Abstract
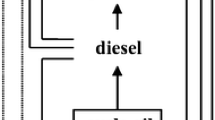
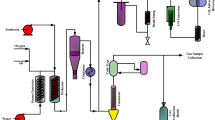
A dynamic model was developed to investigate the impact of operating conditions on the main output variables of the fluidized catalytic cracking (FCC) process with a high-efficiency regenerator and to determine the optimal amounts of operating variables, at the Abadan refinery FCC unit in Iran. To determine the rate constants in the developed kinetic model and other related constants in the developed model, a wide range of industrial data were gathered from the targeted process over several months. Through applying an adjusted dynamic model, the effect of gradual increases in feed preheat temperature (350–500 K) on the yield of gasoline and LCO was investigated, and increases in both yields were observed. The effects of sudden changes in feed preheat temperature, feed and regenerated catalyst flow rate on gasoline yield were also examined. The results showed that a sudden 6.9% increase in feed, a sudden 30K decrease in temperature and a sudden 1.12% decrease in catalyst flow rate resulted in 2%, 0.27% and 0.5% decreases in gasoline, respectively. Furthermore, potential methods for neutralizing these negative effects on the gasoline yield were investigated. Finally, the operating conditions were optimized to improve the gasoline and octane number. Three different optimization cases were studied. The profitability of the unit increased about $2.5–3.8 million per year. A reduction in energy consumption of 12,500 to 21,000 Gj/yr was achieved. The amount of feed and the catalyst flow rate were also decreased by 1.5% and 0.2%–0.9%, respectively.
Similar content being viewed by others
Abbreviations
- A:
-
cross-sectional area [m]
- Aij :
-
pre-exponential factor of the reaction lump i→lump j [m3/(s kg) or s−1]
- Ci :
-
molar concentration of lump i [mol/m3]
- Ĉi :
-
mass concentration of lump i [kg/m3]
- CP :
-
specific heat capacity [j/kg K]
- D:
-
diameter [m]
- Eij :
-
activation energy of the reaction lump i lump j [J/mol]
- EC :
-
energy parameter of the carbon combustion reaction [J/mol]
- E saj :
-
activation energy of the gas-solid reaction [J/mol]
- E gaj :
-
activation energy of the gas reaction [J/mol]
- F:
-
mass flow rate [kg/s]
- FG:
-
flue gas
- Gc :
-
solid mass flux [kg/m2 s]
- GLN:
-
gasoline
- g:
-
gravity acceleration [m/s2]
- H:
-
enthalpy [m/s2]
- kC :
-
rate coefficient of the carbon combustion reaction
- k gj :
-
kinetic rate constant of the gas reaction j [mol s−1 m−3]
- k sj :
-
kinetic rate constant of the gas-solid reaction j [mol s−1 kg−1 cat]
- L:
-
height [m]
- LCO:
-
light cycle oil
- LPG:
-
liquefied petroleum gas
- Mw:
-
molecular weight [kg/mol]
- MRE:
-
mean relative error
- N:
-
molar flow rate [mol/s]
- nij :
-
order of the reaction lump i→lump j
- P:
-
pressure [Pa]
- Qloss :
-
heat loss [J/s]
- Q 0r :
-
Global reaction heat at standard conditions [J/s]
- R:
-
Universal gas constant [J/mol K]
- rC :
-
intrinsic carbon combustion rate [mol (kg catalyst)−1 s−1]
- rij :
-
rate of reaction lump i→ lump j [kg/m3 s]
- ri :
-
rate of formation of i [kg/m3 s]
- r gj :
-
rate of gas reaction j [mol/m3 s]
- r sj :
-
rate of gas-solid reaction j [mol/kg s]
- RMSE:
-
root mean square error
- Sp. Gr.:
-
specific gravity
- T:
-
temperature [K]
- t:
-
time [s]
- u:
-
superficial velocity [m/s]
- U:
-
overall heat transfer coefficient [J/m2 s K]
- V:
-
volume [m3]
- W:
-
inventory [kg]
- xi :
-
mass fraction of i
- Y catck :
-
catalytic coke content of catalyst [kg coke/kg catalyst]
- yi :
-
molar fraction of i
- Yi :
-
component i (in coke) content of catalyst
- z:
-
axial coordinate[m]
- ∆H:
-
heat of reaction [J/kg]
- ∆Hcrk :
-
heat of cracking per unit mass of VGO [J/kg]
- α c :
-
deactivation constant [kg catalyst/kg catalytic coke]
- σ :
-
intrinsic CO2/CO molar ratio in coke [dimensionless]
- ε :
-
volume fraction [dimensionless]
- φ :
-
catalyst deactivation function [dimensionless]
- ρ :
-
density [kg/m3]
- \(\vartheta _{ji}^s\) :
-
stoichiometric coefficient of lump i in gas-solid reaction j [dimensionless]
- \(\vartheta _{ji}^g\) :
-
stoichiometric coefficient of lump i in gas reaction j [dimensionless]
- a:
-
air
- c:
-
catalyst
- ck:
-
coke
- FB:
-
freeboard section
- g:
-
gas
- LF:
-
lift section
- m:
-
mean
- ov:
-
feed vapor
- rc:
-
recirculated catalyst
- RG:
-
regenerator section
- rs:
-
riser
- surr:
-
surrounding
- tf:
-
total feed
- VGO:
-
vacuum gas oil
- 1:
-
refers to the lift and the combustor section
- 2:
-
refers to the dense bed and freeboard
References
R. Sadeghbeigi, Fluid catalytic cracking handbook, Elsevier (1995).
V. W. Weekman Jr. and D. M. Nace, AIChE J., 16, 397 (1970).
L. S. Lee, Y. W. Chen, T. N. Huang and W. Y. Pan, Can. J. Chem. Eng., 67, 615 (1989).
J. Ancheyta-Juárez F. López-Isunza, E. Aguilar-Rodríguez and J. C. Moreno-Mayorga, Ind. Eng. Chem. Res., 36, 5170 (1997).
M. Fabulic Ruszkowski, Z. Gomzi and T. Tomic, Chem. Biochem. Eng. Q., 20, 61 (2006).
G. Bollas, A. Lappas, D. Iatridis and I. Vasalos, Catal. Today, 127, 31 (2007).
J. Ancheyta-Juárez and J. A. Murillo-Hernández, Energy Fuels, 14, 373 (2000).
L. Yen, R. Wrench and A. Ong, Oil Gas J.; (United States), 86 (1988).
J. Ancheyta-Juarez and R. Sotelo-Boyas, Energy Fuels, 14, 1226 (2000).
K. Sertić-Bionda, Z. Gomzi and M. Mužic, Chem. Eng. Commun., 197, 275 (2009).
A. Blasetti and H. de Lasa, Ind. Eng. Chem. Res., 36, 3223 (1997).
H. Farag, A. Blasetti and H. de Lasa, Ind. Eng. Chem. Res., 33, 3131 (1994).
I. Pitault, M. Forissier and J. R. Bernard, Can. J. Chem. Eng., 73, 498 (1995).
S. Sadighi, A. Ahmad and M. Rashidzadeh, Korean J. Chem. Eng., 27, 1099 (2010).
Y. M. John, M. A. Mustafa, R. Patel and I. M. Mujtaba, Fuel, 235, 1436 (2019).
A. G. Sani, H. A. Ebrahim and M. Azarhoosh, Fuel, 225, 322 (2018).
H. Ali and S. Rohani, Chem. Eng. Technol.: Ind. Chem.-Plant Equipment-Process Eng.-Biotechnol., 20, 118 (1997).
A. Arbel, Z. Huang, I. H. Rinard, R. Shinnar and A. V. Sapre, Ind. Eng. Chem. Res., 34, 1228 (1995).
S. M. Jacob, B. Gross, S. E. Voltz and V. W. Weekman Jr., AIChE J., 22, 701 (1976).
A. Secchi, M. Santos, G. Neumann and J. Trierweiler, Comput. Chem. Eng., 25, 851 (2001).
S. Kim, J. Urm, D. S. Kim, K. Lee and J. M. Lee, Korean J. Chem. Eng., 35, 2327 (2018).
I.-S. Han and C.-B. Chung, Chem. Eng. Sci., 56, 1951 (2001).
Y. M. John, R. Patel and I. M. Mujtaba, Comput. Chem. Eng., 106, 730 (2017).
T. Takatsuka, S. Sato, Y. Morimoto and H. Hashimoto, Int. Chem. Eng., 27, 107 (1987).
J. Fernandes, J. J. Verstraete, C. C. Pinheiro, N. Oliveira and F. R. Ribeiro, Comput. Aided Chem. Eng., 20, 589 (2005).
J. L. Fernandes, C. I. Pinheiro, N. Oliveira and F. R. Ribeiro, Comput. Aided Chem. Eng., 21, 1575 (2006).
J. L. Fernandes, C. I. Pinheiro, N. M. Oliveira, A. I. Neto and F. R. Ribeiro, Chem. Eng. Sci., 62, 6308 (2007).
J. L. Fernandes, L. H. Domingues, C. I. Pinheiro, N. M. Oliveira and F. R. Ribeiro, Fuel, 97, 97 (2012).
I.-S. Han, J. B. Riggs and C.-B. Chung, Chem. Eng. Process.: Process Intensification, 43, 1063 (2004).
J. R. Hernández-Barajas, R. Vázquez-Román and D. Salazar-Sotelo, Fuel, 85, 849 (2006).
R. B. Kasat, D. Kunzru, D. Saraf and S. K. Gupta, Ind. Eng. Chem. Res., 41, 4765 (2002).
J. Souza, J. Vargas, O. Von Meien, W. Martignoni and J. Ordonez, J. Chem. Technol. Biotechnol.: Int. Res. Process, Environ. Clean Technol., 84, 343 (2009).
E. Almeida Nt and A. Secchi, Brazilian J. Chem. Eng., 28, 117 (2011).
A. T. Jarullah, N. A. Awad and I. M. Mujtaba, Fuel, 206, 657 (2017).
C. Chen, N. Lu, L. Wang and Y. Xing, Comput. Chem. Eng., 150, 107336 (2021).
J. Biswas and I. Maxwell, Studies Surf. Sci. Catal., 49, 1263 (1989).
J. Biswas and I. Maxwell, Appl. Catal., 58, 1 (1990).
J. Biswas and I. Maxwell, Appl. Catal., 58, 19 (1990).
T. Myrstad, Appl. Catal. A: Gen., 155, 87 (1997).
H. Gonzalez, J. Ramirez, A. Gutierrez-Alejandre, P. Castillo, T. Cortez and R. Zarate, Catal. Today, 98, 181 (2004).
K.-H. Lee, Y.-W. Lee, J.-D. Kim, K.-S. Jeon and B.-H. Ha, Korean J. Chem. Eng., 14, 445 (1997).
M. Davoodpour, R. Tafreshi, A. A. Khodadadi and Y. Mortazavi, Korean J. Chem. Eng., 34, 681 (2017).
J. S. Magee and M. M. Mitchell, Fluid catalytic cracking: Science and technology, Elsevier (1993).
J. L. Fernandes, J. J. Verstraete, C. I. Pinheiro, N. M. Oliveira and F. R. Ribeiro, Chem. Eng. Sci., 62, 1184 (2007).
C. Daly, N. Tidjani, G. Martin and J. Roesler, Détermination des paramétres cinétiques dans la régénération des catalyseurs de FCC, Technical Report No. 56010, Institut Français du Pétrole (2001).
M. Zwinkels and L. Nougier, FCC regenerator simulation model, Technical Report No. 44143, Institut Français du Pétrole (1997).
G. Wang, S. Lin, W. Mo, C. Peng and G. Yang, Ind. Eng. Chem. Process Des. Dev., 25(3), 626 (1986).
B. Alsadik, Adjustment models in 3D geomatics and computational geophysics: With MATLAB examples, Elsevier (2019).
D. Pugliese, F. Bella, V. Cauda, A. Lamberti, A. Sacco, E. Tresso and S. Bianco, ACS Appl. Mater. Interfaces, 5, 11288 (2013).
Acknowledgement
The financial support provided by the Research and Development of Abadan refinery complex is greatly appreciated.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yaghoubi, K., Gilani, N., Abghari, S.Z. et al. Study of gradual and sudden operating condition variations to optimize energy and mass consumption of an industrial fluidized catalytic cracking (FCC) unit with a high-efficiency regenerator. Korean J. Chem. Eng. 39, 1673–1687 (2022). https://doi.org/10.1007/s11814-022-1151-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-022-1151-y