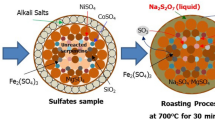
Abstract
In the present study, the leaching behavior of scandium from limonitic laterites under sulfation roasting–water leaching (SAL) was explored. The mineralogical analysis of limonitic laterites showed that scandium was associated with the iron phase. The roasting temperature played an important role in the iron phase conversion during the sulfation roasting process. With an increase in the roasting temperature from 100 to 800 °C, the iron phase (goethite, magnetite, and hematite) gradually converted to monoclinic Fe2(SO4)3 (400 °C), then to rhombohedral Fe2(SO4)3 (600 °C), and finally to hematite (800 °C). Due to the iron phase conversion mechanism, the leaching efficiency of scandium gradually increased first and then decreased with the increase of roasting temperature from 100 to 800 °C. When limonitic laterites roasted at temperature of 600 °C for 2 h with sulfuric acid/laterite ratio of 1/4 (mL/g), a total of 81.15% of scandium and only 0.37% of iron were extracted into leaching solution at 30 °C with the liquid-to-solid ratio of 4:1 (mL/g) for 1 h with agitation. The SAL process enables the selective and efficient enrichment of scandium from low-grade limonitic laterites with lower costs for equipment and operation compared to high-pressure acid leaching (HPAL), lower acid consumption, and lower dissolution of iron compared to atmospheric pressure acid leaching (AL).
Graphical Abstract











Similar content being viewed by others
References
Chasse M, Griffin WL, O’Reilly SY, Calas G (2017) Scandium speciation in a world-class lateritic deposit. Geochem Perspect Lett 3(2):105–113. https://doi.org/10.7185/geochemlet.1711
Kim J, Azimi G (2020) An innovative process for extracting scandium from nickeliferous laterite ore: carbothermic reduction followed by NaOH cracking. Hydrometallurgy 191:1–11. https://doi.org/10.1016/j.hydromet.2019.105194
Luo J, Li G, Rao M, Peng Z, Zhang Y, Jiang T (2015) Atmospheric leaching characteristics of nickel and iron in limonitic laterite with sulfuric acid in the presence of sodium sulfite. Miner Eng 78:38–44. https://doi.org/10.1016/j.mineng.2015.03.030
Meshram P, Abhilash PBD (2019) Advanced review on extraction of nickel from primary and secondary sources. Miner Process Extr Metall Rev 40(3):157–193. https://doi.org/10.1080/08827508.2018.1514300
Van der Ent A, Baker AJM, van Balgooy MMJ, Tjoa A (2013) Ultramafic nickel laterites in Indonesia (Sulawesi, Halmahera): Mining, nickel hyperaccumulators and opportunities for phytomining. J Geochem Explor 128:72–79. https://doi.org/10.1016/j.gexplo.2013.01.009
Yan K, Liu L, Zhao H, Tian L, Xu Z, Wang R (2021) Study on extraction separation of thioarsenite acid in alkaline solution by CO32-type Tri-n-octylmethyl-ammonium chloride. Front Chem 8:1–13. https://doi.org/10.3389/fchem.2020.592837
Garces-Granda A, Lapidus GT, Restrepo-Baena OJ (2018) The effect of calcination as pre treatment to enhance the nickel extraction from low-grade laterites. Miner Eng 120:127–131. https://doi.org/10.1016/j.mineng.2018.02.019
Xiao J, Xiong W, Zou K, Chen T, Wang Z (2021) Extraction of nickel from magnesia-nickel silicate ore. J Sustain Metall 7:642–652. https://doi.org/10.1007/s40831-021-00364-0
Guo X, Zhang C, Tian Q, Yu D (2021) Liquid metals dealloying as a general approach for the selective extraction of metals and the fabrication of nanoporous metals: a review. Mater Today Commun 26(4):102007. https://doi.org/10.1016/j.mtcomm.2020.102007
Makuza B, Tian Q, Guo X, Chattopadhyay K, Yu D (2021) Pyrometallurgical options for recycling spent lithium-ion batteries: a comprehensive review. J Power Sources 491:229–622. https://doi.org/10.1016/j.jpowsour.2021.229622
Chang Y, Zhai X, Li B, Yan F (2010) Removal of iron from acidic leach liquor of lateritic nickel ore by goethite precipitate. Hydrometallurgy 101(1–2):84–87. https://doi.org/10.1016/j.hydromet.2009.11.014
Zhao D, Ma B, Shi B, Zhou Z, Wang C (2020) Mineralogical characterization of limonitic laterite from Africa and its proposed processing route. J Sustain Metall 6(3):491–503. https://doi.org/10.1007/s40831-020-00290-7
Tian Q, Dong B, Guo X, Xu Z, Wang Q, Li D, Yu D (2021) Comparative atmospheric leaching characteristics of scandium in two different types of laterite nickel ore from Indonesia. Miner Eng 173:107212. https://doi.org/10.1016/j.mineng.2021.107212
Pintowantoro S, Widyartha AB, Setiyorini Y, Abdul F (2021) Sodium thiosulfate and natural sulfur: novel potential additives for selective reduction of limonitic laterite ore. J Sustain Metall 7(2):481–494. https://doi.org/10.1007/s40831-021-00352-4
Liu K, Chen Q, Hu H (2009) Comparative leaching of minerals by sulphuric acid in a Chinese ferruginous nickel laterite ore. Hydrometallurgy 98(3–4):281–286. https://doi.org/10.1016/j.hydromet.2009.05.015
Schwertmann U, Schulze DG, Murad E (1982) Identification of ferrihydrite in soils by dissolution kinetics, differential X-ray diffraction, and Mossbauer spectroscopy. J Soil Sci Soc Am 46(4):869–875. https://doi.org/10.2136/sssaj1982.03615995004600040040x
Fan C, Zhai X, Yan F, Chang Y, Li B, Zhang TA (2010) Extraction of nickel and cobalt from reduced limonitic laterite using a selective chlorination–water leaching process. Hydrometallurgy 105(1–2):191–194. https://doi.org/10.1007/s40831-021-00352-4
Kaya S, Dittrich C, Stopic S, Friedrich B (2017) Concentration and separation of Sc from Ni laterite ore processing streams. Metals 7(12):557. https://doi.org/10.3390/met7120557
Ferizoglu E, Kaya S, Topkaya YA (2018) Solvent extraction behaviour of scandium from lateritic nickel-cobalt ores using different organic reagents. Physicochem Probl Miner Process 54(2):538–545. https://doi.org/10.5277/ppmp1855
Guo X, Li D, Park KH, Tian Q, Wu Z (2009) Leaching behavior of metals from a limonitic nickel laterite using a sulfation–roasting–leaching process. Hydrometallurgy 99(3–4):144–150. https://doi.org/10.1016/j.hydromet.2009.07.012
Li J, Chen Z, Shen B, Xu Z, Zhang Y (2017) The extraction of valuable metals and phase transformation and formation mechanism in roasting-water leaching process of laterite with ammonium sulfate. J Clean Prod 140:1148–1155. https://doi.org/10.1016/j.jclepro.2016.10.050
Oxley A, Barcza N (2013) Hydro–pyro integration in the processing of nickel laterites. Miner Eng 54:2–13. https://doi.org/10.1016/j.mineng.2013.02.012
Zhang L, Guo X, Tian Q, Li D, Zhong S, Qin H (2022) Improved thiourea leaching of gold with additives from calcine by mechanical activation and its mechanism. Miner Eng 178:107403. https://doi.org/10.1016/j.mineng.2022.107403
Ribeiro P, Neumann R, Santos I, Rezende MC, Radino-Rouse P, Dutra A (2019) Nickel carriers in laterite ores and their influence on the mechanism of nickel extraction by sulfation-roasting-leaching process. Miner Eng 131:90–97. https://doi.org/10.1016/j.mineng.2018.10.022
Wang W, Du S, Guo L, Tang J, Lu Y, Dong L, Wang W, Du S, Guo L, Tang J (2017) Extraction of nickel from Ramu laterite by sulphation roasting-water leaching. In: 3rd International Conference on Chemical Materials and Process (ICCMP 2017) 1879(1):050004. https://doi.org/10.1063/1.5000474
Anawati J, Yuan R, Kim J, Azimi G (2020) Selective recovery of scandium from nickel laterite ore by acid roasting–water leaching. In: Anawati J, Yuan R, Kim J, Azimi G (eds.) Rare metal technology 2020, Springer, Cham, pp 77–90. https://doi.org/10.1007/978-3-030-36758-9_8
Reid S, Tam J, Yang M, Azimi G (2017) Technospheric mining of rare earth elements from bauxite residue (red mud): process optimization, kinetic investigation, and microwave pretreatment. Sci Rep 7(1):15252. https://doi.org/10.1038/s41598-017-15457-8
Rivera RM, Ulenaers B, Ounoughene G, Binnemans K, Van Gerven T (2018) Extraction of rare earths from bauxite residue (red mud) by dry digestion followed by water leaching. Miner Eng 119:82–92. https://doi.org/10.1016/j.mineng.2018.01.023
Liu Z, Zong Y, Hongxu LI, Jia D, Zhao Z (2017) Selectively recovering scandium from high alkali Bayer red mud without impurities of iron, titanium and gallium. J Rare Earths 9(35):844–941. https://doi.org/10.1016/S1002-0721(17)60992-X
Narayanan RPN, Kazantzis NK, Emmert MH (2018) Selective process steps for the recovery of scandium from jamaican bauxite residue (red mud). ACS Sustain Chem Eng 6(1):1478–1488. https://doi.org/10.1021/acssuschemeng.7b03968
Onghena B, Borra CR, Gerven TV, Binnemans K (2017) Recovery of scandium from sulfation-roasted leachates of bauxite residue by solvent extraction with the ionic liquid betainium bis(trifluoromethylsulfonyl)imide. Sep Purif Technol 176:208–219. https://doi.org/10.1016/j.seppur.2016.12.009
Gamaletsos PN, Godelitsas A, Filippidis A, Pontikes Y (2018) The rare earth elements potential of Greek bauxite active mines in the light of a sustainable REE demand. J Sustain Metall 5:20–47. https://doi.org/10.1007/s40831-018-0192-2
Borra CR, Blanpain B, Pontikes Y, Binnemans K, Gerven TV (2016) Recovery of rare earths and other valuable metals from bauxite residue (red mud): a review. J Sustain Metall 2:365–386. https://doi.org/10.1007/s40831-016-0068-2
Borra CR, Blanpain B, Pontikes Y, Binnemans K, Gerven TV (2016) Smelting of bauxite residue (red mud) in view of iron and selective rare earths recovery. J Sustain Metall 2(1):28–37. https://doi.org/10.1007/s40831-015-0026-4
Borra CR, Blanpain B, Pontikes Y, Binnemans K, Gerven TV (2016) Recovery of rare earths and major metals from bauxite residue (red mud) by alkali roasting, smelting, and leaching. J Sustain Metall 3(2):393–404. https://doi.org/10.1007/s40831-016-0103-3
Gentzmann MC, Schraut K, Vogel C, Gbler HE, Adam C (2021) Investigation of scandium in bauxite residues of different origin. Appl Geochem 126:104898. https://doi.org/10.1016/j.apgeochem.2021.104898
Zhang XK, Zhou KG, Chen W, Lei QY, Huang Y, Peng CH (2019) Recovery of iron and rare earth elements from red mud through an acid leaching-stepwise extraction approach. J Cent South Univ 26(2):458–466. https://doi.org/10.1007/s11771-019-4018-6
Zhou KG, Teng CY, Zhang XK, Peng CH, Chen W (2018) Enhanced selective leaching of scandium from red mud. Hydrometallurgy 182:57–63. https://doi.org/10.1016/j.hydromet.2018.10.011
Ding W, Bao SX, Zhang YM, Xiao JH (2022) Efficient selective extraction of scandium from red mud. Miner Process Extr Metall Rev. https://doi.org/10.1080/08827508.2022.2047044
Li G, Ye Q, Deng B, Luo J, Rao M, Peng Z, Jiang T (2018) Extraction of scandium from sc-rich material derived from bauxite ore residues. Hydrometallurgy 176:62–68. https://doi.org/10.1016/j.hydromet.2018.01.007
Ochsenkuhnpetropulu M, Lyberopulu T, Parissakis G (1995) Selective separation and determination of scandium from yttrium and lanthanides in red mud by a combined ion exchange/solvent extraction method. Anal Chim Acta 315:231–237. https://doi.org/10.1016/0003-2670(95)00309-N
Abhilash HS, Schippers A (2021) Distribution of scandium in red mud and extraction using Gluconobacter oxydans. Hydrometallurgy 202:105621. https://doi.org/10.1016/j.hydromet.2021.105621
Zhang D, Chen H, Nie Z, Xia J, Li E, Fan X, Zheng L (2020) Extraction of Al and rare earths (Ce, Gd, Sc, Y) from red mud by aerobic and anaerobic bi-stage bioleaching. Chem Eng J 401:125914. https://doi.org/10.1016/j.cej.2020.125914
Borra CR, Mermans J, Blanpain B, Pontikes Y, Binnemans K, Gerven TV (2016) Selective recovery of rare earths from bauxite residue by combination of sulfation, roasting and leaching. Miner Eng 92:151–159. https://doi.org/10.1016/j.mineng.2016.03.002
Botelho Junior AB, Romano Espinosa DC, Soares Tenorio JA (2021) Extraction of scandium from critical elements-bearing mining waste: silica gel avoiding in leaching reaction of bauxite residue. J Sustain Metall 7:1627–1642. https://doi.org/10.1007/s40831-021-00434-3
Swamy YV, Kar BB, Mohanty JK (2003) Physico-chemical characterization and sulphatization roasting of low-grade nickeliferous laterites. Hydrometallurgy 69(1):89–98. https://doi.org/10.1016/S0304-386X(03)00027-6
Anawati J, Azimi G (2019) Recovery of scandium from Canadian bauxite residue utilizing acid baking followed by water leaching. Waste Manage 95:549–559. https://doi.org/10.1016/j.wasman.2019.06.044
Harris CT, Peacey JG, Pickles CA (2011) Selective sulphidation of a nickeliferous lateritic ore. Miner Eng 24(7):651–660. https://doi.org/10.1016/j.mineng.2010.10.008
Tagawa H (1984) Thermal decomposition temperatures of metal sulfates. Thermochim Acta 80(1):23–33. https://doi.org/10.1016/0040-6031(84)87181-6
Siriwardane RV, Poston J Jr, Fisher EP, Shen MS, Miltz AL (1999) Decomposition of the sulfates of copper, iron (II), iron (III), nickel, and zinc: XPS, SEM, DRIFTS, XRD, and TGA study. Appl Surf Sci 152(3–4):219–236. https://doi.org/10.1016/S0169-4332(99)00319-0
Long GJ, Longworth G, Battle P, Cheetham AK, Thundathil RV, Beveridge D (1979) A study of anhydrous iron (III) sulfate by magnetic susceptibility, Moessbauer, and neutron diffraction techniques. Inorg Chem 18(3):624–632. https://doi.org/10.1021/ic50193a021
Coombs P, Munir Z (1989) The decomposition of iron (III) sulfate in air. J Therm Anal 35(3):967–976. https://doi.org/10.1007/BF02057253
Mason CW, Gocheva I, Hoster HE, Denis Y (2014) Iron (III) sulfate: a stable, cost effective electrode material for sodium ion batteries. Chem Commun 50(18):2249–2251. https://doi.org/10.1039/c3cc47557c
Yu D, Utigard TA, Barati M (2014) Fluidized bed selective oxidation-sulfation roasting of nickel sulfide concentrate: Part II. Sulfation roasting. Metall Mater Trans B 45(2):662–674. https://doi.org/10.1007/s11663-013-9959-9
Yagmurlu B, Dittrich C, Friedrich B (2016) Precipitation trends of scandium in synthetic red mud solutions with different precipitation agents. J Sustain Metall 3(1):90–98. https://doi.org/10.1007/s40831-016-0098-9
Sadri F, Kim R, Ghahreman A (2021) Behavior of light and heavy rare-earth elements in a two-step Fe and Al removal process from rare-earth pregnant leach solutions. J Sustain Metall 7(3):1327–1342. https://doi.org/10.1007/s40831-021-00423-6
Peters EM, Kaya S, Dittrich C, Forsberg K (2019) Recovery of scandium by crystallization techniques. J Sustain Metall 5(1):48–56. https://doi.org/10.1007/s40831-019-00210-4
McDonald RG, Whittington BI (2008) Atmospheric acid leaching of nickel laterites review Part I. Sulphuric acid technologies. Hydrometallurgy 91(1–4):35–55. https://doi.org/10.1016/j.hydromet.2007.11.009
Wan X, Taskinen P, Shi J, Jokilaakso A (2021) A potential industrial waste–waste co-treatment process of utilizing waste SO2 gas and residue heat to recover Co, Ni, and Cu from copper smelting slag. J Hazard Mater 414:125541. https://doi.org/10.1016/j.jhazmat.2021.125541
Acknowledgements
The authors gratefully acknowledge the financial support from the National Key R&D Program of China (Grant Nos. 2019YFC1907402 and 2018YFC1902501) and National Natural Science Foundation of China (Grant Nos. 51922108, 52074363, and 52104355).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
The contributing editor for this article was Zhi Sun.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dong, B., Tian, Q., Guo, X. et al. Leaching Behavior of Scandium from Limonitic Laterite Ores Under Sulfation Roasting–Water Leaching. J. Sustain. Metall. 8, 1078–1089 (2022). https://doi.org/10.1007/s40831-022-00551-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-022-00551-7