Abstract
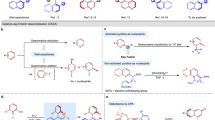
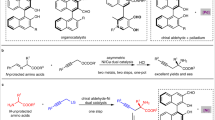
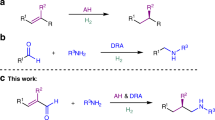
Chiral hydroxylamines are vital substances in bioscience and versatile subunits in the preparation of a variety of functional molecules. However, asymmetric and non-asymmetric synthetic approaches to these compounds are far from satisfactory. Although atom-economic metal-catalysed asymmetric hydrogenations have been studied for over 50 years, the asymmetric hydrogenation of oximes to the corresponding chiral hydroxylamines remains challenging because of the labile N–O bond and inert C=N bond. Here we report an environmentally friendly, earth-abundant, transition-metal nickel-catalysed asymmetric hydrogenation of oximes, affording the corresponding chiral hydroxylamines with up to 99% yield, 99% e.e. and with a substrate/catalyst ratio of 1,000. Computational results indicate that the weak interactions between the catalyst and substrate play crucial roles not only in the transition states, but also during the approach of the substrate to the catalyst, by selectively reducing the reaction barriers and thus improving the reaction efficiency and securing the generation of chirality.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the Article and its Supplementary Information. For the experimental procedures, data for the NMR and HPLC analyses and cartesian coordinates of the optimized structures, see the Supplementary Methods in the Supplementary Information. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition nos. CCDC 2059388 (1a) and 2059391 (2a). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Rappoport, Z. & Lebman, J. F. The Chemistry of Hydroxylamines, Oximes and Hydroxamic Acids (Wiley-VCH, 2008).
Pattabiraman, V. R. & Bode, J. W. Rethinking amide bond synthesis. Nature 480, 471–479 (2011).
Tan, K. L. Temporary intramolecularity. Nat. Chem. 4, 253–254 (2012).
Paudyal, M. P. et al. Dirhodium-catalyzed C-H arene amination using hydroxylamines. Science 353, 1144–1147 (2016).
Tang, W. & Zhang, X. New chiral phosphorus ligands for enantioselective hydrogenation. Chem. Rev. 103, 3029–3069 (2003).
Johnson, N. B., Lennon, I. C., Moran, P. H. & Ramsden, J. A. Industrial-scale synthesis and applications of asymmetric hydrogenation catalysts. Acc. Chem. Res. 40, 1291–1299 (2007).
Xie, J.-H., Zhu, S.-F. & Zhou, Q.-L. Transition metal-catalyzed enantioselective hydrogenation of enamines and imines. Chem. Rev. 111, 1713–1760 (2011).
Chen, Q.-A., Ye, Z.-S., Duan, Y. & Zhou, Y.-G. Homogeneous palladium-catalyzed asymmetric hydrogenation. Chem. Soc. Rev. 42, 497–511 (2013).
Zhang, Z., Butt, N. A. & Zhang, W. Asymmetric hydrogenation of nonaromatic cyclic substrates. Chem. Rev. 116, 14769–14827 (2016).
Seo, C. S. G. & Morris, R. H. Catalytic homogeneous asymmetric hydrogenation: successes and opportunities. Organometallics 38, 47–65 (2019).
Maj, A. M., Suisse, I. & Agbossou-Niedercorn, F. Asymmetric hydrogenation of 2,3-dihydro-1H-inden-1-one oxime and derivatives. Tetrahedron Asymmetry 27, 268–273 (2016).
Huang, K., Li, S., Chang, M. & Zhang, X. Rhodium-catalyzed enantioselective hydrogenation of oxime acetates. Org. Lett. 15, 484–487 (2013).
Krasik, P. & Alper, H. The ruthenium catalyzed asymmetric hydrogenation of oximes using binap as the chiral ligand. Tetrahedron Asymmetry 3, 1283–1288 (1992).
Xie, Y., Mi, A., Jiang, Y. & Liu, H. Enantioselective hydrogenation of ketone oxime catalyzed by Ir-DPAMPP complex. Synth. Commun. 31, 2767–2771 (2001).
Knowles, W. S. & Sabacky, M. J. Catalytic asymmetric hydrogenation employing a soluble, optically active, rhodium complex. Chem. Commun. 1968, 1445–1446 (1968).
Knowles, W. S. Asymmetric hydrogenations (Nobel Lecture). Angew. Chem. Int. Ed. 41, 1998–2007 (2002).
Noyori, R. Asymmetric catalysis: science and opportunities (Nobel Lecture). Angew. Chem. Int. Ed. 41, 2008–2022 (2002).
Bolotin, D. S., Bokach, N. A., Demakova, M. Y. & Kukushkin, V. Y. Metal-involving synthesis and reactions of oximes. Chem. Rev. 117, 13039–13122 (2017).
Chu, Y., Shan, Z., Liu, D. & Sun, N. Asymmetric reduction of oxime ethers promoted by chiral spiroborate esters with an O3BN framework. J. Org. Chem. 71, 3998–4001 (2006).
Huang, X. et al. Asymmetric synthesis of primary amines via the spiroborate-catalyzed borane reduction of oxime ethers. Org. Lett. 9, 1793–1795 (2007).
Huang, K. et al. Highly enantioselective borane reduction of heteroaryl and heterocyclic ketoxime ethers catalyzed by novel spiroborate ester derived from diphenylvalinol: application to the synthesis of nicotine analogues. J. Org. Chem. 73, 4017–4026 (2008).
Huang, K., Ortiz-Marciales, M., Stepanenko, V., Jesús, M. D. & Correa, W. A practical and efficient route for the highly enantioselective synthesis of mexiletine analogues and novel β-thiophenoxy and pyridyl ethers. J. Org. Chem. 73, 6928–6931 (2008).
Mohr, J. & Oestreich, M. B(C6F5)3-catalyzed hydrogenation of oxime ethers without cleavage of the N-O bond. Angew. Chem. Int. Ed. 53, 13278–13281 (2014).
Mas-Roselló, J., Smejkal, T. & Cramer, N. Iridium-catalyzed acid-assisted asymmetric hydrogenation of oximes to hydroxylamines. Science 368, 1098–1102 (2020).
Guin, D. & Gruebele, M. Weak chemical interactions that drive protein evolution: crowding, sticking and quinary structure in folding and function. Chem. Rev. 119, 10691–10717 (2019).
Fraser, H. B., Hirsh, A. E., Steinmetz, L. M., Scharfe, C. & Feldman, M. W. Evolutionary rate in the protein interaction network. Science 296, 750–752 (2002).
Wagner, J. P. & Schreiner, P. R. London dispersion in molecular chemistry reconsidering steric effects. Angew. Chem. Int. Ed. 54, 12274–12296 (2015).
Thomas, A. A. et al. Mechanistically guided design of ligands that significantly improve the efficiency of CuH-catalyzed hydroamination reactions. J. Am. Chem. Soc. 140, 13976–13984 (2018).
Ge, L. et al. Iron-catalysed asymmetric carboazidation of styrenes. Nat. Catal. 4, 28–35 (2021).
Liu, C. et al. Manganese-catalyzed asymmetric hydrogenation of quinolines enabled by π-π interaction. Angew. Chem. Int. Ed. 60, 5108–5113 (2021).
Chen, J. et al. Pd(OAc)2-catalyzed asymmetric hydrogenation of sterically hindered N-tosylimines. Nat. Commun. 9, 5000 (2018).
Zhang, J. et al. Chemo- and enantioselective hydrogenation of ɑ-formyl enamides: an efficient access to chiral ɑ-amido aldehydes. Angew. Chem. Int. Ed. 58, 11505–11512 (2019).
Chen, J. & Gridnev, I. D. Size is important: artificial catalyst mimics behavior of natural enzymes. iScience 23, 100960 (2020).
Li, B., Chen, J., Zhang, Z., Gridnev, I. D. & Zhang, W. Nickel-catalyzed asymmetric hydrogenation of N-sulfonyl imines. Angew. Chem. Int. Ed. 58, 7329–7334 (2019).
Hu, Y. et al. Nickel-catalyzed asymmetric hydrogenation of 2-amidoacrylates. Angew. Chem. Int. Ed. 59, 5371–5375 (2020).
Liu, D. et al. Ni-catalyzed asymmetric hydrogenation of N-aryl imino esters for the efficient synthesis of chiral α-aryl glycines. Nat. Commun. 11, 5935 (2020).
Hu, Y. et al. Cobalt‐catalyzed asymmetric hydrogenation of C=N bond enabled by assisted coordination and non‐bonding interaction. Angew. Chem. Int. Ed. 58, 15767–15771 (2019).
Zhang, J. et al. Asymmetric hydrogenation of γ-branched allylamines for the efficient synthesis of γ-chirogenic amines. Nat. Sci. 1, e10021 (2021).
Li, Y.-Y., Yu, S.-L., Shen, W.-Y. & Gao, J.-X. Iron‑, cobalt- and nickel-catalyzed asymmetric transfer hydrogenation and asymmetric hydrogenation of ketones. Acc. Chem. Res. 48, 2587–2598 (2015).
Zhang, Z., Butt, N. A., Zhou, M., Liu, D. & Zhang, W. Asymmetric transfer and pressure hydrogenation with earth‐abundant transition metal catalysts. Chin. J. Chem. 36, 443–454 (2018).
Alig, L., Fritz, M. & Schneider, S. First-row transition metal (de)hydrogenation catalysis based on functional pincer ligands. Chem. Rev. 119, 2681–2751 (2019).
Liu, Y., Dong, X.-Q. & Zhang, X. Recent advances of nickel-catalyzed homogeneous asymmetric hydrogenation. Chin. J. Org. Chem. 40, 1096–1104 (2020).
Agbossou-Niedercorn, F. & Michon, C. Bifunctional homogeneous catalysts based on first row transition metals in asymmetric hydrogenation. Coordin. Chem. Rev. 425, 213523 (2020).
Friedfeld, M. R. et al. Cobalt precursors for high-throughput discovery of base metal asymmetric alkene hydrogenation catalysts. Science 342, 1076–1080 (2013).
Zuo, W., Lough, A. J., Li, Y. F. & Morris, R. H. Amine(imine)diphosphine iron catalysts for asymmetric transfer hydrogenation of ketones and imines. Science 342, 1080–1083 (2013).
Friedfeld, M. R., Zhong, H., Ruck, R. T., Shevlin, M. & Chirik, P. J. Cobalt-catalyzed asymmetric hydrogenation of enamides enabled by single-electron reduction. Science 360, 888–893 (2018).
Acknowledgements
We dedicate this work to Professor Tsuneo Imamoto for his 80th birthday. We thank the National Key R&D Program of China (no. 2018YFE0126800, W.Z.), National Natural Science Foundation of China (nos. 21620102003, W.Z.; 21991112, W.Z.; 21772119, J.C.; 21702134, J.C.) and Shanghai Municipal Education Commission (no. 201701070002E00030, W.Z.) for financial support. We thank the Instrumental Analysis Center of SJTU for characterization.
Author information
Authors and Affiliations
Contributions
W.Z. directed the project. B.L. conducted most of the synthetic experiments. D.L. conducted some of the synthetic experiments. I.D.G. conducted the density functional theory computational study. J.C., B.L., W.Z. and I.D.G. wrote the original draft of the manuscript. J.C., W.Z., B.L. and I.D.G. reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Tomas Smeijkal, Jianrong Zhou and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–7 and Tables 1–8. Synthesis and characterization data, supplementary discussion, computational and procedural details, crystallographic data, NMR spectra, HPLC traces.
Supplementary Data 1
Crystallographic data for compound 1a; CCDC reference 2059388
Supplementary Data 2
Crystallographic data for compound 2a; CCDC reference 2059391
Supplementary Data 3
Contains the Cartesian coordinates of computational structures
Rights and permissions
About this article
Cite this article
Li, B., Chen, J., Liu, D. et al. Nickel-catalysed asymmetric hydrogenation of oximes. Nat. Chem. 14, 920–927 (2022). https://doi.org/10.1038/s41557-022-00971-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-022-00971-8
This article is cited by
-
A 13-million turnover-number anionic Ir-catalyst for a selective industrial route to chiral nicotine
Nature Communications (2023)
-
Electrosynthesis of amino acids from NO and α-keto acids using two decoupled flow reactors
Nature Catalysis (2023)
-
Divergent synthesis of chiral amines via Ni-catalyzed chemo- and enantioselective hydrogenation of alkynone imines
Science China Chemistry (2023)
-
Asymmetric synthesis of bedaquiline based on bimetallic activation and non-covalent interaction promotion strategies
Science China Chemistry (2022)