsch, B. & Ballou, D. P. Kinetic mechanisms of the oxygenase from a two-component enzyme, p-hydroxyphenylacetate 3-hydroxylase from Acinetobacter baumannii*. J. Biol. Chem. 281, 17044–17053 (2006).
Pimviriyakul, P., Thotsaporn, K., Sucharitakul, J. & Chaiyen, P. Kinetic mechanism of the dechlorinating flavin-dependent monooxygenase HadA*. J. Biol. Chem. 292, 4818–4832 (2017).
Prongjit, M., Sucharitakul, J., Wongnate, T., Haltrich, D. & Chaiyen, P. Kinetic mechanism of pyranose 2-oxidase from Trametes multicolor. Biochemistry 48, 4170–4180 (2009).
Granik, V. G., Graevskaya, I. P. & Ryabova, S. Y. Heterocyclization of 2-indolinone derivatives (a review). Pharm. Chem. J. 31, 646–662 (1997).
Andorfer, M. C. et al. Understanding flavin-dependent halogenase reactivity via substrate activity profiling. ACS Catal. 7, 1897–1904 (2017).
Agarwal, V. et al. Biosynthesis of polybrominated aromatic organic compounds by marine bacteria. Nat. Chem. Biol. 10, 640–647 (2014).
Mori, S., Pang, A. H., Thamban Chandrika, N., Garneau-Tsodikova, S. & Tsodikov, O. V. Unusual substrate and halide versatility of phenolic halogenase PltM. Nat. Commun. 10, 1255 (2019).
Menon, B. R. K. et al. RadH: a versatile halogenase for integration into synthetic pathways. Angew. Chem. Int. Ed. 56, 11841–11845 (2017).
Markova, K. et al. Decoding the intricate network of molecular interactions of a hyperstable engineered biocatalyst. Chem. Sci. 11, 11162–11178 (2020).
Taylor, M. G. & Massey, V. Decay of the 4a-hydroxy-FAD intermediate of phenol hydroxylase. J. Biol. Chem. 265, 13687–13694 (1990).
Schnepel, C., Minges, H., Frese, M. & Sewald, N. A high-throughput fluorescence assay to determine the activity of tryptophan halogenases. Angew. Chem. Int. Ed. 55, 14159–14163 (2016).
Bell, E. L. et al. Biocatalysis. Nat. Rev. Methods Prim. 1, 46 (2021).
Yi, D. et al. Recent trends in biocatalysis. Chem. Soc. Rev. 50, 8003–8049 (2021).
Intasian, P. et al. Enzymes, in vivo biocatalysis, and metabolic engineering for enabling a circular economy and sustainability. Chem. Rev. 121, 10367–10451 (2021).
Jurcik, A. et al. CAVER Analyst 2.0: analysis and visualization of channels and tunnels in protein structures and molecular dynamics trajectories. Bioinformatics 34, 3586–3588 (2018).
Chovancova, E. et al. CAVER 3.0: a tool for the analysis of transport pathways in dynamic protein structures. PLoS Comput. Biol. 8, e1002708 (2012).
PyMOL: Schrodinger, LLC. The PyMOL Molecular Graphics System, Version 1.8 (2015).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996).
Santos-Aberturas, J., Dörr, M. & Bornscheuer, U. T. in Protein Engineering: Methods and Protocols (eds Bornscheuer, U. T. & Höhne, M.) 157–170 (Springer, 2018).
Sullivan, B., Walton, A. Z. & Stewart, J. D. Library construction and evaluation for site saturation mutagenesis. Enzym. Microb. Technol. 53, 70–77 (2013).
Maenpuen, S. et al. Creating flavin reductase variants with thermostable and solvent-tolerant properties by rational-design engineering. ChemBioChem 21, 1481–1491 (2020).
Dolinsky, T. J., Nielsen, J. E., McCammon, J. A. & Baker, N. A. PDB2PQR: an automated pipeline for the setup of Poisson–Boltzmann electrostatics calculations. Nucleic Acids Res. 32, W665–W667 (2004).
MacKerell, A. D. et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B 102, 3586–3616 (1998).
Phillips, J. C. et al. Scalable molecular dynamics with NAMD. J. Comp. Chem. 26, 1781–1802 (2005).
Pongpamorn, P. et al. Identification of a hotspot residue for improving the thermostability of a flavin-dependent monooxygenase. ChemBioChem 20, 3020–3031 (2019).
Acknowledgements
We thank the Thailand Science Research Innovation and NSRF via the Program Management Unit for Human Resources and Institutional Development, Research and Innovation (grant no. B05F640089) and the Royal Academy of Engineering (for their support to P. Chaiyen), the Vidyasirimedhi Institute of Science and Technology (VISTEC) (for their support to K.P., A.P., S.V., C.K. and P. Chaiyen), the Thailand Science Research Innovation and National Research Council of Thailand (Royal Golden Jubilee PHD/0135/2557 grant to A. P. and P. Chaiyen) and Chiang Mai University for partial support to N. Lawan. We acknowledge the VISTEC-NSTDA fellowship (to C. Kantiwiriyawanitch, P. Chaiyen and P. Chitnumsub), and thank the Czech Ministry of Education for financial support to J. Damborsky (grant nos. CZ.02.1.01/0.0/0.0/16_026/0008451 and LM2018121). We thank S. Maenpuen (Burapha University) for providing stopped-flow and rapid-quench flow technical support, and V. Pongsupasa (VISTEC) for providing a thermostable C1-A58P enzyme for the thermostability assays. We thank U. Bornscheuer and M. Dörr (University of Greifswald) for valuable advice related to enzyme engineering procedures. We thank S. Ketrat, S. Nutanong and School of Information Science and Technology, VISTEC for computing facilities. The figures were created using materials from PyMOL and BioRender.com.
Author information
Authors and Affiliations
Contributions
K.P., A.P. and P. Chaiyen conceived and designed the study. A.P., S.V. and K.P. performed the tunnel analysis and rational design of enzyme engineering. K.P. and A.P. conducted the library creation and screening. K.P., A.P. and C.K. performed protein production and purification, and enzymatic assays. K.P. and A.P. performed the transient kinetics experiments with contributions from J.S. N.L. performed the computational analysis. S.V., K.P. and N.L. analysed the molecular dynamics simulations. S.V., N.L., J.S., J.D., P. Chitnumsub, and K.-H.v.P., analysed data and reviewed the manuscript. K.P., A.P. and P. Chaiyen prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Roland Ludwig, Christian Schnepel and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Table 1, Figs. 1–57 and References.
Supplementary Data 1
Initial structure molecular dynamics of WT.
Supplementary Data 2
Molecular dynamics snapshot of WT_400K at 4 ns.
Supplementary Data 3
Initial structure molecular dynamics of V82I.
Supplementary Data 4
Molecular dynamics snapshot of V82I_400K at 4 ns.
Supplementary Data 5
Initial structure molecular dynamics of WT_HOBr.
Supplementary Data 6
Molecular dynamics snapshot of WT_HOBr at 19.4 ns.
Supplementary Data 7
Initial structure molecular dynamics of V82I_HOBr.
Supplementary Data 8
Molecular dynamics snapshot of V82I_HOBr at 19.4 ns.
Supplementary Data 9
Molecular dynamics snapshot of V82I_HOBr at 102 ns.
Supplementary Data 10
Initial structure molecular dynamics of WT_Phenol.
Supplementary Data 11
Molecular dynamics snapshot of WT_Phenol at 8 ns.
Supplementary Data 12
Initial structure molecular dynamics of V82I_Phenol.
Supplementary Data 13
Molecular dynamics snapshot of V82I_Phenol at 8 ns.
Rights and permissions
About this article
Cite this article
Prakinee, K., Phintha, A., Visitsatthawong, S. et al. Mechanism-guided tunnel engineering to increase the efficiency of a flavin-dependent halogenase. Nat Catal 5, 534–544 (2022). https://doi.org/10.1038/s41929-022-00800-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-022-00800-8
This article is cited by
-
Site-selective chlorination of pyrrolic heterocycles by flavin dependent enzyme PrnC
Communications Chemistry (2024)
-
Discovery and mechanism-guided engineering of BHET hydrolases for improved PET recycling and upcycling
Nature Communications (2023)
-
Regulation of P450 TleB catalytic flow for the synthesis of sulfur-containing indole alkaloids by substrate structure-directed strategy and protein engineering
Science China Chemistry (2023)
-
Pimchai Chaiyen’s biography
Biophysical Reviews (2022)