Abstract
Carbon sequestration in unmineable coal seams has been proposed as one of the most attractive technologies to mitigate carbon dioxide (CO2) emissions in which CO2 is stored in the microporous structure of the coal matrix in an adsorbed state. The CO2 adsorption process is hence considered one of the more effective methodologies in environmental sciences. Thus, adsorption isotherm measurements and modelling are key important scientific measures required in understanding the adsorption system, mechanism, and process optimization in coalbeds. In this paper, three renowned and reliable adsorption isotherm models were employed including Langmuir, Freundlich, and Temkin for pure CO2 adsorption data, and the extended-Langmuir model for multicomponent, such as flue gas mixture-adsorption data as investigated in this research work. Also, significant thermodynamics properties including the standard enthalpy change (\(\Delta H^\circ\)), entropy change (\(\Delta S^\circ\)), and Gibbs free energy (\(\Delta G^\circ\)) were assessed using the van’t Hoff equation. The statistical evaluation of the goodness-of-fit was done using three (3) statistical data analysis methods including correlation coefficient (R2), standard deviation (σ), and standard error (SE). The Langmuir isotherm model accurately represent the pure CO2 adsorption on the coals than Freundlich and Temkin. The extended Langmuir gives best experimental data fit for the flue gas. The thermodynamic evaluations revealed that CO2 adsorption on the South African coals is feasible, spontaneous, and exothermic; and the adsorption mechanism is a combined physical and chemical interaction between the adsorbate and the adsorbent.
Similar content being viewed by others
1 Introduction
Considering the rising quantities of carbon dioxide (CO2) in the atmosphere resulting in global warming, a systematic plan for developing cost-effective and energy-efficient technologies to reduce CO2 emissions is urgently needed (Wang et al. 2013). About 80% of global commercial energy needs come from fossil fuels, including coal, oil, and gas (Gadonneix et al. 2013). CO2 emissions from burning fossil fuels have been reported as a significant contributor to elevated anthropogenic levels of CO2 in the atmosphere (Shojai Kaveh et al. 2012; Wang et al. 2013). In 2019, fossil fuels and industry accounted for over 80% of the overall global CO2 emissions, with coal-fired power plants contributing 30% of the total (IEA 2019).
Anthropogenic CO2 reportedly accounts for over 63% of all contributors to climate change in the atmosphere. The rate of CO2 emissions since 2000 was higher than the rate of CO2 emissions expected by most fossil-fuel-intensive scenarios established by the Intergovernmental Panel on Climate Change in the late 1990s (IPCC 2020). Carbon capture and storage (CCS) can potentially reduce atmospheric CO2 emissions when burning fossil fuels. This alternative involves disposal methods, such as injection of CO2 into deep saline aquifers, unmineable coal seams, and depleted gas and oil reservoirs. CO2 injected into a coal reservoir flows through the cleat network, diffuses through the carbon matrix and is stored on the microporous surfaces of the coal in a sorbed state (Shojai Kaveh et al. 2012). In the context of the geological storage of CO2, a few studies (Mabuza and Premlall 2014; Mazumder et al. 2006; Pini et al. 2009; Shojai Kaveh et al. 2012; Syed et al. 2013; Zhang et al. 2016; Lukhele et al. 2017) consider the direct injection of flue gases from power plants or other flue gas emitting industries.
The sorption capacity of CO2 or flue gas on coal can be measured through adsorption isotherm data, which was collected through a series of repeated laboratory experimental tests to ensure validation of results. The modelling of adsorption isotherm data has become an essential and useful methodology for predicting adsorption performance, which is critical for understanding the pathways of the adsorption mechanism, and the design of adsorption systems (Chen 2015). Proper interpretation and understanding of adsorption isotherms are critical to improving adsorption mechanism pathways and effective design of adsorption system. As such, regression analysis has been one amongst the foremost applied tools for outlining the best fitting adsorption models because it quantifies the distribution of adsorbates, analyses the adsorption system, and verifies the consistency of theoretical assumptions of adsorption isotherm model (Ayawei et al. 2017). The Langmuir, Freundlich, and Temkin, are two-parameter isotherm models that are commonly used in modelling the adsorption data for pure gases at constant temperature, with the Extended-Langmuir used in modelling the adsorption data for multicomponent gases (Dada et al. 2012). The modelled experimental adsorption isotherm generates a non-linear curve that describes the adsorption process; and the mathematical correlation described in the modelling analysis is essential for operational design and practical applications of the adsorption system (Foo and Hameed 2010).
Recently, many researchers have further explored the adsorption and transportation of gases on coals with different degrees of maturity. Wang et al. (2020) found that coal has a high adsorption capacity as well as strong adsorption potential, especially for CO2. In a multicomponent gas containing CO2, CH4, N2, and He, Zhou et al. (2020) found that the adsorption capacity decreased in the following order: CO2 > CH4 > N2, > He, with the CO2 adsorption having the noticeable negative effect on the strength of the coal, while the He has negligible effect on coal strength due to its non-adsorptive nature (Wang et al. 2014). Furthermore, the diffusivity of CO2 on high rank coals is higher than low rank coals, suggesting a positive CO2 diffusivity corelation with increased coal maturity, more especially in vitrinite rich coals (Keshavarz et al. 2017). In addition to this, gas adsorption on coals from the view of thermodynamics aspects provides significant information related to the mechanism of adsorption of gases on coal surfaces (Tang and Ripepi 2017). Thermodynamic properties including the standard enthalpy change (\(\Delta H^\circ\)), Gibbs free energy change (\(\Delta G^\circ\)), the entropy change (\(\Delta S^\circ\)), and isosteric heat of adsorption give adequate physical insight to interpret the sorption process (Yan et al. 2019). Hao et al. (2021) evaluated the thermodynamic properties of CH4/CO2/N2 adsorption on Zaozhuang anthracite coal and showed that the gas adsorption is exothermic physisorption (\(\Delta H^\circ < 40\; {\text{kJ/mol}}\)), thermodynamically spontaneous (\(\Delta G^\circ < 0\)), and the gas molecules are highly ordered (\(\Delta S^\circ < 0\)) on the coal surface. Raganati et al. (2018) reported that adsorption systems which have standard enthalpy change in the range \(20 < \Delta H^\circ < 40 \;{\text{kJ/mol}}\) are attributed to a combined nature of physisorption and chemisorption mechanism.
This research paper presents the comprehensive theoretical modelling of the adsorption data generated by Mabuza et al. (2018), in which pure CO2 and a flue gas representative mixture of a typical SA coal-fired power plant has been used to evaluate the sorption on the microporous coal surface for CO2 storage in unmineable coal seams at isothermal temperatures of 30 ºC, 40 ºC, 50 ºC, and 60 ºC and pressure up to 9.0 MPa. The accuracy in parameters prediction for the isotherm models were compared and are discussed accordingly. The statistical evaluation of the goodness-of-fit was done using the correlation coefficient (R2), standard deviation (σ), and standard error (SE). The results of modelling this adsorption data are expected to expand on the theoretical basis of gas–solid adsorption in carbon capture and storage for environmental protection.
2 Methodology
2.1 Origin of the coal samples and preparation
The coal samples examined were collected from two different South African coalfields located in Mpumalanga (Ermelo—Coal EML) and KwaZulu-Natal (Somkhele—Coal SML). A report by Viljoen et al. (2010) has described these coalfields as potential CO2 storage sites in the future. The samples were sealed in airtight zip-lock bags and placed in the refrigerator (under 3 ºC temperature condition) after being flushed with nitrogen gas. This coal storage practise was conducted to avoid spontaneous oxidation and excessive exposure to the atmosphere, and it is a practise recommended by Ören and Şensöğüt (2018). Coal EML is classified as medium rank C (bituminous C) while Coal SML is high rank C (Anthracite C). Coal EML is inertinite-rich (74.2 vol%, inc. mm), while Coal SML is vitrinite-rich (84.0 vol%, inc. mm). Details of the petrography of the two coals may be acquired from Mabuza et al. (2020). Each sample was obtained as 50 mm lumps that were divided using the coning and quartering method in accordance with the ISO 14780—solid biofuels—sample preparation (2017). It was then crushed with a TENCAN—XPC jaw crusher to an average grain size of 2 mm. These samples were used for pure CO2 and flue gas sorption experiments.
2.2 Experimental sorption data
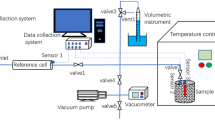
The experimental high-pressure sorption data for pure CO2 and flue gas (at nominal molar feed composition of 12 mol% CO2, 5.5 mol% O2, 82 mol% N2, 0.38 mol% SO2, and 0.12 mol% NO2) sorption on Coal EML and Coal SML at temperatures ranging from 30 ºC to 60 ºC and pressures up to 9.0 MPa was acquire through a series of batch tests using a high-pressure volumetric sorption system (HPVSS). Details of the experimental setup and experimental procedures to generate the sorption data are given by Mabuza et al. (2018) in their work on low–high temperature flue gas direct injection in south African bituminous and anthracite coals: sorption capacity assessment.
The adsorbed-phase density is usually assumed to be constant across the experimental range (Kim et al. 2011). In this study the value of 22.6 mmol/cm3 for CO2 was adopted since it has been found to be a reasonable estimation and was previously successfully applied by numerous other authors (Fitzgerald et al. 2005; He et al. 2010; Kim et al. 2011). The adsorbed-phase density estimates do, however, affect the calculated absolute adsorption isotherm (Fitzgerald et al. 2005).
2.3 Non-linear and linear forms of the isotherm models
Three well-known empirical adsorption isotherm models were employed for sorption verification in this study to model the sorption of pure CO2 on two South African coals (Coal EML and Coal SML). Those isotherm models included (1) Langmuir model, (2) Freundlich model, and (3) Temkin model.
These isotherm models are typically represented as two parameter models. As reported by Ho et al. (2002), the simplest method to determine isotherm constants for two parameter isotherms is to transform the isotherm variables so that the equation is converted to a linear form and then to apply linear regression.
In the Langmuir adsorption model, the adsorbed amount, Ns, is correlated to the monolayer coverage, Nsm, as follows:
where P is the system pressure and KL is Langmuir constant and highly depends on the energy of adsorption. This expression can be reorganized as follows:
For the experimental data, P/Ns versus P can be plotted. The plot will be linear, and the slope is equal to 1/Nsm (Birdi 2017). The Langmuir constants (KL and Nsm) are fundamental factors that determine the field development of coal reservoirs. Langmuir monolayer coverage (Nsm) is a direct indicator of the gas storage capacity and is positively related to coal rank. The affinity of a gas for a solid surface as well as the energy stored in a coal formation are both related to the Langmuir constant KL which is inversely related to coal rank (Yang et al. 2019). Nsm is proportional to the number of sorption sites available and is also dependent on the coal composition and surface complexity (Cai et al. 2013).
The Freundlich isotherm equation is given by the following expression (Al-Wahbi and Dammag 2011; Dada et al. 2012):
Linearization of the above equation via logarithmic plot yields (Dada et al. 2012):
where KF is Freundlich isotherm constant, 1/n is the adsorption intensity, Pe is the equilibrium pressure, and qe is the amount of gas adsorbed at equilibrium pressure. The Freundlich coefficients n and KF are acquired from the plots of (ln qe) versus (In Pe) (Oladoja et al. 2008). The magnitude of exponent (n) provides an indication of the capacity and favourability of the adsorbate/adsorbent system (Al-Wahbi and Dammag 2011). According to Treybal (1980) values of n between 1 and 10 represent favourable adsorption. When the slope (1/n) ranges between 0 and 1 is a measure of adsorption intensity or surface heterogeneity, becoming more heterogeneous as its value gets closer to zero. While a value below unity indicates chemisorption process where 1/n over one is an indication of cooperative adsorption (Haghseresht and Lu 1998).
The Temkin isotherm model equation is generally applicable in the following form (Temkin and Pyzhev 1940):
The linear form of this expression is:
where
where T is the absolute temperature, R is the universal gas constant, AT is the equilibrium binding constant corresponding to the maximum binding energy, bT is Temkin isotherm constant and the constant BT is related to the heat of adsorption (dimensionless) (Al-Wahbi and Dammag 2011).
The Extended-Langmuir (EL) adsorption isotherm model was used to correlate the measured flue gas sorption data. According to Reeves and Koperna (2008), the EL model predictions require single-component isotherm measurements. The EL adsorption isotherm model has a semi-empirical nature and it is given by (Kapoor et al. 1990). By applying the Langmuir isotherm to a N component mixture and assuming no interaction among the adsorbate molecules, the EL model is:
where Nm,i is the maximum amount of adsorption of component i, Bi is the Langmuir constant at a specified temperature for component i, P is the pressure, and yi being the molar fraction of component i in the gas phase (Wei et al. 2007).
2.4 Statistical evaluation of the adsorption isotherm models goodness-of-fit
Several hundred papers are published yearly reporting liquid–solid and gas–solid adsorption data. In general, the data is analysed using a variety of standard models such as the Langmuir, Freundlich, Temkin, and extended Langmuir. The validity of these models is often assessed empirically via their ability to fit the data, independently of their physicochemical soundness (Douven et al. 2015). For Langmuir, Freundlich, and Temkin models, a common error function often used is called the normalized standard deviation of sorption models (Song et al. 2016). The normalized standard deviation describes the variation in experimental measurements in comparison with the calculated values. This function is expressed as:
where \(S\left( \% \right)\) is the normalized standard deviation, Q is the number of moles, the subscripts ‘exp’ and ‘calc’ are the experimental and calculated values, respectively, and N is the number of data points (Wu et al. 2009).
For Extended-Langmuir model, an error function known as the absolute (∆n) and relative deviations error (R) analyses (Eqs. (10) and (11)) are often used to measure the deviations between the flue gas sorption experimental data and the Extended-Langmuir model data. The functions are expressed as follows:
2.5 Adsorption thermodynamic properties
Thermodynamic parameters including the standard enthalpy change (\(\Delta H^\circ\)), Gibbs free energy change (\(\Delta G^\circ\)), and the entropy change (\(\Delta S^\circ\)) are evaluated for adsorption systems to establish the nature of adsorption (i.e., adsorbate-adsorbent interactions), either physisorption or chemisorption (Raganati et al. 2018). Physisorption arises from van der Waals forces due to weak interactions, while chemisorption is characterized by strong chemical interactions due to chemical bonding as a consequence of electrons transfer between the adsorbate and adsorbent (Peköz and Donadio 2016). The theoretical data obtained from the Langmuir isotherm models is combined with thermodynamic laws to determine these thermodynamics parameters by applying the following equations (Kumar Singh and Anil Kumar 2018; Wilson and Handan Tezel 2021):
where R is the universal ideal gas constant, T is the absolute temperature, and KL is the Langmuir constant. Substituting Eq. (12) into Eq. (13) results in the van’t Hoff equation:
This linear equation is used to construct the van’t Hoff plots, where \({\text{ln}}K_{L}\) represents the y-axis and 1/T the x-axis. The parameters \(\Delta H^\circ\) and \(\Delta S^\circ\) are determined from the slope and intercept, respectively, while \(\Delta G^\circ\) may be calculated directly from Eq. (13).
The standard enthalpy change (\(\Delta H^\circ )\) determines whether the adsorption process is exothermic or endothermic by a negative or positive value, respectively. In an exothermic adsorption process, energy is released to the surroundings in form of heat, this is alluded while in an endothermic process energy is absorbed from the surroundings. Furthermore, for a physisorption process, the value of \(\Delta H^\circ\) is less than 20 kJ/mol, and it is greater than 40 kJ/mol for a chemisorption process (Ahmed et al. 2019). With reference to \(\Delta S^\circ\), the value is indicative of the order (organization) of adsorbate at the solid–gas interface during the adsorption process. Such that, when \(\Delta S^\circ < 0\) the adsorbate is less random (highly ordered) on the solid surface, and for \(\Delta S^\circ > 0\) the adsorbate is more random (less ordered). For Gibbs free energy change (\(\Delta G^\circ )\), the adsorption process is said to take place spontaneously and feasible when the value is less than zero (i.e., \(\Delta G^\circ < 0)\). Otherwise for (\(\Delta G^\circ > 0)\), the process is non-spontaneous and non-feasible (Du et al. 2021).
3 Results and discussion
3.1 Pure CO2 sorption isotherms modelling
The Langmuir isotherm model was applied to estimate maximum adsorption capacity corresponding to complete monolayer coverage, Nsm, on the coal surface. Figures 1 and 2 illustrate pure CO2 experimental sorption isotherms for Coal EML and Coal SML with Langmuir isotherm model regression at the various temperatures. It is observed that there is a good correlation between experimental and Langmuir theoretical (calculated) data, this is validated by the Langmuir isotherm model regression fitting well with the CO2 experimental data with a minimal average relative. This means that Langmuir isotherm model can describe the present pure CO2 sorption data of the two coal samples. Moreover, this good correlation between experimental and theoretical data suggests that the maximum sorption capacity corresponding to complete monolayer coverage, Nm, on the coal surfaces can be accurately estimated using the Langmuir equation.
The Freundlich model was applied to estimate the adsorption intensity, 1/n, of the CO2 on the coal surface. Figures 3 and 4 show pure CO2 experimental sorption isotherms for Coal EML and Coal SML with Freundlich isotherm model regression. It is observed that there is a poor correlation between the experimental and Freundlich theoretical data. The Freundlich isotherm regression does not fit well with the pure CO2 experimental data, this is more evident beyond the subcritical pressures (> 6.0 MPa). This behaviour is attributed to the limitations of the isotherm model at high pressure of the gas. According to Kumar et al. (2010), under high pressure gas experimental applications, the experimental isotherm deviates from the Freundlich isotherm model while approaching the saturation or while approaching the limiting pressure conditions. This means that the Freundlich isotherm cannot explain the sorption behaviour at high pressure regions.
The Freundlich equation, experimentally, was previously shown that the amount of adsorption changes directly with pressure until saturation is attained. Beyond that point, even when a higher pressure is applied, the rate of adsorption saturates (Kang et al. 2014). Thus, adsorbents produce isotherms that satisfy the Freundlich equation in the low to intermediate pressure range, but the agreement is frequently poor at high pressures (Adelodun et al. 2017). The Freundlich isotherm model, unlike the Langmuir model, does not approach a limiting adsorptive capacity as the pressure approaches infinity, hence, resulting in these limitations. This indicates that there is no saturation limit for the Freundlich isotherm model (Raganati et al. 2018). Consequently, the Freundlich isotherm model tend to fail at higher pressures. This behaviour suggests that the Freundlich isotherm model cannot be used to describe or represent the current pure CO2 adsorption experimental data; consequently, the adsorption intensity, 1/n, of CO2 on the coal surfaces cannot be accurately estimated using the Freundlich equation.
The Temkin adsorption isotherm model was applied to evaluate the adsorption potential, bT, of the CO2 on the coal surface. Figures 5 and 6 present pure CO2 experimental sorption isotherm of Coal EML and Coal SML, respectively, with Temkin isotherm model regression. As was the case with the Freundlich isotherm model, there is a poor correlation between the experimental and Temkin theoretical data. This phenomenon is mostly observed at pressures close to supercritical (> 7.39 MPa). This behaviour is in complete contrast with literature, according Kim et al. (2004) as well as Foo and Hameed (2010), Temkin equation is excellent for predicting the gas phase equilibrium on solids (when organization in a tightly packed structure with identical orientation is not necessary); however, conversely complex adsorption systems including the liquid-phase adsorption isotherms are usually not appropriate to be represented. This contrast indicates that the Temkin isotherm model cannot be used to describe the pure CO2 sorption experimental data measured under the conditions of temperature and pressure probed; as a result, the adsorption potential, bT, of CO2 on the coals surfaces cannot be accurately estimated using the Temkin equation.
3.2 Statistical evaluation and model parameters for Langmuir, Freundlich, and Temkin
Table 1 presents the Langmuir, Freundlich, and Temkin models constants, parameters, and correlation coefficients for Coal EML and Coal SML for the temperature range under investigation. The table also presents the statistical analysis including the normalized standard deviation, and standard error for the two coals. For the Langmuir model, the coefficient of correlation, R2, is above 0.9 at low temperatures (30 ºC to 40 ºC) and below 0.9 at high temperatures (50 ºC and 60 ºC) this means that the measured sorption data fitted the linear regression better at low temperatures than at high temperatures. The standard deviation, σ, for Coal EML does not exceed ± 0.85 and for Coal SML exceeds ± 0.85 but less than ± 1.107 for all the temperature range. While the standard error, SE, for Coal EML is less than 0.3 and in excess of 0.3 but less than 0.4 for Coal SML. This means that there is less variation or dispersion of the experimental values when compared to the theoretical values.
The correlation factor acquired from the Freundlich model regression is above 0.9 for both coals throughout the entire temperature range. The corresponding adsorption intensity, 1/n, ranges between 0.567 to 0.739 for Coal EML, and between 0.616 to 0.720 for Coal SML. It is observed that the adsorption intensity increases with an increase in temperature for both coals. According to Ghazi et al. (2013) adsorption intensity value greater than 1.0 implies weak bond between the adsorbate and the adsorbent. This means that there is a stronger bond between the CO2 and the coal since adsorption is less than 1.0 throughout the temperature range. However, this bond is the strongest at low temperatures and tend to weaken at higher temperatures. This means that more energy is required for adsorption at higher temperatures. This phenomenon aligns well with the gas–solid adsorption theory that adsorption is an exothermic process. Since the adsorption process exothermic, low temperatures allow the process to take place favourably by increasing the gas–solid bond strength. The standard deviation is less than ± 1.268 for both coals for the entire temperature range.
The correlation factor obtained from the Temkin model regression is also above 0.9 for both coals for the entire temperature range. The Temkin constant related to the heat of adsorption (amount of energy that is released), bT, ranges between 1.395 kJ/mol and 3.076 kJ/mol for Coal EML and between 1.592 kJ/mol and 2.718 kJ/mol for Coal SML. This heat of adsorption increases with an increase in temperature. The positive values (bT = ΔQ = − ΔH) means that the adsorption process is exothermic for the entire temperature range. However, the increase in temperature favours the reverse process; hence, a decrease in the adsorption capacity of CO2 on the coals as reported by Mabuza et al. (2017). The standard deviation is less than ± 1.6 for Coal EML and less that ± 1.2 for Coal SML for the entire temperature range.
Figure 7 compares the experimental and theoretical maximum sorption capacity of CO2 on Coal EML and Coal SML at 30 ºC, 40 ºC, 50 ºC, and 60 ºC. This comparison is important to distinguish the degree of discrepancies (Da) between the experimental and theoretical sorption capacity in order to validate the reliability of the adsorption models used. Viljoen et al. (2010) only reported the maximum potential CO2 that can be stored in various coal seams in South Africa; however, they did not specify how much can be stored in an adsorbed state. Thus, experiments and models were necessary to estimate and predict the maximum amount that can be stored in an adsorbed state.
From the graphs presented in Fig. 7, the percentage discrepancy for Langmuir and Temkin isotherm models values when compared to the experimental values is less than 10% for both coals and across the entire temperature range. However, similar comparison yielded discrepancies in excess of 10% for the Freundlich isotherm model at 40 ºC, 50 ºC, and 60 ºC. According to de Myttenaere et al. (2016) there is no upper limit on a “mean absolute percent error” in statistical and scientific studies. There is only the necessary (human) judgment on whether the data referred to can be useful or not. As such, a Da value more than 10% was considered high in this study, the basis of this deduction is both experiential and was also adopted from literature (Guang et al. 1995; McKay et al. 2014). Thus, Freundlich isotherm model may not be used to describe the experimental data for both coals, especially at elevated temperatures.
3.3 Flue gas sorption modelling
The Extended-Langmuir (EL) adsorption isotherm model was used to correlate the measured flue gas sorption data. According to Reeves and Koperna (2008), the EL model predictions require single-component isotherm measurements. The EL adsorption isotherm model has a semi-empirical nature. Figures 8 and 9 present the flue gas experimental sorption isotherms with EL isotherm model regression for Coal EML and Coal SML, respectively, at 30 ºC, 40 ºC, 50 ºC, and 60 ºC. The EL theoretical data fits well on the experimental data with a calculated average relative deviation, R, of less than 10% for both coal samples. This suggests that the EL isotherm model can fairly describe the flue gas sorption behaviour on the coals.
Figure 10 presents the relationship of the Extended Langmuir isotherm model parameters for the various flue gas species obtained by linear fitting for Coal EML with temperature at 30 ºC, 40 ºC, 50 ºC, and 60 ºC. Similar trends were acquired for and Coal SML for the same relationship. In the 30–60 °C range, the maximum adsorption amount of the two coal samples decreases with an increase in temperature as shown in Fig. 10a. The most credible explanation for this negative effect is that adsorption is an exothermic process. Since the adsorption process is exothermic, low temperatures allow the process to take place favourably by increasing the gas–solid bond strength due to increased adsorbed phase volume (Krooss et al. 2002). However, the variation of the Langmuir adsorption constant with temperature is complicated and non-monotonic as shown in Fig. 10b. Chen et al. (2011) observed similar trends when modelling adsorption data of Chinese Changcui and Qinan coals and concluded that the relationship between the maximum adsorption amount, Nm,i, and temperature, is described by an exponential function.
The relative deviation between the experimental and theoretical maximum flue gas sorption capacity for Coal EML and Coal SML is less than 6% for most temperatures investigated, except for Coal SML at 30 ºC recording a relative deviation close to 10% as shown in Fig. 11. This indicates that there is minimal difference between the experimental and the theoretical values; thus, the Extended-Langmuir adsorption isotherm model may be used to predict the flue gas sorption isotherms at temperatures of 30 ºC, 40 ºC, 50 ºC, and 60 ºC, and at pressures ranging from 1.0 MPa to 9.0 MPa.
3.4 Adsorption thermodynamic properties
Figure 12 shows the van’t Hoff plot generated from CO2 adsorption data for Coal EML and Coal SML at temperatures ranging from 30 °C to 60 °C. The standard enthalpy change (\(\Delta H^\circ\)), the entropy change (\(\Delta S^\circ {/}R\)), and Gibbs free energy change (\(\Delta G^\circ\)) values were calculated from the van’t Hoff plot slope (\(\Delta H^\circ {/}R\)), the intercept (\(\Delta S^\circ {/}R\)), and the Eq. (13), respectively, these parameters are listed in Table 2. The \(\Delta G^\circ\) is negative at all the investigated adsorption temperature range, this indicates that the CO2 adsorption process on Coal EML and Coal SML occurs spontaneous and feasible. The \(\Delta G^\circ\) magnitude decreases with an increase in temperature (i.e., \(\Delta G^\circ\) approaches zero or becomes less negative), this means that the adsorption process is more favourable at low temperatures. This is attributable to the fact that at higher temperatures, the physical bonds between the adsorbent and CO2 molecules weaken, and the opposite of adsorption (i.e., desorption) is favoured. This implies that the equilibrium shifts in the reversal direction (i.e., from adsorption to desorption) (Ammendola et al. 2020). Furthermore, CO2 molecules have higher kinetic energy at higher temperatures which result in reduced physical interaction with the coals surfaces (Yu et al. 2018).
The standard heat change (\(\Delta H^\circ\)) reveals that the adsorption process is exothermic for both the coals since the values are negative. The \(- \Delta H^\circ\) values are larger than 20 kJ/mol but less than 40 kJ/mol; thus, the adsorption systems are neither within a well-defined physisorption region (\(\Delta H^\circ < 20 \,{\text{kJ/mol}})\) nor the chemisorption region (\(\Delta H^\circ > 40 \,{\text{kJ/mol}})\). This implies that the CO2 adsorption processes have a dual nature of physical and chemical adsorption on the two coals investigated. Gabruś et al. (2021) also made similar findings on Polish hard coals in the Upper Silesia Coal Basin. They alluded that the combined adsorption nature is due to the heterogeneous morphology of coal texture, as well as the extreme coal bed seam conditions. Lastly, the negative standard entropy change (\(\Delta S^\circ\)) suggests that the magnitude of randomness of the CO2 molecules decreased (i.e., became more ordered) during the adsorption process. This is alluded to the reduced freedom of movement of the CO2 molecules as they adhere to the surface of the coals. In principle, CO2 molecules transform from random state (in gaseous phase) to an organized state (solid-like phase on the surface of an adsorbent) during the adsorption process.
4 Conclusions
The pure CO2 experimental data was fitted on three conventional adsorption isotherm models (Langmuir, Freundlich, and Temkin) and the flue gas experimental data was fitted on Extended-Langmuir adsorption isotherm model. There is a negative effect of temperature on sorption capacity of pure CO2 and flue gas on the coal samples. This suggests that the sorption process was exothermic; as a result, decreasing the sorbed phase volume with increasing temperature. Generally, the maximum sorption capacity of the coals with respect to the flue gas individual components decreased in the following order: CO2 > N2 > O2 > SO2 > NO2 on molar basis. This indicates that the coals have a preferential sorption for CO2. Langmuir adsorption isotherm model was found to describe the pure CO2 sorption process better than Freundlich and Temkin adsorption isotherm models. This is an indication of the occurrence of the monolayer coverage adsorption mechanism. Extended Langmuir adsorption isotherm model was found to describe the flue gas sorption process on both the coal samples, and the maximum amount adsorbed decreases with temperature. However, the variation of Langmuir adsorption constant with temperature in non-monotonic. The thermodynamic study showed that the CO2 adsorption on the coals is feasible, spontaneous, and exothermic; also, the adsorption mechanism is dual in nature with physical and chemical interactions taking place.
Availability of data and materials
The raw data that support the findings of this study may be made available by the corresponding upon request.
References
Adelodun AA, Ngila JC, Kim D-G, Jo Y-M (2017) Isotherm, thermodynamic and kinetic studies of selective CO2 adsorption on chemically modified carbon surfaces. Aerosol Air Qual Res 16(12):3312–3329. https://doi.org/10.4209/aaqr.2016.01.0014
Ahmed A-M, Ali A, Ghazy AH (2019) Adsorption separation of nickel from wastewater by using olive stones. Adv J Chem Sec A 2(1):79–93. https://doi.org/10.29088/sami/AJCA.2019.2.7993
Al-Wahbi AAM, Dammag HAQ (2011) Removal of methylene blue from aqueous solutions using Yemen Bentonite. Diyala J Eng Sci 4(1):30–53
Ammendola P, Raganati F, Chirone R, Miccio F (2020) Fixed bed adsorption as affected by thermodynamics and kinetics: yellow tuff for CO2 capture. Powder Technol 373(1):446–458. https://doi.org/10.1016/j.powtec.2020.06.075
Ayawei N, Ebelegi AN, Wankasi D (2017) Modelling and interpretation of adsorption isotherms. J Chem 1:1–11. https://doi.org/10.1155/2017/3039817
Birdi KS (2017) Surface chemistry and geochemistry of hydraulic fracturing. Taylor & Francis Group, LLC, Boca Raton
Cai Y, Liu D, Pan Z, Yao Y, Li J, Qiu Y (2013) Pore structure and its impact on CH4 adsorption capacity and flow capability of bituminous and subbituminous coals from Northeast China. Fuel 103(1):258–268. https://doi.org/10.1016/j.fuel.2012.06.055
Chen S, Jin L, Chen X (2011) The effect and prediction of temperature on adsorption capability of coal/CH4. Procedia Eng 26(1):126–131. https://doi.org/10.1016/j.proeng.2011.11.2149
Chen X (2015) Modeling of expermiental adsorption isotherm data. Information 6(1):14–22. https://doi.org/10.3390/info6010014
Dada AO, Olaleka AP, Olatunya AM, Dada O (2012) Langmuir, Freundlich, Temkin and Dubinin-Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. J Appl Chem 3(1):38–45. https://doi.org/10.9790/5736-0313845
De Myttenaere A, Golden B, Le Grand B, Rossi F (2016) Mean absolute percentage error for regression models. Neurocomputing 192(1):38–48. https://doi.org/10.1016/j.neucom.2015.12.114
Douven S, Paez CA, Gommes CJ (2015) The range of validity of sorption kinetic models. J Colloids Interface Sci 448(1):437–450. https://doi.org/10.1016/j.jcis.2015.02.053
Du X, Cheng Y, Liu Z, Yin H, Wu T, Huo L, Shu C (2021) CO2 and CH4 adsorption on different rank coals: a thermodynamics study of surface potential, Gibbs free energy change and entropy loss. Fuel 283:118886. https://doi.org/10.1016/j.fuel.2020.118886
Fitzgerald JE, Pan Z, Sudibandriyo M, Robinson JRL, KaM G, Reeves S (2005) Adsorption of methane, nitrogen, carbon dioxide and their mixtures on wet Tiffany coal. Fuel 84(18):2351–2363. https://doi.org/10.1016/j.fuel.2005.05.002
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156(1):2–10. https://doi.org/10.1016/j.cej.2009.09.013
Gabruś E, Wojtacha-Rychter K, Aleksandrzak T, Smoliński A, Król M (2021) The feasibility of CO2 emission reduction by adsorptive storage on Polish hard coals in the Upper Silesia Coal Basin: an experimental and modeling study of equilibrium, kinetics and thermodynamics. Sci Total Environ 796(1):149064. https://doi.org/10.1016/j.scitotenv.2021.149064
Gadonneix P, Nadeau M-J, Kim Y, David, Birnbaum L, Cho H-E, Choudhury A, Roy, Da Costa Carvalho Neto J, Dauger J-M, Meyers K, Sambo A, Statham BA, Lleras J, Antonio, Vargas , Ward G, Wu X, Zatari TM, Frei C (2013) World Energy Resources: 2013 Survey. London, World Energy Council
Ghazi M, Chan N, Weng, Haliza A, Rahman, Nor A, Zakaria (2013) Freundlich isotherm equilibrium equastions in determining effectiveness a low cost absorbent to heavy metal removal in wastewater (Leachate) at Teluk Kitang Landfill, Pengkalan Chepa, Kelantan, Malaysia. J Geogr Earth Sci 1(1):1–8. https://doi.org/10.15640/jges
Guang W, Baraldo M, Furlanut M (1995) Calculating percentage prediction error: a user’s note. Pharmacol Res 32(4):241–248. https://doi.org/10.1016/S1043-6618(05)80029-5
Haghseresht F, Lu GQ (1998) Adsorption characteristics of phenolic compounds onto coal-reject-derived adsorbents. Energy Fuels 12(6):1100–1107. https://doi.org/10.1021/ef9801165
Hao M, Qiao Z, Zhang H, Wang Y, Li Y (2021) Thermodynamic analysis of CH4/CO2/N2 adsorption on anthracite coal: investigated by molecular simulation. Energ Fuel 35(5):4246–4257. https://doi.org/10.1021/acs.energyfuels.0c04337
He J, Shi Y, Ahn S, Kang JW, Lee C-H (2010) Adsorption and desorption of CO2 on Korean coal under subcritical to supercritical conditions. J Phys Chem B 114(14):4854–4861. https://doi.org/10.1021/jp911712m
Ho YS, Porter JF, Mckay G (2002) Equilibrium isotherm studies for the sorption of divalent metal ions onto peat: copper, nickel and lead single component systems. Water Air Soil Pollut 141(1):1–33. https://doi.org/10.1023/a:1021304828010
IEA (2019) Global Energy & CO2 Status Report 2019—The latest trends in energy and emissions. Paris, IEA
IPCC (2020) Climate Change and Land—An IPCC Special Report on climate change, desertification, land degradation, sustainable land management, food security, and greenhouse gas fluxes in terrestrial ecosystems. In: Masson-Delmotte V, Portner H-O, Skea J, Slade R, Ferrat M, Neogi S, Pereira J, Portugal, Kissick K, Buendia E, Calvo, Connors S, Haughey E, Pathak M, Vyas P, Belkacemi M, Zhai P, Roberts D, Shukla P, R, van Diemen R, Luz S, Petzold J, Huntley E, Malley J (ed). Cambridge and New York, IPCC
ISO (2017) Solid biofuels—Sample preparation, in ISO 14780. Geneva, Switzerland: International Organization for Standardization
Kang S-P, Shin J-Y, Lim J-S, Lee S (2014) Experimental measurement of the induction time of natural gas Hydrate and its prediction with polymeric kinetic inhibitor. Chem Eng Sci 116(1):817–823. https://doi.org/10.1016/j.ces.2014.04.035
Kapoor A, Ritter JA, Yang RT (1990) An extended Langmuir model for adsorption of gas mixtures on heterogeneous surfaces. Langmuir 6(3):660–664. https://doi.org/10.1021/la00093a022
Keshavarz A, Sakurovs R, Grigore M, Sayyafzadeh M (2017) Effect of maceral composition and coal rank on gas diffusion in Australian coals. Int J Coal Geol 173(1):65–75. https://doi.org/10.1016/j.coal.2017.02.005
Kim HJ, Shi Y, He J, Lee H-H, Lee C-H (2011) Adsorption characteristics of CO2 and CH4 on dry and wet coal from subcritical to supercritical conditions. Chem Eng J 171(1):45–53. https://doi.org/10.1016/j.cej.2011.03.035
Kim Y, Kim C, Choi S, Rengraj S, Yi J (2004) Arsenic removal using mesoporous alumina prepared via a templating method. Environ Sci Technol 38:924–931. https://doi.org/10.1021/es0346431
Krooss BM, Van Bergen F, Gensterblum Y, Siemons N, Pagnier HJM, David P (2002) High-pressure methane and carbon dioxide adsorption on dry and moisture-equilibrated Pennsylvanian coals. Int J Coal Geol 51(2):69–92. https://doi.org/10.1016/S0166-5162(02)00078-2
Kumar KV, De Castro MM, Martinez-Escandell M, Molina-Sabio M, Rodriguez-Reinoso F (2010) A continuous binding site affinity distribution function from the freundlich isotherm for the supercritical adsorption of hydrogen on activated carbon. J Phys Chem C 114(32):13759–13765. https://doi.org/10.1021/jp104014f
Kumar Singh V, Anil Kumar E (2018) Comparative studies on CO2 adsorption isotherms by solid adsorbents. Mater Today Proc 5(11, Part 2):23033–23042. https://doi.org/10.1016/j.matpr.2018.11.032
Lukhele KD, Premlall K, Shongwe M, Mabuza MM, Daramola MO (2017) Effect of SO2 co-feeding on CO2 adsorption capacity of South African coals during CO2 sequestration. Pet Coal 59(2):177–186
Mabuza MM, Premlall K, Onyango MS, Daramola MO (2017) Sorption of flue gas mixture on South African bituminous and anthracite coals: CO2 sequestration and modelling study. In: Proceedings of the international pittsburgh coal conference, Pittsburgh, Pennsylvania, pp 1–15
Mabuza M, Premlall K (2014) Assessing impure CO2 adsorption capacity on selected South African Coals: comparative study using low and high concentrated simulated flue gases. Energy Proc 51(1):308–315. https://doi.org/10.1016/j.egypro.2014.07.037
Mabuza M, Premlall K, Daramola MO (2020) Physicochemical characterization of South African coals upon short-term flue gas exposure using conventional and advanced techniques. Mater Sci Energy Technol 3:25–35. https://doi.org/10.1016/j.mset.2019.09.008
Mabuza M, Premlall K, Onyango M, Daramola MO (2018) Low-high temperature flue gas direct injection in South African bituminous and anthracite coals: sorption capacity assessment. Curr Sci India 115(4):682–691. https://doi.org/10.18520/cs/v115/i4/682-691
Mazumder S, Van Hemert P, Busch A, Wolf KHAA, Tejera-Cuesta P (2006) Flue gas and pure CO2 sorption properties of coal: a comparative study. Int J Coal Geol 67(4):267–279. https://doi.org/10.1016/j.coal.2005.12.001
Mckay G, Mesdaghinia A, Nasseri S, Hadi M, Solaimany Aminabad M (2014) Optimum isotherms of dyes sorption by activated carbon: fractional theoretical capacity & error analysis. Chem Eng J 251(1):236–247. https://doi.org/10.1016/j.cej.2014.04.054
Oladoja NA, Aboluwoye CO, Oladimeji YB (2008) Kinetics and isotherm studies on methylene blue adsorption onto ground palm kernel coat. Turk J Eng Env Sci 32(1):303–312
Ören Ö, Şensöğüt C (2018) Determination of safe storage types for coals with regard to their susceptibility to spontaneous combustion—Tuncbilek coal case. Int J Coal Prep Util 38(6):290–301. https://doi.org/10.1080/19392699.2016.1252339
Peköz R, Donadio D (2016) Effect of van der Waals interactions on the chemisorption and physisorption of phenol and phenoxy on metal surfaces. J Chem Phys 145(10):104701. https://doi.org/10.1063/1.4962236
Pini R, Ottiger S, Storti G, Mazzotti M (2009) Pure and competitive adsorption of CO2, CH4 and N2 on coal for ECBM. Energy Proc 1(1):1705–1710. https://doi.org/10.1016/j.egypro.2009.01.223
Raganati F, Alfe M, Gargiulo V, Chirone R, Ammendola P (2018) Isotherms and thermodynamics of CO2 adsorption on a novel carbon-magnetite composite sorbent. Chem Eng Res Des 134(1):540–552. https://doi.org/10.1016/j.cherd.2018.04.037
Reeves SR, Koperna GJ (2008) Geologic sequestration of CO2 in deep, unmineable coalbeds: an integrated research and commercial-scale field demonstration project. Houston, Texas, US Department of Energy
Shojai Kaveh N, Wolf KH, Ashrafizadeh SN, Rudolph ESJ (2012) Effect of coal petrology and pressure on wetting properties of wet coal for CO2 and flue gas storage. Int J Greenh Gas Con 11(Supplement):S91–S101. https://doi.org/10.1016/j.ijggc.2012.09.009
Song G, Zhu X, Chen R, Liao Q, Ding Y-D, Chen L (2016) An investigation of CO2 adsorption kinetics on porous magnesium oxide. Chem Eng J 283(1):175–183. https://doi.org/10.1016/j.cej.2015.07.055
Syed A, Durucan S, Shi J-Q, Korre A (2013) Flue gas injection for CO2 storage and enhanced coalbed methane recovery: mixed gas sorption and swelling characteristics of coals. Energy Proc 37(1):6738–6745. https://doi.org/10.1016/j.egypro.2013.06.607
Tang X, Ripepi N (2017) High pressure supercritical carbon dioxide adsorption in coal: adsorption model and thermodynamic characteristics. J CO2 Util 18(1):189–197. https://doi.org/10.1016/j.jcou.2017.01.011
Temkin MJ, Pyzhev V (1940) Recent modifications to Langmuir isotherms. Acta Physiochim URSS 12(1):217–222
Treybal RE (1980) Mass transfer operation. McGraw-Hil, London
Viljoen JHA, Stapelberg FDJ, Cloete M (2010) Technical report on the geological storage of carbon dioxide in South Africa. In: Downes G, van der Spuy D, van Eck S, van Heerden A (ed). Silverton, Pretoria, Council for Geoscience
Wang C, Wang C, Wei M, Gong B, Tan Y (2014) Deformation transition of intact coal induced by gas injection. Int J Min Sci Technol 24(6):833–838. https://doi.org/10.1016/j.ijmst.2014.10.016
Wang L, Yang Y, Shen W, Kong X, Li P, Yu J, Rodrigues AE (2013) Experimental evaluation of adsorption technology for CO2 capture from flue gas in an existing coal-fired power plant. Chem Eng Sci 101(1):615–619. https://doi.org/10.1016/j.ces.2013.07.028
Wang R, Wang Q, Niu Q, Pan J, Wang H, Wang Z (2020) CO2 adsorption and swelling of coal under constrained conditions and their stage-change relationship. J Nat Gas Sci Eng 76(1):103205. https://doi.org/10.1016/j.jngse.2020.103205
Wei XR, Wang GX, Massarotho P, Golding SD, Rudolph V (2007) A review on recent advances in the numerical simulation for coalbed-methane-recovery process. SPE Res Eval & Eng 10(6):657-666. https://doi.org/10.2118/93101-PA
Wilson SMW, Handan Tezel F (2021) Adsorption separation of CF4, O2, CO2, and COF2 from an excimer gas mixture. Sep Purif Technol 258:117659. https://doi.org/10.1016/j.seppur.2020.117659
Wu F-C, Tseng R-L, Juang R-S (2009) Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. Chem Eng J 153(1–3):1–8. https://doi.org/10.1016/j.cej.2009.04.042
Yan M, Bai Y, Li S-G, Lin H-F, Yan D-J, Shu C-M (2019) Factors influencing the gas adsorption thermodynamic characteristics of low-rank coal. Fuel 248(1):117–126. https://doi.org/10.1016/j.fuel.2019.03.064
Yang Y, Liu S, Zhao W, Wang L (2019) Intrinsic relationship between Langmuir sorption volume and pressure for coal: experimental and thermodynamic modeling study. Fuel 241(1):105–117. https://doi.org/10.1016/j.fuel.2018.12.008
Yu S, Bo J, Meijun Q (2018) Molecular dynamic simulation of self- and transport diffusion for CO2/CH4/N2 in low-rank coal vitrinite. Energy Fuels 32(3):3085–3096. https://doi.org/10.1021/acs.energyfuels.7b03676
Zhang D, Zhang J, Huo P, Wang Q, Wang H, Jiang W, Tao J, Zhu L (2016) Influences of SO2, NO, and CO2 exposure on pore morphology of various rank coals: implications for coal-fired flue gas sequestration in deep coal seams. Energy Fuels 30(7):5911–5921. https://doi.org/10.1021/acs.energyfuels.6b00220
Zhou W, Gao K, Xue S, Han Y, Shu C-M, Huang P (2020) Experimental study of the effects of gas adsorption on the mechanical properties of coal. Fuel 281(1):118745. https://doi.org/10.1016/j.fuel.2020.118745
Acknowledgements
The authors acknowledge the National Research Foundation (NRF) of South Africa for the financial support provided to undertake this research successfully. The findings and conclusions expressed in this publication are solely those of the authors and do not necessarily reflect the views of the Foundation.
Author information
Authors and Affiliations
Contributions
All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
We assert that none of the information in the paper has been published or is being considered for publication elsewhere, and that there are no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mabuza, M., Premlall, K. & Daramola, M.O. Modelling and thermodynamic properties of pure CO2 and flue gas sorption data on South African coals using Langmuir, Freundlich, Temkin, and extended Langmuir isotherm models. Int J Coal Sci Technol 9, 45 (2022). https://doi.org/10.1007/s40789-022-00515-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40789-022-00515-y