Abstract
In recent years, many studies on population pharmacokinetics of linezolid have been conducted. This comprehensive review aimed to summarize population pharmacokinetic models of linezolid, by focusing on dosage optimization to maximize the probability of attaining a certain pharmacokinetic-pharmacodynamic parameter in special populations. We searched the PubMed and EMBASE databases for population pharmacokinetic analyses of linezolid using a parametric non-linear mixed-effect approach, including both observational and prospective trials. Of the 32 studies, 26 were performed in adults, four in children, and one in both adults and children. High between-subject variability was determined in the majority of the models, which was in line with the variability of linezolid concentrations previously detected in observational studies. Some studies found that patients with renal impairment, hepatic failure, advanced age, or low body weight had higher exposure and adverse reactions rates. In contrast, lower concentrations and therapeutic failure were associated with obese patients, young patients, and patients who had undergone renal replacement techniques. In critically ill patients, the inter-individual and intra-individual variability was even greater, suggesting that this population is at an even higher risk of underexposure and overexposure. Therapeutic drug monitoring may be warranted in a large proportion of patients given that the Monte Carlo simulations demonstrated that the one-size-fits-all labeled dosing of 600 mg every 12 h could lead to toxicity or therapeutic failure for high values of the minimum inhibitory concentration of the target pathogen. Further research on covariates, including renal function, hepatic function, and drug–drug interactions related to P-glycoprotein could help to explain variability and improve linezolid dosing regimens.
Similar content being viewed by others
High variability of linezolid exposure has been observed, which could result in therapeutic failure and toxicity with standard dosing. |
Linezolid population pharmacokinetic models conducted in special populations are very useful for improving linezolid dosing regimens because they consider both population and pathogen characteristics. |
Some covariates have been found to influence linezolid pharmacokinetics but unexplained variability still remains high, so further research is required to improve the predictive performance of these models. |
1 Introduction
Linezolid is an oxazolidinone antibiotic used for treating serious resistant Gram-positive bacterial infections, such as methicillin-resistant Staphylococcus aureus, penicillin-resistant Streptococcus, and vancomycin-resistant Enteroccocus [1,2,3], which blocks protein synthesis by binding to the 50S portion of the ribosome. Linezolid can be administered either intravenously or orally owing to its absolute bioavailability of close to 100% [4].
The labeled dosage of linezolid (600 mg every 12 h is based on several dose-finding trials that were conducted predominantly on healthy volunteers. Minimum trough concentrations (Cmin) and mean steady-state concentrations were considered effective against Gram-positive pathogens, when taking into account their minimum inhibitory concentration (MIC). No correlation was observed between adverse effects and linezolid concentrations [5,6,7], nevertheless, some differences in the pharmacokinetic (PK) parameters were identified when comparing special populations, such as patients with renal impairment [8], patients with hepatic impairment [8], and elderly patients [9] to healthy subjects.
Adverse reactions related to mitochondrial toxicity, such as optic neuropathy, lactic acidosis, and in particular, hematological toxicity are the main concern. Reported rates of hematological adverse reactions in clinical trials were significantly lower than those observed in subsequent observational studies, especially for thrombocytopenia [10, 11]. Some factors associated with higher linezolid concentrations and thrombocytopenia occurrence included renal insufficiency [12,13,14,15,16], hepatic impairment [13], longer treatment duration [13], low baseline platelet count [17,18,19], higher daily dosage [20], and high total bilirubin [19] (Table S1 of the Electronic Supplementary Material [ESM]).
Large interindividual variability has been associated with linezolid exposure, which could increase the incidence of safety issues and compromise treatment efficacy. Several authors proposed the need for linezolid concentrations to be monitored [21,22,23] given that some studies reported that the concentrations in almost half of patients treated with standard doses were outside the desired range [23, 24].
This percentage could be higher in special populations, which would lead to an unacceptable risk of both underexposure and overexposure. Physiological factors such as renal impairment, hepatic failure, advanced age and low body weight (WT) have been correlated with higher concentrations and adverse reaction rates. On the contrary, renal replacement techniques, younger age, excess WT, and obesity have been associated with low concentrations and therapeutic failure. There was an even higher inter-individual and intra-individual variability among critically ill patients, suggesting that this population is at an even higher risk of underexposure and overexposure [21].
In this scenario, population PK (popPK) models are a very useful tool for improving linezolid dosing regimens in special populations, maximizing the probability of target attainment (PTA) of the proposed PK/pharmacodynamic (PD) indexes. This review aims to compile all published popPK models of linezolid, focusing on dosing simulations and the influence of covariates to optimize treatment.
2 Methods
2.1 Search Strategy
The EMBASE and PubMed databases were searched from inception to February 2022 using the following terms: ‘linezolid’ AND (‘population pharmacokinetics’ OR ‘pharmacometrics’ OR ‘pharmacokinetic model’ OR ‘popPK’ OR ‘nonlinear mixed effect model’ OR ‘NONMEM’). The reference lists from the relevant studies were analyzed for additional literature.
2.2 Inclusion/Exclusion Criteria
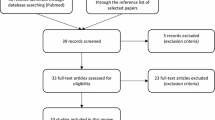
All popPK models of linezolid that met the following inclusion criteria were included in the review: (1) studied population: pediatric and adult patients; (2) treatment: linezolid administered either orally or intravenously; and (3) PK analysis: a non-linear, mixed-effect PK modeling approach was used. The following studies were excluded: (1) reviews and in vitro and animal studies; (2) papers not written in English; and (3) studies in which non-compartmental or non-parametric approaches were employed (Fig. 1).
2.3 Data Extraction
Three authors performed data extraction using a data collection form to collect the following variables: first author, year of publication, number of patients, participant characteristics (patients/healthy subjects, race/country, age, sex, WT, adult/pediatric, pathology), route of administration, number of observations, observations per patient, modeling software, structural and statistical model, tested and retained covariates, and model evaluation method. The model evaluation methods were divided into three types based on the increasing order of quality: basic internal, advanced internal, and external model evaluation [25].
3 Therapeutic Drug Monitoring
Linezolid peak plasma concentration is usually achieved within 1–2 h following oral administration [26]. The volume of distribution (Vd) is close to total body water, approximately 50–60 L [27], and its protein binding to albumin is relatively low, with a range from 10.5 to 31% [5, 28,29,30,31,32,33,34,35]. It shows good tissue distribution (Fig. 2) [Table S2 of the ESM].
Linezolid metabolism is complex as two major metabolites are formed by oxidation pathways that are not mediated by cytochrome P450 but by reactive oxygen species [36,37,38]. Nevertheless, interactions related to cytochrome P450 3A4/3A5 and, above all, P-glycoprotein have been proposed [10, 22, 39,40,41,42,43,44,45,46,47] (Table S3 of the ESM) (Fig. 2).
There was a linear relationship between the Cmin and the area under the curve in 24 h (AUC0–24h); therefore, trough concentrations could be used as a predictor of drug exposure. The correlation between the observed and predicted AUC was high. The predicted AUC ranged from 0.7-fold to 1.3-fold and from 0.76-fold to 1.5-fold of the real AUC values, respectively [48, 49]. Post-2-hour, post-5-hour, or post-6-hour sampling has also been proposed in oral administration to assess peak plasma concentration and characterize delayed absorption [24, 50].
Furthermore, it is essential to read into this value the infectious context, considering the MIC of this antibiotic against the isolated microorganism. Different PK/PD targets have been proposed as a ratio of AUC0–24h/MIC = 50–100 [51], 80–120 [52], and a percentage of time above the MIC of greater than 85% during the entire dosing interval in order to maximize efficacy and prevent the development of antibiotic resistance [2, 51, 52]. However, a Cmin of higher than 7.5–22.1 mg/L was significantly correlated with the occurrence of adverse effects [53,54,55], especially hematological toxicity, peripheral neuropathy, mitochondrial toxicity, and optic neuropathy [56]. Therefore, a target therapeutic range of Cmin between 2 and 7 mg/L for steady-state linezolid has been proposed for treating Gram-positive bacterial infections [57]. In the case of tuberculosis (TB) infections, a Cmin target of < 2 mg/L has also been proposed because of its lower MIC, usually ≤ 1 mg/L, even in extensively drug-resistant TB [58].
The strong correlation between exposure and efficacy and in particular toxicity supports the use of linezolid therapeutic drug monitoring with the following proposed PK/PD indexes: AUC0–24h/MIC = 80–120, percentage of time above the MIC ≥85%, and Cmin between 2 and 7 mg/L [2, 24, 51, 52, 59,60,61]. A recent position paper by several scientific societies (ESICM, ESCMID, IATDMCT and ISAC) pointed out that therapeutic drug monitoring is clearly recommended for linezolid in critically ill patients [57] (Fig. 3).
4 Characteristics of popPK Models
The initial database search yielded 1179 publications, of which a total of 32 studies involving 2572 participants met the inclusion criteria. The population characteristics are summarized in Tables 1 and 2, respectively. Studies were published between 2005 and 2021, and the number of subjects ranged from 5 to 603, with a median of 44.5. Of the 32 publications, 27 were conducted in adult patients [19, 34, 37, 53, 58, 62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80], four in pediatric patients [81,82,83,84] and one in both populations [33]. Intravenous administration was the only route in 16 of the publications [34, 37, 62, 65, 66, 69,70,71,72, 74, 75, 79,80,81,82, 84], while both intravenous and oral formulations were included in 11 studies [18, 19, 33, 53, 63, 64, 67, 68, 77, 78, 85]. In five studies, only oral formulations were allowed [58, 73, 76, 83, 86].
The model characteristics of the included studies are summarized in Table 3. The number of observations ranged from 44 to 2539 (median 242), and the median observations per patient was 3.9. The majority of the studies used the NONMEM software to develop the popPK model, with the exception of five studies: two used Phoenix NLME [70, 84], one used WinNonMix [65], one used Monolix [73], one used S-ADAPT [86], and one used Pumas [80]. The basic internal validation method of goodness of fit was performed in all of the included models, while bootstrapping was used in 17 of the studies [33, 53, 64, 67, 70, 71, 73,74,75, 77,78,79, 81, 82, 84,85,86]. The vast majority also included advanced internal validation methods, such as visual predictive check or prediction-corrected visual predictive check [18, 19, 33, 34, 37, 53, 58, 63, 68,69,70,71,72,73,74, 76, 77, 79, 81,82,83,84,85,86], normalized prediction distribution errors [18, 34, 72, 81], or case-deletion diagnostics [67]. In two studies, an external validation was also performed [71, 72].
Simulations were performed in 24 out of 32 studies to determine the optimal dosing regimens. Most of the studies used the ratio between AUC0–24h and MIC and/or the percentage of time over the MIC in a dosing interval (percentage of time above the MIC) as the PK/PD target. Several studies also used Cmin as a surrogate marker of AUC0–24h [19, 37, 53, 70, 72, 73].
The final structural model, PK parameters, model variability, and tested and retained covariates are summarized in Table 4. In the studies that included the oral route of administration, absorption was described as a first-order process. The median value of the absorption rate constant was 1.22 (0.192–1.91)/h. In two studies, a fixed value of the absorption rate constant from the literature was set to 0.583/h [53, 67], and an absorption lag time parameter was included in two models [63, 73]. Oral bioavailability was > 90% regardless of the formulation, with the exception of one model developed in patients with cystic fibrosis, perhaps owing to the characteristic malabsorption, in which the bioavailability was 85.1% [68].
In adults, the population pharmacokinetics of linezolid was described by a one-compartment model in 15 studies [18, 19, 37, 53, 58, 64, 67, 69, 70, 72, 73, 75, 76, 80, 85] and by a two-compartment model in 13 [33, 34, 62, 63, 65, 66, 68, 71, 74, 77,78,79, 84, 86] studies. Population PK models with sparse data (mainly peak and trough sampling schema) tended to fit to a one-compartment model because of the lack of sampling points, while rich data models best fitted to a two-compartment model. The median (range) estimated value of the Vd was 45.1 L (20.2–284.4 L). Elimination was mainly described as a linear process with the exception of three studies [77, 79, 86]. Furthermore, two studies included an inhibition of clearance (CL) over time [63, 68]. The median (range) value for CL was 6.2 L/h (2.5–11.2 L/h). Typical values were reported for a patient with a total WT of 70 kg and a creatinine CL (CLCR) of 80 mL/min.
In the pediatric population, three models fitted to a one-compartment with linear elimination [81,82,83], while one model fitted to a two-compartment model [84]. The median (range) estimated value of CL was 0.119 L/h/kg (0.0676–0.12 L/h/kg) and the Vd was 0.782 L/kg (0.385–0.836 L/kg).
Between-subject variability (BSV) was described by an exponential model in all cases, and it was relatively high. In adults, the median (range) values of BSV in CL and Vd (or central volumen of distribution [Vc]) were 41.7% (12.7–108.2%) and 34.9% (8.89–142.1%), respectively. In three studies, an inter-occasion variability in CL was included with median (range) values of 33.3% (16.1–64.7%) [34, 69, 86]. In the pediatric population, BSV values were similar among the different models with median (range) values of 38.7% (37–52.5%) and 32% (28.1–55.8%) for CL and Vd, respectively.
With regard to residual variability, a proportional error model was used in 14 studies [34, 53, 64, 68, 70, 73,74,75, 77, 79, 80, 84,85,86] with median (range) values of 16.1% (4.76–53.9%), an additive error model was used in two [67, 82] with median (range) values of 1.28 mg/L (1.13–1.43 mg/L), and a combined error model was used in 16 studies [18, 19, 33, 37, 58, 62, 63, 66, 69,70,71,72, 76, 78, 81, 83] with median (range) values of 16.5% (4.13–56.4%) and 0.255 mg/L (0.005–1.43 mg/L) for the proportional and additive error model, respectively.
Many factors were tested as possible covariates of the PK parameters. The most commonly identified significant covariates on linezolid CL were WT, renal function (CLCR), or estimated glomerular filtration rate (eGFR), renal replacement techniques and hepatic function (presence of cirrhosis, liver transplantation/resection, prothrombin activity, and LiMAx [maximal liver function capacity] value) while WT, lean body WT, body surface area, and peritonitis were identified as covariates of Vd. In the pediatric population, WT and CLCR were identified as covariates of CL. Furthermore, post-natal age (PNA) was also a significant covariate on the CL in a popPK study in premature infants [82] and aspartate transaminase in critically ill pediatric patients [84].
5 Special Populations
5.1 Altered Renal Function
Initial studies of linezolid pharmacokinetics in patients with renal dysfunction or end-stage renal disease requiring hemodialysis suggested that dose adjustment was not necessary because of the similar concentration profiles observed compared to those of healthy subjects [8]. However, several subsequent studies correlated impaired renal function with increased concentrations of linezolid and its metabolites, as well as the occurrence of adverse reactions [13,14,15,16, 19, 55, 59, 87, 88].
In 2003, Meagher et al. published the first popPK model that identified renal function as a significant covariate of total linezolid CL (not included in results). They found that CLCR and ideal body WT explained 16% of linezolid total average CL [89].
Despite reported renal CL of linezolid of about 30%, the impact of renal function could be more than expected a priori. According to Matsumoto et al.’s model, predicted CL ranged between 2.2 L/h up to 6.5 L/h, almost a three-fold difference, from the lowest to the highest CLCR value (6.3–173.1 mL/min) [53]. A target Cmin range of 3.6–8.2 mg/L was also proposed. The lower limit was based on the trough concentration required to attain an AUC/MIC target >100 for the highest MIC observed in their hospital (2 mg/L). The higher limit was estimated based on the Cmin associated with a 50% probability of developing thrombocytopenia, which was time dependent. Accordingly, in the model performed by Tsuji et al. [75] in low WT patients with renal dysfunction, the CL ranged from 1.8 to 3.8 L/h, an approximately two-fold difference. Furthermore, Ide et al. [74] studied patients with sepsis with and without renal impairment and observed great differences between the typical CL values between those populations (2.06 vs 6.36 L/h), and these were very close to those predicted by Matsumoto et al. An even higher influence of renal function was reported in Sasaki et al.’s model, in which the predicted CL ranged between 1 and 8.1 L/h; however, some of the patients also had liver cirrhosis [67]. Nevertheless, other factors such as the lower WT of the Japanese population must be considered, as non-renal CL values could be lower than expected, increasing the influence of renal function in total CL.
Other authors that assessed the influence of renal function were Boak et al., who developed a PK-toxicodynamic model, in which CLCR was a significant covariate on total linezolid CL. Based on the average AUC0–24h of their population, 90%, 50%, and 7% of the patients would have achieved the target of AUC/MIC >100 for MIC values of 1, 2, and 4 mg/L, respectively [18].
Tsuji et al. further developed a PK-toxicodynamic model, measuring both total and unbound linezolid concentrations. Renal function, age, and total body WT were significant covariates of linezolid CL. The authors concluded that renal function significantly affected linezolid renal CL and age slightly affected nonrenal CL. Inhibition of platelet formation was proposed as the main thrombocytopenia mechanism. Mean values of renal and non-renal CL were 1.44 L/h and 1.86 L/h; therefore, endorsing the possibility of the higher influence of renal function in total linezolid CL in Japanese patients [33]. Alternatively, Crass et al. developed a popPK analysis to identify an alternate dosing strategy in renal impairment. With standard dosing, only 33% of patients with eGFR < 60 mL/min would achieve therapeutic trough concentrations (2–8 mg/L), mainly owing to the increased probability of supratherapeutic concentrations. Therefore, the authors suggested reducing the dose in this group of patients to 300 mg every 12 h to increase the PTA up to 65%. Furthermore, they also suggested increasing the dose to 450–600 mg every 8 h in patients with an eGFR ≥ 90 mL/min to ensure a ≥ 90% PTA [19]. It is important to note that the differences between the mean values of non-renal and renal linezolid CL were higher (3.43 L/h/1.89m2 vs 1.77 L/h/1.89m2, respectively) compared with the aforementioned studies.
In the same vein, Wang et al., also developed a model proposing dose adjustments based on their CLCR. The exponent of CLCR on the total CL was 0.36, indicating the considerable influence of renal function, the same value as reported by Zhang et al. [70]. They proposed the need for higher doses of linezolid in normal renal function and continuous infusion of 2400 mg every 24 h in the case of augmented renal CL. None of the regimens was found to be optimal for MIC = 4 mg/L [72].
Another important point to note is that the majority of the popPK models tested renal function as a potential covariate, as creatinine level, CLCR, or eGFR, nonetheless, less than half (12 out of 29) ended up including it as a significant covariate in the model. Most were performed in Japanese patients or in populations with other significant comorbidities, such as hepatic impairment.
In conclusion, renal function appears to be a relevant factor influencing linezolid exposure, as CLCR and eGFR have been identified as covariates of linezolid CL in several published popPK models. In patients with diminished renal CL, higher concentrations of linezolid would be expected, resulting in overexposure. To the contrary, lower concentrations of linezolid would be expected in patients with augmented renal CL, resulting in underexposure. The physiological characteristics of the population could also significantly impact the relative impact of renal CL on total linezolid CL. Dose reductions may be necessary in patients with impaired renal function. However, higher doses may be necessary in patients with eGFR of greater than 90 mL/min.
5.2 Renal Replacement Therapies
Because of its low molecular WT, low protein binding, and relatively low Vd, linezolid is likely to be removed by intermittent hemodialysis (IHD) and continuous renal replacement therapies (CRRT). The impact of IHD on linezolid PK parameters was assessed by Brier et al. (not included in results), who described an apparent increase in oral CL from 4.59 L/h on off-dialysis days to 7.8 L/h on on-dialysis days. Despite 30% of the dose being removed in a dialysis session, no dosing recommendations were deemed necessary, but a supplemental dose was recommended during the first hemodyalisis session [8].
Fiaccadori et al. reported substantial linezolid removal during IHD (32.3%) and sustained low-efficiency dialysis (33.9%), while continuous venous-venous hemodialysis removed 17.5% of the dose. Subtherapeutic concentrations of linezolid were noticed, especially with CRRT and sustained low-efficiency dialysis.
The impact of the different CRRT was also assessed. Meyer et al. (not included in results) conducted a multi-dose study in anuric critically ill patients undergoing continuous venovenous hemofiltration (CVVH) with highly permeable polysulfone membranes. The PK parameters were comparable to healthy subjects and patients without renal impairment, supporting the standard dosage of 600 mg twice daily in these patients [90].
Swoboda et al., analyzed the differences in the popPK parameters in patients with sepsis with normal renal function compared to anuric patients with sepsis undergoing extended dialysis (ED). They concluded that linezolid plasma concentrations can be reduced to subtherapeutic values during ED. Patients with sepsis with and without ED may require higher doses. Body WT, history of liver transplantation or resection, and dialysis were significant covariates [66].
Roger et al., analyzed the differences between CVVH and continuous venovenous hemodiafiltration (CVVHDF) [not included in the results]. No statistical differences were found in terms of CL; however, the mean CLCVVHDF was 20.5% higher than CLCVVH (5.9 vs 4.5 L/h). Increasing WT and decreasing sequential organ failure assessment were associated with higher linezolid CL. The sequential organ failure assessment score reflects the level of organ dysfunction of different systems by measuring several physiological parameters. Those related to the cardiovascular system (mean arterial pressure and need of vasoactive agents) and hepatic function (bilirubin level) probably contribute the most to impaired linezolid CL, owing to decreased cardiac output and subsequent renal excretion, and decreased linezolid metabolism. The authors concluded that patients who undergo CVVH and CVVHDF have a low PTA at standard doses, especially with a body WT of > 90 kg and an MIC ≥ 2 mg/L [91].
On the contrary, Ide et al.’s study also considered the differences between patients with sepsis who underwent CRRT (eight with CVVHDF and two with continuous venous-venous hemodialysis). The differences between linezolid CL in patients with renal dysfunction and those who underwent CRRT were not as large as in the previous studies (2.06 L/h vs 2.74 L/h). The authors suggested that the low CRRT dose could have had an impact on the lower CL values observed [74].
In summary, renal replacement therapies including IHD, CVVH, CVVHDF, and sustained low-efficiency dialysis seem to remove significant amounts of linezolid, around 30% of the dose. The parameters and duration of the renal replacement therapy could also play an important role in total linezolid CL and should be considered. Subtherapeutic concentrations of linezolid were observed more frequently than supratherapeutic concentrations in this subgroup of patients, particularly in those with higher body WT and MICs, in which higher doses might be required.
5.3 Hepatic Impairment
According to the summary of product characteristics, the recommended linezolid dose for mild-to-moderate hepatic impairment is 600 mg twice daily. However, this recommendation was based on a single study with only seven patients with mild-to-moderate hepatic impairment. Although no statistical differences were found in terms of the concentrations and PK parameters, it may not be reliable to state that dose adjustments were not warranted for this population given the limitations of the very small sample size [92]. No dose recommendations for severe hepatic impairment were included.
As was the case in renal impairment, post-commercialization studies observed increased concentrations of linezolid and a higher incidence of adverse reactions in patients with hepatic impairment. Ikuta et al. [93] reported an incidence of 48.8% for thrombocytopenia, with chronic liver disease being the only significant risk factor. Zhang et al. [94] found a 57.1% incidence of thrombocytopenia in patients with acute-on-chronic liver failure. In a case-control study conducted by Luque et al., 76.9% of the subjects had supratherapeutic trough concentrations; however, the upper limit of the target therapeutic range (10 mg/L) was higher than the currently recommended limit (7 mg/L) [24, 57]. The median steady-state Cmin was 20.6 mg/L and this was similar among different degrees of hepatic impairment. Liver cirrhosis was the only risk factor associated with supratherapeutic levels (odds ratio 11.4). Around half of the patients experienced hematological toxicity (53.8%). Clinically relevant thrombocytopenia (< 100 × 103 platelets/µL) was significantly associated with high trough concentrations (23.6 vs 4 mg/L) and hyperlactatemia (19.9 vs 3.4 mg/L) [95].
As previously mentioned, Sasaki et al. [67] found that the presence of liver cirrhosis leads to a 45.2% reduction in total linezolid CL. Nevertheless, only four patients with liver cirrhosis (Child-Pugh class C) were included in this study. Swoboda et al. [66] also found that liver transplantation or resection was a significant factor affecting linezolid CL, with it dropping by 60%.
Two popPK models were specifically developed in patients with hepatic impairment. Wicha et al., used the maximal liver function capacity (LiMAx) test, which aims to determine liver function by assessing cytochrome P450 1A2 activity measuring the ratio of exhaled 13C and 12C following an intravenous injection of 13C-labeled methacetin [96, 97]. They first separately assessed CLrenal and CLnon-renal, in which CLCR and LiMAx were significant covariates, respectively. However, when analyzing total linezolid CL, the LiMAx value was the only significant covariate. Patients with LiMAx values < 100 μg/kg/h were at a high risk of linezolid overexposure [69].
Recently, Zhang et al., developed a popPK model to improve dosing in this population. The majority of patients in this study were diagnosed with liver failure or cirrhosis, Child-Pugh class C (66.7%). Creatinine CL and prothrombin activity were significant covariates on CL. In patients with prothrombin activity < 20%, the estimated probability of supratherapeutic trough concentrations was 64.4%. Authors suggested that 300 mg every 12 h would achieve a PK/PD target of AUC/MIC > 80 in 91.9% of patients, even for high MIC values of 4 mg/L [70].
Although the metabolism of linezolid is mediated by non-enzymatic pathways, the liver appears to play a key role in linezolid CL. Observational studies found higher linezolid concentrations and hematological toxicity rates in patients with liver failure. When developing popPK models in this population, one of the main limitations is finding reliable liver function markers. Published models considered the presence of cirrhosis, the prothrombin activity, and the novel LiMax approach as covariates. Decreased linezolid CL values and high rates of overexposure were observed in this population. Dose reductions were proposed to attain the PK/PD target even for high MIC values.
5.4 Elderly Patients
Pivotal studies concluded that age did not alter the PK parameters and that dosage adjustments would not be necessary in this group of patients [9]. Nevertheless, some observational studies observed a significant effect on linezolid PK parameters. In a retrospective observational study by Cattaneo et al., a correlation between age and serum creatinine with Cmin was observed. Elderly patients, particularly those aged > 80 years, were at a higher risk of overexposure [21]. The authors subsequently reported increasing trough concentrations correlated with age, with a 30% increase per decade from 50 years on. This study had an important limitation that could lead to an overestimation of age effect on linezolid concentrations, which was the absence of information on renal CL [98].
Abe et al. performed a popPK analysis to determine the influence of age and body WT. Clearance diminished as age increased from about 60 years. A 3.5-fold difference in exposure was observed between a patient aged ≥ 80 years with a body WT ≤ 50 kg and another aged <60 years and weighing ≥ 50 kg [64]. Xie et al., also identified an inverse correlation between age and linezolid CL [34] and Tinelli et al., carried out a study specifically on this population. In the first Cmin measurement, all of them had supratherapeutic trough concentrations. After reducing the dose to 300 mg every 12 h, 85% of subsequent Cmin was within the therapeutic range [99]. Recently, Cheng et al. also found higher concentrations in elderly patients, correlating this with hematological toxicity [100].
Despite there being no specific dosing recommendations for the elderly population, age appears to be a significant factor that affects linezolid exposure. This might be related to some age-related factors that affect linezolid exposure. Changes in the body composition of elderly patients can decrease the Vd. Furthermore, renal and hepatic functions worsen, decreasing CL. It is important to mention that age was tested as a potential covariate in most of the models; however, it was only significant in a few models. This might be explained by the fact that other covariates such as body WT or renal function could explain the decreasing CL values better than the patient’s age.
In summary, increased age, particularly over 60 years, was correlated with higher linezolid concentrations and toxicity. Dose reductions might be needed, especially in patients with a lower body WT and other comorbidities.
5.5 Overweight and Obese Patients
The relationship between linezolid CL and body WT has been documented in several studies, some of them focusing on overweight and obese patients. Cojutti et al. developed a popPK model in which greater CL values and lower AUC were noticed in obese grade III vs overweight patients (not included in results). Estimated glomerular filtration rate was the only significant covariate on CL, while WT was a covariate on both central and peripheral Vd. The authors proposed 450 mg every 8 h when eGFR was ≥ 60 mL/min/1.73m2. However, when MIC was ≥ 2 mg/L, the majority of the tested regimens would lead to low PTA, except 600 mg every 8 h, which gave an unacceptable high risk of overexposure (> 65%) [47].
In Xie et al.’s popPK model, the simulations concluded that giving standard doses to obese patients would lead to a low PTA, especially in patients aged < 60 years, and this was in line with Cojutti et al.’s findings [34]. In addition, Ehmann et al. performed a study comparing the PTA of non-obese and obese patients eligible for surgical intervention. Lower linezolid exposure was associated with obese patients. Interestingly, the body size descriptor that better related to distribution was the lean body WT, and the mean arterial pressure was found to be correlated with linezolid CL. The authors proposed a dose increase, shorter dosage intervals, and/or prolonging administrations by up to 4 h to maximize the PTA, especially when MIC = 2 mg/L. None of the tested regimens achieved an adequate PTA when MIC = 4 mg/L [79]. In contrast to Ehmann et al.’s results, total body WT was the descriptor that most influenced PK parameters, especially the Vd in Blackman et al.’s model. However, the results of simulations provided similar conclusions, as a low PTA was estimated for MIC ≥ 2 mg/L. In fact, 600 mg every 8 h could achieve an optimal PTA for MIC = 2 mg/L, but not in the case of 4 mg/L [80].
In summary, overweight and obese patients experienced lower exposure to linezolid and seemed to be at a higher risk of treatment failure. Both Vd and linezolid CL could increase in overweight and obese patients. Volume of distribution was allometrically scaled to WT in all models; however, both ideal body WT and total body WT were used as body size descriptors. Higher doses might be required in this population, and the risk of therapeutic failure is greater with decreasing age and when MIC values are ≥ 2 mg/L. The doses needed to attain PK/PD targets could lead to a high risk of overexposure for the higher MIC values.
5.6 Cystic Fibrosis
Keel et al. studied linezolid pharmacokinetics in eight patients diagnosed with cystic fibrosis based on a previously published popPK model that included a theoretical inhibition compartment to explain non-linear CL of linezolid over time [63]. Only lean body WT was a significant covariate for CL in this study. Clearance after the first dose and a 9-day treatment period was calculated, with a mean 38.9% reduction, from 9.7 to 6.1 L/h. The mean parameter values were similar to those recorded in healthy subjects, despite bioavailability (85% vs 100%), possibly owing to characteristic malabsorption of this group of patients [68].
5.7 Critically Ill Patients
Critically ill patients are known to have several clinically relevant PK and physiological alterations that may alter drug concentrations, compromising therapeutic success. Furthermore, the high prevalence of severe infections with high mortality rates still represents a challenge, with linezolid playing a crucial role, given that up to 50% of bloodstream infections in critical care units are caused by Gram-positive bacteria [101, 102]. Therefore, it is a key element to optimize linezolid exposure in this group of patients.
A few studies observed large BSV to linezolid exposure in critically ill patients. Dong et al. remarked that highly variable linezolid PK/PD properties were observed in this group [103]. In the same vein, Zoller et al. reported 100-fold differences in trough concentrations (from < 0.13 to 14.49 mg/L) and AUC0–24 (50.1–453.9 mg·h/L). High intra-subject variability was also noted, as only 17% of patients attained trough concentrations within the desired range for the entire study period. Importantly, a large proportion of the patients had linezolid trough concentrations and AUC0–24 below the target [104]. To the contrary, most of the patients with out-of-range concentrations fell above the upper limit in the 10-year therapeutic drug monitoring program experience reported by Pea et al. [23]. This could be explained by the differences in the Cmin target, 2–7 mg/L compared with 2–10 mg/L in the Zoller et al. study. Decreased protein binding in patients with hypoalbuminemia was also observed [35, 105] and non-linearity of CL over time [30].
Töpper et al., developed a popPK model in surgical critically ill patients. Despite no simulations being performed, a high BSV was noted (108.2% in CL and 53.7% in Vd), and the majority of patients experienced underexposure or overexposure to linezolid. The authors proposed the possible role of drug–drug interactions to partially explain the high variability, especially with proton pump inhibitors and levothyroxine [37].
Recently, Soraluce et al. developed a popPK model in critically ill patients, including those subjected to CRRT. Creatinine CL was the only significant covariate on CL. The extracorporeal CL was calculated by multiplying the effluent flow by the sieving coefficient. The mean sieving coefficient was 0.8 and this did not vary depending on the technique or membrane employed. The PK/PD target was not achieved in many patients, especially for MICs of 2 and 4 mg/L. Authors proposed a continuous infusion of 50 mg/h, given that 85% of patients would achieve the target of Css > MIC for values of 2 mg/L. Nevertheless, for MIC values of 4 mg/L, only 50% of patients achieved the target even with continuous infusion administration [71]. Taubert et al., found interesting significant covariates on linezolid CL, which may reflect the disease and pathophysiological status of critically ill patients: acute respiratory distress syndrome (ARDS), fibrinogen, and lactate. On the one hand, lactate may inversely reflect the organ perfusion and hemodynamic stability, and on the other hand, fibrinogen may indirectly reflect hepatic function. Nonetheless, the most relevant covariate was ARDS, the presence of which would increase linezolid CL by 82%. The authors proposed that the large amount of reactive oxygen species in the lungs of patients with ARDS was a potential underlying mechanism [78]. Wang et al. developed a popPK model to perform dose adjustments according to CLCR values. Mean values of CL and Vd were very similar to those reported by Soraluce et al. Based on simulations, they proposed doses of 600 mg every 12 h for CLCR ~ 40 mL/min, 600–900 mg every 12 h for CLCR ~ 80 mL/min, and a continuous infusion of 2400 mg/day in patients with augmented renal CL.
In summary, critically ill patients appear to be at a high risk of both underexposure and overexposure because of the physiopathological changes experienced by this population, in which the attainment of the PK/PD target is particularly relevant given the high prevalence of severe infections with high mortality rates. Covariates related to the physiopathological status of these patients have been found to be correlated with linezolid CL, such as ARDS diagnosis or lactate and fibrinogen levels; however, further research is needed. Higher-than-standard linezolid doses may be required in patients with augmented renal CL, renal replacement therapy, or when treating high MIC infections. Continuous infusion has also been proposed in order to maximize the PTA when MIC is ≥ 2 mg/L.
5.8 Patients with TB
In addition to Gram-positive infections, linezolid is also widely used for treating TB infections, especially for resistant strains. Lower doses are used but for longer periods of time.
Three popPK models were performed in patients with TB [58, 73, 76], which mainly differed in terms of the PK/PD targets and the PTA results of simulations because of differences in MIC. Large variability in the linezolid CL values was noted. In Abdelwahab et al.’s [58] popPK model developed in South African patients, CL was 3.57 L/h, which was considerably lower than the 6.06 L/h reported by Alghamdi et al. [73] and the 7.69 L/h reported by Tietjen et al. [76]. As the rest of the baseline characteristics were similar, the ethnicity could have a significant influence, as most of the patients in the Abdelwahab model were black or mixed race.
Accordingly, Abdelwahab et al. suggested that a 600-mg daily regimen would be enough to attain the PK/PD target in the South African population, while Tietjen et al., proposed a 600-mg twice-daily regimen. In the same vein, Alghamdi et al., predicted that daily doses of 900–1200 mg would ensure efficacy but potentially with more toxicity.
5.9 Pediatric Patients
A phase I single-dose study including 58 children aged from 3 months to 16 years found significant differences in drug disposition. The mean values of CL and Vd were greater than in adults. As a result, exposure to linezolid measured by dose-normalized AUC was 35% of the mean value in adults (3.72 mg·h/L vs 10.51 mg·h/L per mg/kg of linezolid). A non-linear correlation between CL and age was observed, and children aged < 40 months had the highest values of CL. On the basis of these data, authors suggested 10 mg/kg every 8–12 h in this population [106].
Subsequently, other trials were conducted in different pediatric subpopulations. A study in neonates and young infants, PNA ≤ 3 months, found that linezolid pharmacokinetics varies substantially in the first week of life depending on PNA. Preterm infants aged < 7 days had similar CL values to adults, while infants aged > 7 days had values that were approximately three-fold greater [107]. Pooled data from another three trials that included children also found age-dependent linezolid CL. While adolescents had similar values to adults, young children had higher CL, up to three-fold, and subsequently, a smaller AUC, and shorter half-lives. Finally, a popPK analysis of data from a phase III trial reported that doses of 10 mg/kg every 8 h in children aged under 12 years resulted in similar linezolid exposure to the exposure in adults who received 600 mg every 12 h [108].
A popPK model by Li et al. conducted in pediatric patients aged from 0 to 12 years identified body WT and eGFR as significant covariates on linezolid CL. They also observed risk of underexposure in children treated with 10 mg/kg every 8 h for MIC ≥ 2 mg/L. The authors suggested increasing doses up to 15–20 mg/kg every 8 h in this population [81].
A PK/PD evaluation performed by Cojutti et al., (not included in results) revealed suboptimal values of Cmin and AUC0–24h with the recommended dosage of 10 mg/kg every 8 h in almost half of the patients. Moreover, a PTA ≥ 90% of AUC/MIC > 100 would only be achieved for MIC ≤ 1 mg/L. Doses of up to 15 mg/kg every 8 h would be necessary for MIC = 2 mg/L, while neither of the dosage regimens tested ensured an acceptable PTA for MIC = 4 mg/L. With regard to significant covariates, none of the demographic characteristics or renal function correlated with Cmin values, while co-medications (phenobarbital, dexamethasone, proton pump inhibitors, and amiodarone) accounted for two-thirds of the variability [109].
Garcia-Prats et al. developed a popPK analysis to determine optimal dosing for treating multi-drug-resistant TB in children. Weight significantly correlated with linezolid CL. They proposed WT-banded once-daily linezolid dosing, with a range from 80 mg for children weighing 5–7 kg up to 600 mg for those weighing > 44kg. The exposure target was an AUC0–24h,ss = 110 mg·h/L based on adult data [83].
A popPK model in preterm infants was also developed. Thibault et al. found that PNA and WT correlated significantly with linezolid CL. Doses of up to 12 mg/kg every 8 h would be necessary in order to achieve PTA ≥90% for MIC = 2 mg/L. None of the regimens tested attained sufficient PTA for MIC = 4 mg/L [82].
Finally, Yang et al. performed a model in critically ill children in which WT and aspartate transaminase were significant covariates of CL. Higher doses of up to 15 mg/kg every 6 h were estimated to treat pathogens with MIC = 2 mg/L [84].
In summary, linezolid CL seems to vary substantially in the pediatric population. In premature infants, PNA was found to be the main covariate influencing CL, the values of which ranged from those recorded in adults when aged < 7 days to three-fold in premature infants aged > 7 days, possibly owing to organ maturation, mainly the liver. This higher CL appears to be maintained in younger children, but it then seems to decrease in adolescents, reaching values similar to adults. Higher-than-standard doses were suggested in younger children and when treating pathogens with high MIC values.
This comprehensive review had some limitations. First, only parametric non-linear mixed-effect models were included in the results, despite the fact that some non-parametric models have also been published [4, 47, 91, 110, 111] owing to the difficulties in performing a direct comparison. Nonetheless, some information from these studies was included in the discussion when considered necessary.
Apart from that, there were some limitations in the PK/PD targets and MIC values as they only consider the plasma concentration of linezolid. An ideal PK/PD target should include concentrations in the site of infection. Finally, regarding the covariate analysis in each population, we mainly focused on the those in which that covariate was significant and included in the model.
6 Conclusions
This review summarized the most relevant information on population pharmacokinetics of linezolid, highlighting special populations that might be at a higher risk of overexposure and underexposure, leading to toxicity or treatment failure. A dosing simulation analysis also helps to optimize linezolid treatment, as it considers relevant covariates that influence linezolid exposure, such as body WT, renal and hepatic function, and age, as well as the MIC of the target pathogens. Therapeutic drug monitoring may be warranted in a large proportion of patients to maximize the probability of attaining the desired PK/PD target. Further studies focusing on potential covariates, such as renal and hepatic function, drug–drug interactions involving P-glycoprotein, and the influence of gestational age in premature infants, are warranted given that unexplained variability remains high. In addition, the predictive performance of models needs to be assessed in the specific population in which the models are to be used.
Change history
03 August 2023
A Correction to this paper has been published: https://doi.org/10.1007/s40262-023-01295-7
References
Swaney SM, Aoki H, Ganoza MC, Shinabarger DL. The oxazolidinone linezolid inhibits initiation of protein synthesis in bacteria. Antimicrob Agents Chemother. 1998;42(12):3251–5.
Dresser LD, Rybak MJ. The pharmacologic and bacteriologic properties of oxazolidinones, a new class of synthetic antimicrobials. Pharmacotherapy. 1998;18(3):456–62.
Shinabarger DL, Marotti KR, Murray RW, Lin AH, Melchior EP, Swaney SM, et al. Mechanism of action of oxazolidinones: effects of linezolid and eperezolid on translation reactions. Antimicrob Agents Chemother. 1997;41(10):2132–6.
Beringer P, Nguyen M, Hoem N, Louie S, Gill M, Gurevitch M, et al. Absolute bioavailability and pharmacokinetics of linezolid in hospitalized patients given enteral feedings. Antimicrob Agents Chemother. 2005;49(9):3676–81.
Stalker DJ, Jungbluth GL. Clinical pharmacokinetics of linezolid, a novel oxazolidinone antibacterial. Clin Pharmacokinet. 2003;42(13):1129–40.
Stalker DJ, Jungbluth GL, Hopkins NK, Batts DH. Pharmacokinetics and tolerance of single- and multiple-dose oral or intravenous linezolid, an oxazolidinone antibiotic, in healthy volunteers. J Antimicrob Chemother. 2003;51(5):1239–46.
Pawsey S, Daley-Yates P, Wajszczuk C. U-1007666 safety, toleration and pharmacokinetics after oral and intravenous administration. Abstract F151. Presented at: First European Congress of Chemotherapy, Federation for the Societies for European Chemotherapy and Infection. 1996 May 14–17; Glasgow, UK.
Brier ME, Stalker DJ, Aronoff GR, Batts DH, Ryan KK, O’Grady M, et al. Pharmacokinetics of linezolid in subjects with renal dysfunction. Antimicrob Agents Chemother. 2003;47(9):2775–80.
Sisson TL, Jungbluth GL, Hopkins NK. Age and sex effects on the pharmacokinetics of linezolid. Eur J Clin Pharmacol. 2002;57(11):793–7.
Vinh DC, Rubinstein E. Linezolid: a review of safety and tolerability. J Infect. 2009;59(Suppl. 1):S59-74.
Gerson SL, Kaplan SL, Bruss JB, Le V, Arellano FM, Hafkin B, et al. Hematologic effects of linezolid: summary of clinical experience. Antimicrob Agents Chemother. 2002;46(8):2723–6.
Soriano A, Gómez J, Gómez L, Azanza JR, Pérez R, Romero F, et al. Efficacy and tolerability of prolonged linezolid therapy in the treatment of orthopedic implant infections. Eur J Clin Microbiol Infect Dis. 2007;26(5):353–6.
Takahashi Y, Takesue Y, Nakajima K, Ichiki K, Tsuchida T, Tatsumi S, et al. Risk factors associated with the development of thrombocytopenia in patients who received linezolid therapy. J Infect Chemother. 2011;17(3):382–7.
Lin Y-H, Wu V-C, Tsai I-J, Ho Y-L, Hwang J-J, Tsau Y-K, et al. High frequency of linezolid-associated thrombocytopenia among patients with renal insufficiency. Int J Antimicrob Agents. 2006;28(4):345–51.
Wu V-C, Wang Y-T, Wang C-Y, Tsai I-J, Wu K-D, Hwang J-J, et al. High frequency of linezolid-associated thrombocytopenia and anemia among patients with end-stage renal disease. Clin Infect Dis. 2006;42(1):66–72.
Morata L, De la Calle C, Gómez-Cerquera JM, Manzanedo L, Casals G, Brunet M, Cobos-Trigueros N, et al. Risk factors associated with high linezolid trough plasma concentrations. Expert Opin Pharmacother. 2016;17(9):1183–7.
Grau S, Morales-Molina JA, Mateu-de Antonio J, Marín-Casino M, Alvarez-Lerma F. Linezolid: low pre-treatment platelet values could increase the risk of thrombocytopenia. J Antimicrob Chemother. 2005;56(2):440–1.
Boak LM, Rayner CR, Grayson ML, Paterson DL, Spelman D, Khumra S, et al. Clinical population pharmacokinetics and toxicodynamics of linezolid. Antimicrob Agents Chemother. 2014;58(4):2334–43.
Crass RL, Cojutti PG, Pai MP, Pea F. Reappraisal of linezolid dosing in renal impairment to improve safety. Antimicrob Agents Chemother. 2019;63(8):e00605-e619.
Niwa T, Suzuki A, Sakakibara S, Kasahara S, Yasuda M, Fukao A, et al. Retrospective cohort chart review study of factors associated with the development of thrombocytopenia in adult Japanese patients who received intravenous linezolid therapy. Clin Ther. 2009;31(10):2126–33.
Cattaneo D, Gervasoni C, Cozzi V, Castoldi S, Baldelli S, Clementi E. Therapeutic drug management of linezolid: a missed opportunity for clinicians? Int J Antimicrob Agents. 2016;48(6):728–31.
Pea F, Furlanut M, Cojutti P, Cristini F, Zamparini E, Franceschi L, et al. Therapeutic drug monitoring of linezolid: a retrospective monocentric analysis. Antimicrob Agents Chemother. 2010;54(11):4605–10.
Pea F, Cojutti PG, Baraldo M. A 10-year experience of therapeutic drug monitoring (TDM) of linezolid in a hospital-wide population of patients receiving conventional dosing: is there enough evidence for suggesting TDM in the majority of patients? Basic Clin Pharmacol Toxicol. 2017;121(4):303–8.
Pea F, Viale P, Cojutti P, Del Pin B, Zamparini E, Furlanut M. Therapeutic drug monitoring may improve safety outcomes of long-term treatment with linezolid in adult patients. J Antimicrob Chemother. 2012;67(8):2034–42.
Sherwin CMT, Kiang TKL, Spigarelli MG, Ensom MHH. Fundamentals of population pharmacokinetic modelling: validation methods. Clin Pharmacokinet. 2012;51(9):573–90.
Welshman IR, Sisson TA, Jungbluth GL, Stalker DJ, Hopkins NK. Linezolid absolute bioavailability and the effect of food on oral bioavailability. Biopharm Drug Dispos. 2001;22(3):91–7.
Sisson TL, Jungbluth GL, Hopkins NK. A pharmacokinetic evaluation of concomitant administration of linezolid and aztreonam. J Clin Pharmacol. 1999;39(12):1277–82.
Dehghanyar P, Bürger C, Zeitlinger M, Islinger F, Kovar F, Müller M, et al. Penetration of linezolid into soft tissues of healthy volunteers after single and multiple doses. Antimicrob Agents Chemother. 2005;49(6):2367–71.
Heinze A, Holzgrabe U. Determination of the extent of protein binding of antibiotics by means of an automated continuous ultrafiltration method. Int J Pharm. 2006;311(1–2):108–12.
Buerger C, Plock N, Dehghanyar P, Joukhadar C, Kloft C. Pharmacokinetics of unbound linezolid in plasma and tissue interstitium of critically ill patients after multiple dosing using microdialysis. Antimicrob Agents Chemother. 2006;50(7):2455–63.
Traunmüller F, Schintler MV, Spendel S, Popovic M, Mauric O, Scharnagl E, et al. Linezolid concentrations in infected soft tissue and bone following repetitive doses in diabetic patients with bacterial foot infections. Int J Antimicrob Agents. 2010;36(1):84–6.
Wiskirchen DE, Shepard A, Kuti JL, Nicolau DP. Determination of tissue penetration and pharmacokinetics of linezolid in patients with diabetic foot infections using in vivo microdialysis. Antimicrob Agents Chemother. 2011;55(9):4170–5.
Tsuji Y, Holford NHG, Kasai H, Ogami C, Heo Y-A, Higashi Y, et al. Population pharmacokinetics and pharmacodynamics of linezolid-induced thrombocytopenia in hospitalized patients. Br J Clin Pharmacol. 2017;83(8):1758–72.
Xie F, Mantzarlis K, Malliotakis P, Koulouras V, Degroote S, Koulenti D, et al. LIMOP Study Collaborators. Pharmacokinetic evaluation of linezolid administered intravenously in obese patients with pneumonia. J Antimicrob Chemother. 2019;74(3):667–74.
Yagi T, Naito T, Doi M, Nagura O, Yamada T, Maekawa M, et al. Plasma exposure of free linezolid and its ratio to minimum inhibitory concentration varies in critically ill patients. Int J Antimicrob Agents. 2013;42(4):329–34.
Douros A, Grabowski K, Stahlmann R. Drug-drug interactions and safety of linezolid, tedizolid, and other oxazolidinones. Expert Opin Drug Metab Toxicol. 2015;11(12):1849–59.
Töpper C, Steinbach CL, Dorn C, Kratzer A, Wicha SG, Schleibinger M, et al. Variable linezolid exposure in intensive care unit patients: possible role of drug-drug interactions. Ther Drug Monit. 2016;38(5):573–8.
Wynalda MA, Hauer MJ, Wienkers LC. Oxidation of the novel oxazolidinone antibiotic linezolid in human liver microsomes. Drug Metab Dispos. 2000;28(9):1014–7.
Egle H, Trittler R, Kümmerer K, Lemmen SW. Linezolid and rifampin: drug interaction contrary to expectations? Clin Pharmacol Ther. 2005;77(5):451–3.
Gebhart BC, Barker BC, Markewitz BA. Decreased serum linezolid levels in a critically ill patient receiving concomitant linezolid and rifampin. Pharmacotherapy. 2007;27(3):476–9.
Gandelman K, Zhu T, Fahmi OA, Glue P, Lian K, Obach RS, et al. Unexpected effect of rifampin on the pharmacokinetics of linezolid: in silico and in vitro approaches to explain its mechanism. J Clin Pharmacol. 2011;51(2):229–36.
Pea F, Cadeo B, Cojutti PG, Pecori D, Bassetti M. Linezolid underexposure in a hypothyroid patient on levothyroxine replacement therapy: a case report. Ther Drug Monit. 2014;36(5):687–9.
Cojutti P, Crapis M, Bassetti M, Hope W, Pea F. Linezolid underexposure in a patient co-treated with venlafaxine. Eur J Clin Pharmacol. 2015;71(10):1285–6.
Slatter JG, Stalker DJ, Feenstra KL, Welshman IR, Bruss JB, Sams JP, et al. Pharmacokinetics, metabolism, and excretion of linezolid following an oral dose of [(14)C]linezolid to healthy human subjects. Drug Metab Dispos. 2001;29(8):1136–45.
Baldelli S, Montrasio C, Cheli S, Viganò O, Bini T, Egidi M, et al. Case of suboptimal linezolid exposure: is there a role for pharmacogenetics? Ther Drug Monit. 2020;42(2):347–8.
Allegra S, Di Paolo A, Cusato J, Fatiguso G, Arrigoni E, Danesi R, et al. A common mdr1 gene polymorphism is associated with changes in linezolid clearance. Ther Drug Monit. 2018;40(5):602–9.
Cojutti P, Pai MP, Pea F. Population pharmacokinetics and dosing considerations for the use of linezolid in overweight and obese adult patients. Clin Pharmacokinet. 2018;57(8):989–1000.
Alffenaar J-WC, Kosterink JGW, van Altena R, van der Werf TS, Uges DRA, Proost JH. Limited sampling strategies for therapeutic drug monitoring of linezolid in patients with multidrug-resistant tuberculosis. Ther Drug Monit. 2010;32(1):97–101.
Srinivas NR, Syed M. Applicability of a single time point strategy for the prediction of area under the concentration curve of linezolid in patients: superiority of Ctrough- over Cmax-derived linear regression models. Drugs R D. 2016;16(1):69–79.
Kamp J, Bolhuis MS, Tiberi S, Akkerman OW, Centis R, de Lange WC, et al. Simple strategy to assess linezolid exposure in patients with multi-drug-resistant and extensively-drug-resistant tuberculosis. Int J Antimicrob Agents. 2017;49(6):688–94.
Andes D, van Ogtrop ML, Peng J, Craig WA. In vivo pharmacodynamics of a new oxazolidinone (linezolid). Antimicrob Agents Chemother. 2002;46(11):3484–9.
Rayner CR, Forrest A, Meagher AK, Birmingham MC, Schentag JJ. Clinical pharmacodynamics of linezolid in seriously ill patients treated in a compassionate use programme. Clin Pharmacokinet. 2003;42(15):1411–23.
Matsumoto K, Shigemi A, Takeshita A, Watanabe E, Yokoyama Y, Ikawa K, et al. Analysis of thrombocytopenic effects and population pharmacokinetics of linezolid: a dosage strategy according to the trough concentration target and renal function in adult patients. Int J Antimicrob Agents. 2014;44(3):242–7.
Hiraki Y, Tsuji Y, Hiraike M, Misumi N, Matsumoto K, Morita K, et al. Correlation between serum linezolid concentration and the development of thrombocytopenia. Scand J Infect Dis. 2012;44(1):60–4.
Matsumoto K, Takeshita A, Ikawa K, Shigemi A, Yaji K, Shimodozono Y, et al. Higher linezolid exposure and higher frequency of thrombocytopenia in patients with renal dysfunction. Int J Antimicrob Agents. 2010;36(2):179–81.
Cattaneo D, Alffenaar J-W, Neely M. Drug monitoring and individual dose optimization of antimicrobial drugs: oxazolidinones. Expert Opin Drug Metab Toxicol. 2016;12(5):533–44.
Abdul-Aziz MH, Alffenaar J-WC, Bassetti M, Bracht H, Dimopoulos G, Marriott D, et al.; Infection Section of European Society of Intensive Care Medicine (ESICM), Pharmacokinetic/Pharmacodynamic and Critically Ill Patient Study Groups of European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Group of International Association of Therapeutic Drug Monitoring and Clinical Toxicology (IATDMCT), Infections in the ICU and Sepsis Working Group of International Society of Antimicrobial Chemotherapy (ISAC). Antimicrobial therapeutic drug monitoring in critically ill adult patients: a position paper. Intensive Care Med. 2020;46(6):1127–53.
Abdelwahab MT, Wasserman S, Brust JCM, Dheda K, Wiesner L, Gandhi NR, et al. Linezolid population pharmacokinetics in South African adults with drug-resistant tuberculosis. Antimicrob Agents Chemother. 2021;65(12):e0138121.
Cattaneo D, Orlando G, Cozzi V, Cordier L, Baldelli S, Merli S, et al. Linezolid plasma concentrations and occurrence of drug-related haematological toxicity in patients with gram-positive infections. Int J Antimicrob Agents. 2013;41(6):586–9.
The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters, version 11.0, 2021. 2021. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_11.0_Breakpoint_Tables.xlsx. Accessed 13 Apr 2022.
Rao GG, Konicki R, Cattaneo D, Alffenaar J-W, Marriott DJE, Neely M; IATDMCT Antimicrobial Scientific Committee. Therapeutic drug monitoring can improve linezolid dosing regimens in current clinical practice: a review of linezolid pharmacokinetics and pharmacodynamics. Ther Drug Monit. 2020;42(1):83–92.
Whitehouse T, Cepeda JA, Shulman R, Aarons L, Nalda-Molina R, Tobin C, et al. Pharmacokinetic studies of linezolid and teicoplanin in the critically ill. J Antimicrob Chemother. 2005;55(3):333–40.
Plock N, Buerger C, Joukhadar C, Kljucar S, Kloft C. Does linezolid inhibit its own metabolism? Population pharmacokinetics as a tool to explain the observed nonlinearity in both healthy volunteers and septic patients. Drug Metab Dispos. 2007;35(10):1816–23.
Abe S, Chiba K, Cirincione B, Grasela TH, Ito K, Suwa T. Population pharmacokinetic analysis of linezolid in patients with infectious disease: application to lower body weight and elderly patients. J Clin Pharmacol. 2009;49(9):1071–8.
Fiaccadori E, Maggiore U, Rotelli C, Giacosa R, Parenti E, Picetti E, et al. Does haemodialysis significantly affect serum linezolid concentrations in critically ill patients with renal failure? A pilot investigation. Nephrol Dial Transplant. 2006;21(5):1402–6.
Swoboda S, Ober MC, Lichtenstern C, Saleh S, Schwenger V, Sonntag H-G, et al. Pharmacokinetics of linezolid in septic patients with and without extended dialysis. Eur J Clin Pharmacol. 2010;66(3):291–8.
Sasaki T, Takane H, Ogawa K, Isagawa S, Hirota T, Higuchi S, et al. Population pharmacokinetic and pharmacodynamic analysis of linezolid and a hematologic side effect, thrombocytopenia, in Japanese patients. Antimicrob Agents Chemother. 2011;55(5):1867–73.
Keel RA, Schaeftlein A, Kloft C, Pope JS, Knauft RF, Muhlebach M, et al. Pharmacokinetics of intravenous and oral linezolid in adults with cystic fibrosis. Antimicrob Agents Chemother. 2011;55(7):3393–8.
Wicha SG, Frey OR, Roehr AC, Pratschke J, Stockmann M, Alraish R, et al. Linezolid in liver failure: exploring the value of the maximal liver function capacity (LiMAx) test in a pharmacokinetic pilot study. Int J Antimicrob Agents. 2017;50(4):557–63.
Zhang S-H, Zhu Z-Y, Chen Z, Li Y, Zou Y, Yan M, et al. Population pharmacokinetics and dosage optimization of linezolid in patients with liver dysfunction. Antimicrob Agents Chemother. 2020;64(6):e00133-e220.
Soraluce A, Barrasa H, Asín-Prieto E, Sánchez-Izquierdo JÁ, Maynar J, Isla A, et al. Novel population pharmacokinetic model for linezolid in critically ill patients and evaluation of the adequacy of the current dosing recommendation. Pharmaceutics. 2020;12(1):54.
Wang X, Wang Y, Yao F, Chen S, Hou Y, Zheng Z, et al. Pharmacokinetics of linezolid dose adjustment for creatinine clearance in critically ill patients: a multicenter, prospective, open-label, observational study. Drug Des Devel Ther. 2021;15:2129–41.
Alghamdi WA, Al-Shaer MH, An G, Alsultan A, Kipiani M, Barbakadze K, et al. Population pharmacokinetics of linezolid in tuberculosis patients: dosing regimen simulation and target attainment analysis. Antimicrob Agents Chemother. 2020;64(10):e01174-e1220.
Ide T, Takesue Y, Ikawa K, Morikawa N, Ueda T, Takahashi Y, et al. Population pharmacokinetics/pharmacodynamics of linezolid in sepsis patients with and without continuous renal replacement therapy. Int J Antimicrob Agents. 2018;51(5):745–51.
Tsuji Y, Yukawa E, Hiraki Y, Matsumoto K, Mizoguchi A, Morita K, et al. Population pharmacokinetic analysis of linezolid in low body weight patients with renal dysfunction. J Clin Pharmacol. 2013;53(9):967–73.
Tietjen AK, Kroemer N, Cattaneo D, Baldelli S, Wicha SG. Population pharmacokinetics and target attainment analysis of linezolid in multidrug-resistant tuberculosis patients. Br J Clin Pharmacol. 2022;88(4):1835–44.
Minichmayr IK, Schaeftlein A, Kuti JL, Zeitlinger M, Kloft C. Clinical determinants of target non-attainment of linezolid in plasma and interstitial space fluid: a pooled population pharmacokinetic analysis with focus on critically ill patients. Clin Pharmacokinet. 2017;56(6):617–33.
Taubert M, Zoller M, Maier B, Frechen S, Scharf C, Holdt L-M, et al. Predictors of inadequate linezolid concentrations after standard dosing in critically ill patients. Antimicrob Agents Chemother. 2016;60(9):5254–61.
Ehmann L, Simon P, Busse D, Petroff D, Dorn C, Huisinga W, et al. Risk of target non-attainment in obese compared to non-obese patients in calculated linezolid therapy. Clin Microbiol Infect. 2020;26(9):1222–8.
Blackman AL, Jarugula P, Nicolau DP, Chui SH, Joshi M, Heil EL, et al. Evaluation of linezolid pharmacokinetics in critically ill obese patients with severe skin and soft tissue infections. Antimicrob Agents Chemother. 2021;65(2):e01619-e1620.
Li S-C, Ye Q, Xu H, Zhang L, Wang Y. Population pharmacokinetics and dosing optimization of linezolid in pediatric patients. Antimicrob Agents Chemother. 2019;63(4):e02387-e2418.
Thibault C, Kassir N, Goyer I, Théorêt Y, Litalien C, Moussa A, et al. Population pharmacokinetics of intravenous linezolid in premature infants. Pediatr Infect Dis J. 2019;38(1):82–8.
Garcia-Prats AJ, Schaaf HS, Draper HR, Garcia-Cremades M, Winckler J, Wiesner L, et al. Pharmacokinetics, optimal dosing, and safety of linezolid in children with multidrug-resistant tuberculosis: combined data from two prospective observational studies. PLoS Med. 2019;16(4):e1002789.
Yang M, Zhao L, Wang X, Sun C, Gao H, Wang X, et al. Population pharmacokinetics and dosage optimization of linezolid in critically ill pediatric patients. Antimicrob Agents Chemother. 2021;AAC.02504-20.
Fang J, Zhang X-S, Zhang C-H, Zhou Z-Y, Han L, Wang Y-X, et al. Model based identification of linezolid exposure–toxicity thresholds in hospitalized patients. Front Pharmacol. 2021;12:2390.
Imperial MZ, Nedelman JR, Conradie F, Savic RM. Proposed linezolid dosing strategies to minimize adverse events for treatment of extensively drug-resistant tuberculosis. Clin Infect Dis. 2021;2021:ciab699. https://doi.org/10.1093/cid/ciab699.
Soriano A, Ortega M, García S, Peñarroja G, Bové A, Marcos M, et al. Comparative study of the effects of pyridoxine, rifampin, and renal function on hematological adverse events induced by linezolid. Antimicrob Agents Chemother. 2007;51(7):2559–63.
Nukui Y, Hatakeyama S, Okamoto K, Yamamoto T, Hisaka A, Suzuki H, et al. High plasma linezolid concentration and impaired renal function affect development of linezolid-induced thrombocytopenia. J Antimicrob Chemother. 2013;68(9):2128–33.
Meagher AK, Forrest A, Rayner CR, Birmingham MC, Schentag JJ. Population pharmacokinetics of linezolid in patients treated in a compassionate-use program. Antimicrob Agents Chemother. 2003;47(2):548–53.
Meyer B, Kornek GV, Nikfardjam M, Karth GD, Heinz G, Locker GJ, et al. Multiple-dose pharmacokinetics of linezolid during continuous venovenous haemofiltration. J Antimicrob Chemother. 2005;56(1):172–9.
Roger C, Muller L, Wallis SC, Louart B, Saissi G, Lipman J, et al. Population pharmacokinetics of linezolid in critically ill patients on renal replacement therapy: comparison of equal doses in continuous venovenous haemofiltration and continuous venovenous haemodiafiltration. J Antimicrob Chemother. 2016;71(2):464–70.
Hendershot P, Jungbluth G, Cammarata S. Pharmacokinetics of linezolid in patients with liver disease. J Antimicrob Chemother. 1999;44(Suppl. A):55.
Ikuta S, Yasui C, Aihara T, Yoshie H, Iida H, Beppu N, et al. Chronic liver disease increases the risk of linezolid-related thrombocytopenia in methicillin-resistant Staphylococcus aureus-infected patients after digestive surgery. J Infect Chemother. 2011;17(3):388–91.
Zhang Y-M, Yu W, Zhou N, Li J-Z, Xu L-C, Xie Z-Y, et al. High frequency of thrombocytopenia in patients with acute-on-chronic liver failure treated with linezolid. Hepatobiliary Pancreat Dis Int. 2015;14(3):287–92.
Luque S, Muñoz-Bermudez R, Echeverría-Esnal D, Sorli L, Campillo N, Martínez-Casanova J, et al. Linezolid dosing in patients with liver cirrhosis: standard dosing risk toxicity. Ther Drug Monit. 2019;41(6):732–9.
Stockmann M, Lock JF, Riecke B, Heyne K, Martus P, Fricke M, et al. Prediction of postoperative outcome after hepatectomy with a new bedside test for maximal liver function capacity. Ann Surg. 2009;250(1):119–25.
Kaffarnik MF, Lock JF, Vetter H, Ahmadi N, Lojewski C, Malinowski M, et al. Early diagnosis of sepsis-related hepatic dysfunction and its prognostic impact on survival: a prospective study with the LiMAx test. Crit Care. 2013;17(5):R259.
Cattaneo D, Fusi M, Cozzi V, Baldelli S, Bonini I, Gervasoni C, et al. Supra-therapeutic linezolid trough concentrations in elderly patients: a call for action? Clin Pharmacokinet. 2021;60(5):603–9.
Tinelli M, Gervasoni C, Piazza M, Terzi R, Cozzi V, Maffezzini E, et al. Is it time to revise linezolid dose in elderly patients? Eur J Clin Pharmacol. 2017;73(10):1335–6.
Cheng C-N, Wu C-C, Kuo C-H, Wang C-C, Wang J-T, Lin Y-T, et al. Impact of high plasma concentrations of linezolid in Taiwanese adult patients: therapeutic drug monitoring in improving adverse drug reactions. J Formos Med Assoc. 2021;120(1 Pt 2):466–75.
Blanco J, Muriel-Bombín A, Sagredo V, Taboada F, Gandía F, Tamayo L, et al.; Grupo de Estudios y Análisis en Cuidados Intensivos. Incidence, organ dysfunction and mortality in severe sepsis: a Spanish multicentre study. Crit Care. 2008;12(6):R158.
Levy MM, Evans LE, Rhodes A. The surviving sepsis campaign bundle: 2018 update. Intensive Care Med. 2018;44(6):925–8.
Dong H, Wang X, Dong Y, Lei J, Li H, You H, et al. Clinical pharmacokinetic/pharmacodynamic profile of linezolid in severely ill intensive care unit patients. Int J Antimicrob Agents. 2011;38(4):296–300.
Zoller M, Maier B, Hornuss C, Neugebauer C, Döbbeler G, Nagel D, et al. Variability of linezolid concentrations after standard dosing in critically ill patients: a prospective observational study. Crit Care. 2014;18(4):R148.
Sazdanovic P, Jankovic SM, Kostic M, Dimitrijevic A, Stefanovic S. Pharmacokinetics of linezolid in critically ill patients. Expert Opin Drug Metab Toxicol. 2016;12(6):595–600.
Kearns GL, Abdel-Rahman SM, Blumer JL, Reed MD, James LP, Jacobs RF, et al.; Pediatric Pharmacology Research Unit Network. Single dose pharmacokinetics of linezolid in infants and children. Pediatr Infect Dis J. 2000;19(12):1178–84.
Kearns GL, Jungbluth GL, Abdel-Rahman SM, Hopkins NK, Welshman IR, Grzebyk RP, et al.; Pediatric Pharmacology Research Unit Network. Impact of ontogeny on linezolid disposition in neonates and infants. Clin Pharmacol Ther. 2003;74(5):413–22.
Vo M, Rubino CM, Cirincione BB, Bruss J, Jungbluth GL. Pharmacokinetic-pharmacodynamic analysis of data from a phase III trial of linezolid IV/PO for the treatment of resistant Gram-positive bacterial infections in children. Clin Pharmacol Ther. 2003;73(2):P74.
Cojutti P, Maximova N, Crichiutti G, Isola M, Pea F. Pharmacokinetic/pharmacodynamic evaluation of linezolid in hospitalized paediatric patients: a step toward dose optimization by means of therapeutic drug monitoring and Monte Carlo simulation. J Antimicrob Chemother. 2015;70(1):198–206.
Luque S, Hope W, Sorli L, Muñoz-Bermudez R, Campillo N, Barceló-Vidal J, et al. Dosage individualization of linezolid: precision dosing of linezolid to optimize efficacy and minimize toxicity. Antimicrob Agents Chemother. 2021;65(6):e02490-e2520.
Bhalodi AA, Papasavas PK, Tishler DS, Nicolau DP, Kuti JL. Pharmacokinetics of intravenous linezolid in moderately to morbidly obese adults. Antimicrob Agents Chemother. 2013;57(3):1144–9.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This work was partially supported by ISCIII co-funded by FEDER (ICI21/00043) and Axencia Galega Innovación (Grupos de Potencial Crecimiento IN607B2020/11). EBV, LGQ, ACB, CMG, and AFF are grateful to the Carlos III Health Institute for financing their personal contracts: CM20/00135, CM20/00024, CM21/00114, JR20/00026, and JR18/00014.
Conflicts of Interest/Competing Interests
None of the authors has any conflicts of interest that are directly relevant to the content of this review.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Authors’ Contributions
Conceptualization, funding acquisition, and supervision were performed by AFF, CMG, and VMS. IZF, MGB, and MCT were responsible for methodology design. Material preparation, data collection, and original draft preparation were performed by EBV, LGQ, and ACB. Review and editing were performed by MGB and MCT. All authors revised the manuscript critically for important intellectual content and approved the final version.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bandín-Vilar, E., García-Quintanilla, L., Castro-Balado, A. et al. A Review of Population Pharmacokinetic Analyses of Linezolid. Clin Pharmacokinet 61, 789–817 (2022). https://doi.org/10.1007/s40262-022-01125-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-022-01125-2