Abstract
In order to expand the methods of lithium intercalation into layered multicomponent matrices, the reaction of lithium hydride with layered telluride Ni3GaTe2 through the stage of formation of mechanocomposites has been studied. Intercalation compounds LixNi3GaTe2 (0 ≤ x ≤ 0.3) have been shown to form when annealing mechanocomposites of the matrix and intercalating agent (LiH) in argon. The channels of hydride ion conversion have been studied and transformations involving the matrix have been described at various temperatures and molar ratios of the matrix and intercalating agent.
Similar content being viewed by others
INTRODUCTION
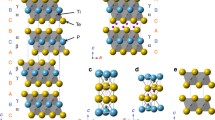
Low-dimensional metal–metal bond systems are interesting objects for study in the field of inorganic chemistry and solid state chemistry, since their presence in crystalline compounds often determines the nontrivial structure and physicochemical properties of the latter. One of these types of compounds is represented by a family of mixed nickel and p metal tellurides with a layered structure and the composition of Ni3MTe2, where M = Ga, In, Ge, Sn, Sb, 0 ≤ x ≤ 1 [1–8]. All these compounds crystallize in the hexagonal syngony with space group P63/mmc and Z = 2, except for Ni2.58SnTe2 described in space group P\(\bar {3}\)1c. The structure of layered compounds is derived from the combination of the NiAs structure type and the Ni2In structure type. It is based on two-dimensionally infinite heterometallic fragments bounded by tellurium atoms along axis c, which, in turn, form a van der Waals gap through weak Te–Te interactions. The most studied is the Ni–Ga–Te system, in which samples Ni2.98GaTe2, Ni2.75GaTe2, Ni2.5GaTe2, and Ni2.3GaTe2 have been synthesized [1, 2]. The structure of Ni2.98GaTe2 is based on alternating \(_{\infty }^{2}\)[Ni2.6Ga] heterometallic fragments bounded by terminal tellurium atoms located along axis c. In this case, the heterometallic fragments refer to the defect structure of Ni2In. The lengths of homo- and heterometallic Ni–Ni and Ni–M bonds are close in value in all Ni3 – xGaTe2 compounds. The Ni(1)–Ga and Ni(1)–Te distances in ternary compounds are similar to distances in the structure of the Ni1.8Ga intermetallic compound, which belongs to the Ni2In structural type (Ni(1)–Ga = 2.625 Å) [2].
In contrast to intermetallic compounds, the nearest Ni(1) octahedral environment is formed by three gallium atoms and three tellurium atoms. This is explained by the fact that the position of arsenic in the initial NiAs structure in our case is occupied by gallium and tellurium in an orderly manner, which leads to its division into two independent positions and tripling of the initial unit cell along axis c. Three gallium atoms occupying the equatorial vertices of the trigonal bipyramid and two tellurium atoms occupying the axial vertices of the trigonal bipyramid form a coordination polyhedron of the Ni(2) atom. The relatively short Ni(2)–Ga distance is similar to the distance in the Ni2Ga intermetallic compound (d(Ni(2)–Ga) = 2.311 Å). The authors [2] explain the partial occupancy of the d metal position by the fact that a short heterometallic bond is not typical for a pair of Ni–Ga elements. We believe that is not a rational explanation of the observed phenomenon. The coordination polyhedron of Ni(3) is an octahedron formed only by tellurium atoms.
The Ni–Te distance in these ternary compounds is similar to the typical interatomic distances found in the structures of a large number of binary nickel tellurides, especially in the Ni1 – xTe solid solution, which is a defective NiAs structure. In [2], the crystal structures of nickel-depleted compounds of the Ni3 – xGaTe2 type (х = 0.25, 0.5) were refined by the Rietveld method.
Note the relatively constant occupancy of the Ni(3) position inside the van der Waals gap (~25–30%), which is accompanied by a decrease in the occupancy of the Ni(2) position inside the heterometallic fragment as the total content of Ni decreases (50% in Ni2.79GaTe2, 36% in Ni2.58GaTe2) (Table 1). An increase in parameter c when passing from Ni2.79GaTe2 to Ni2.58GaTe2 is explained by a decrease in the occupancy of the Ni(3) site, since the van der Waals gap becomes more pronounced and “shrinks” less due to the presence of nickel atoms in it in part of the cells. The authors [2] also believe that the Ni(2) position in Ni3 – хGaTe2 cannot be completely free. When trying to synthesize a compound with the composition Ni2GaTe2, a ternary phase containing Ni2.3GaTe2 and Ga2Te3 was obtained. This confirms the existence of a lower limit for the content of Ni in compounds of the Ni3 – xGaTe2 type. The appearance of the superstructure is associated with the ordered filling of the Ni(3) position in plane ab, which is observed for compounds of the composition Ni3 – xGaTe2, where 0.5 ≤ x ≤ 0.65; 3/4 nickel atoms are regularly absent in the Ni(2) position, in contrast to the Ni3GaTe2 compound, where the Ni(3)' and Ni(3)" positions are completely occupied (Fig. 1) [2]. In a supercell with doubled parameters a and b, the former Ni(2) positions in the heterometallic layer are transformed into Ni(2)' and Ni(2)'' positions. In the proposed symmetry, the first position is occupied, while the second one remains vacant, which leads to the appearance of heterometallic fragments of the \(_{\infty }^{2}\)[Ni2.25Ga] type. Considering that the occupation of the Ni(3) position in the van der Waals gap is ~30% (according to the Rietveld refinement data), the authors [2] believe that the ideal composition for observing this type of ordering is close to Ni2.55GaTe2. This is consistent with the upper limit of the nickel content for which this type of superstructure has been identified. At х < 0.45, a “standard” cell of the Ni2.98GaTe2 type is observed with parameter c tripled compared to NiAs. The authors [2] assume that the occupancy of the nickel position inside the van der Waals gap is constant. However, in a real structure, the total occupancy may vary, changing the ratio between the occupancy of two d metal positions and the total nickel content [2].
The study of the intercalation process into structures with a layered structure is important both for theoretical inorganic chemistry and physicochemistry of solids and for the targeted synthesis of functional inorganic materials, including model ones. Of particular interest is the use of intercalates of layered alkali metal chalcogenides for the preparation of nanomaterials, in particular electrodes for metal-ion batteries, upon their treatment with protic solvents [9–12]. For the intercalation of lithium into layered matrix structures, elemental metallic Li [13], organometallic lithium compounds n-butyllithium [14, 15], tert-butyllithium [16], lithium hydroxide [12], as well as solutions of metallic lithium in liquid ammonia [9] are currently used. It should be noted that none of these methods can provide products with a given composition. This circumstance determines the possibility of a deeper study of the properties of intercalates with different contents of the intercalant atom.
The first example of the use of lithium hydride as an intercalating agent was intercalation into graphite, which is widely used as a model object [17, 18]. Subsequently, LiH was used to synthesize a technologically important product, lithiated spinel Li1 + xMn2O4 – δ, which is used as an electrochemically active material in rechargeable lithium-ion batteries, as a result of which single-phase spinel products with a record lithium content were obtained [19, 20]. It was found that hydrogen and water are released as by-products, the ratio of which varies depending on the molar ratio of the intercalating agent and the matrix, as well as on the annealing mode.
Thus, the expansion of systems in which lithium hydride can be used as an intercalating agent and the study of the transformations occurring in this case are urgent problems for both inorganic chemistry and materials science in general [21–28].
EXPERIMENTAL
Precursors. In this work, we used LiH with the content of the main substance of at least 98% (according to volumetric analysis). Nickel (powder, 99.98%), gallium (ingot), and tellurium (powder, 99.999%) were used as starting materials for the synthesis of Ni3GaTe2. Nickel was preliminarily annealed for several hours at 550°C in a flow of dry hydrogen of chromatographic purity to reduce the oxide layer. The initial ternary Ni3GaTe2 matrix was synthesized according to the standard high-temperature ampoule method: a stoichiometric mixture of initial simple substances was placed in a calcined quartz ampoule 8–15 mm in diameter and 50–100 mm long, which was then evacuated to a residual pressure of 5–10 × 10–3 mm Hg, sealed in the flame of an oxygen burner, and annealed in a furnace at 1023 K for 168 h. According to the X-ray powder diffraction data, the Ni3GaTe2 sample is single-phase. X-ray powder diffraction studies were performed using the equipment of the Center for Collective Use of the Institute of General and Inorganic Chemistry of the Russian Academy of Sciences (a Bruker D8 Advance X-ray diffractometer, CuKα radiation, a Ni filter, a Lynxeye detector, reflection geometry) as part of the State Assignment of the Kurnakov Institute of General and Inorganic Chemistry of the Russian Academy of Sciences in the field of fundamental scientific research.
The diffraction patterns of lithium-containing samples were recorded in a fluoroplastic cell. The samples were covered with a Captone polyamide film, which was fixed with a clamping ring. Operations with substances sensitive to air components (unloading and loading grinding jars, working with lithium hydride, sample preparation of lithiated samples for X-ray powder diffraction studies) were carried out in a sealed SPECS GB 22M glove box (10 and 5 ppm O2 and H2O, respectively); samples were placed in low-background cuvettes with oriented silicon single crystal substrates (angle range 2θ = 5°–90°, step 0.01023°, accumulation time 0.15–0.3 s).
Elemental analysis (Li, Ga, Ni, Te) was performed using an iCAP 6300 Duo emission spectrometer with inductively coupled plasma. To transfer the sample to a solution, a weighed portion of the analyte was treated with aqua regia, for the preparation of which hydrochloric and nitric acids of special purity were used. The hydrogen content was determined gravimetrically by burning a sample of the test substance in a flow of high purity oxygen with trapping of the water vapor formed in U-shaped tubes with anhydrous Mg(ClO4)2. The relative error of determination by this method is 1.5%. In order to avoid the loss of hydrogen to be determined in the form of a simple substance, afterburning of the gases entering the U-shaped tube with Mg(ClO4)2 over the CuO wire was carried out.
Ball milling was carried out using a Retsch MM400 vibrating ball mill with grinding jars (V = 25 mL) lined with ZrO2 and grinding balls (5 mm) made of ZrO2. The ratio of the mass of the processed sample of the substance to the mass of the balls was maintained at the level of 20 : 1.
pH was measured using an IPL-201 Multitest liquid analyzer with a pX measurement range (–2…+20) with an error of ±0.02 pX (pH). A weighed portion of the LixNi3GaTe2 preparation to be hydrolysed was chosen to be sufficient for the formation of a 0.01 M LiOH solution after hydrolysis was completed. For hydrolysis, bidistilled water was used, the pH value was measured with constant stirring of the intercalate suspension in water with a magnetic stirrer.
RESULTS AND DISCUSSION
To perform intercalation, a given amount of lithium hydride was subjected to ball milling (25 min, 30 Hz), then the required amount of a single-phase Ni3GaTe2 sample (matrix) was added to the grinding jar, and the resulting mixture was activated for 3–5 min in the same mode. This order of activation was chosen in view of the possibility of mechanolysis of telluride, which has a layered structure. In the present work, we studied mixtures with the molar fraction of the introduced LiH equal to 0.1, 0.2, 0.3, 0.5, and 0.7. The mechanocomposites obtained in this way had a given calculated ratio of elements (Table 2) and a phase composition corresponding to a mechanical mixture (Fig. 2). The mechanocomposites extracted from the grinding jar were transferred into alundum crucibles (l = 35 mm, dint = 6 mm, dext = 8 mm) and placed in a flow-through quartz tube-reactor. Annealing was carried out in a high purity argon atmosphere (p(O2) ~ 0.0001 atm). On the diffraction patterns of the samples annealed at 523 K (2 h), LiH reflections are still observed, and the elemental composition remains almost unchanged, only the hydrogen content slightly decreases, which indicates its release in the molecular form. An increase in the annealing time to 24–48 h and a temperature to 747 K in the case of LiH with a molar fraction not exceeding x = 0.3 leads to the formation of single-phase LixNi3GaTe2 samples with the structure of the original Ni3GaTe2 matrix retained (Fig. 2). The samples thus obtained do not contain hydrogen (Table 2). During lithium intercalation, the Ni3GaTe2 unit cell undergoes slight contraction, for example, for x = 0.3, a = 3.9557(5) Å, c = 15.6555(18) Å, V = 212.51(4) Å3).
With an increase in the molar fraction of LiH in mixtures (>0.3) or temperatures above 823 K, along with complete evolution of hydrogen, decomposition of the matrix is observed: a mirror of elemental tellurium is formed on the walls of the reaction quartz tube, which is also noticeable from the change in the elemental composition (Table 2). Mass transfer of tellurium is possible both as a result of evaporation from the matrix and as a result of the thermolysis of H2Te, the source of hydrogen for the formation of which is the hydride ion introduced with LiH.
Figure 3 shows the results of hydrolysis of intercalates, indicating that hydrolysis proceeds with a pronounced induction period. The pH value begins to increase due to the formation of LiOH after a certain time after the introduction of the LixNi3GaTe2 preparation into water, which cannot be expected from the reaction of LiH or metallic Li localized on the surface of the sample crystallites or in the form of a mechanical mixture with the matrix with water.
CONCLUSIONS
Lithium hydride LiH was first used as an intercalating agent to prepare pure samples of intercalates of layered multicomponent telluride LixNi3GaTe2 with the structure of the initial matrix and a given content of lithium by a solid-phase reaction. The possibilities and limitations of this method are determined, and accompanying transformations are described in a wide range of molar fractions of LiH in the initial mixtures and temperatures. The data obtained suggest a statistical distribution of lithium in the van der Waals gap of the matrix. For a more detailed study of intercalates, it is necessary to study single-crystal intercalated samples.
REFERENCES
O. N. Litvinenko, A. N. Kuznetsov, A. V. Olenev, et al., Russ. Chem. Bull. 56, 1945 (2007). https://doi.org/10.1007/s11172-007-0301-z
A. A. Isaeva, O. N. Makarevich, A. N. Kuznetsov, et al., Eur. J. Inorg. Chem. 1395 (2010). https://doi.org/10.1002/ejic.200901027
A.-K. Larsson, L. Noren, R. L. Withers, et al., J. Solid State Chem. 180, 2723 (2007). https://doi.org/10.1016/j.jssc.2007.07.020
L. Noren, R. L. Withers, and F. J. Brink, J. Alloys Compd. 353, 133 (2003). https://doi.org/10.1016/S0925-8388(02)01309-9
H.-J. Deiseroth and K. Aleksandrov, C. Reiner, et al., Eur. J. Inorg. Chem. 8, 1561 (2006). https://doi.org/10.1002/ejic.200501020
H.-J. Deiseroth, F. Sprirovski, C. Reiner, et al., Z. Kristallogr. New Cryst. Struct. 222, 169 (2007). https://doi.org/10.1524/ncrs.2007.0070
T. Dankwort, V. Duppel, H.-J. Deiseroth, et al., Sci. Technol. 31, 7 (2016). https://doi.org/10.1088/0268-1242/31/9/094001
T. K. Reynolds, R. F. Kelley, and F. J. DiSalvo, J. Alloys Compd. 366, 136 (2004). https://doi.org/10.1016/j.jallcom.2003.07.008
S. Ding, S. K. Bux, D. J. King, et al., J. Mater. Chem. 19, 2588 (2009). https://doi.org/10.1039/B820226E
A. Raza, J. Z. Hassan, M. Ikram, et al., Adv. Mater. Interfaces 8, 2002205 (2021). https://doi.org/10.1002/admi.202002205
L. Zhang, C. Chen, J. Zhou, et al., Adv. Funct. Mater. 30, 2004139 (2020). https://doi.org/10.1002/adfm.202004139
L. Ren, X. Qi, Y. Liu, et al., J. Mater. Chem. 22, 4921 (2012). https://doi.org/10.1039/c2jm15973b
T. Yajima, M. Koshiko, Y. Zhang, et al., Nat. Comm. 7, 13809 (2016). https://doi.org/10.1038/ncomms13809
R. Guzmán, J. Morales, and J. L. Tirado, J. Mater. Chem. 3, 1271 (1993). https://doi.org/10.1039/jm9930301271
D. Yang and R. F. Frindt, J. Phys. Chem. Solids 57, 1113 (1996). https://doi.org/10.1016/0022-3697(95)00406
J. H. Golden, F. J. DiSalvo, and J. M. J. Fréchet, Mater. Sci. Forum. 152–153, 209 (1994). https://doi.org/10.4028/www.scientific.net/msf.152-153.209
L. Elansari, R. Marassl, R. Tossicl, et al., Mol. Cryst. Liq. Cryst. Sci. Technol., Sect. A 310, 309 (1998). https://doi.org/10.1080/10587259808045354
S. Konar, U. Häusserman, and G. Svensson, Chem. Mater. 27, 2566 (2015). https://doi.org/10.1021/acs.chemmater.5b00235
G. A. Buzanov, G. D. Nipan, K. Yu. Zhizhin, et al., Patent RF RU2591154, Bull. Izobret. No. 19, published June 10, 2016.
G. A. Buzanov, G. D. Nipan, K. Yu. Zhizhin, et al., Russ. Chem. Bull. 471, 330 (2016). https://doi.org/10.1134/s0012500816110082
O. V. Lapshin, E. V. Boldyreva, and V. V. Boldyrev, Russ. J. Inorg. Chem. 66, 433 (2021). https://doi.org/10.1134/S0036023621030116
A. A. Davydova, E. V. Raksha, V. A. Glazunova, et al., Russ. J. Inorg. Chem. 66, 324 (2021). https://doi.org/10.1134/S0036023621030062
L. S. Pechen, E. V. Makhonina, A. E. Medvedeva, et al., Russ. J. Inorg. Chem. 66, 777 (2021). https://doi.org/10.1134/S0036023621050144
M. G. Shelyapina, I. P. Lushpinskaya, S. A. Kurnosenko, et al., Russ. J. Gen. Chem. 90, 760 (2020). https://doi.org/10.1134/S1070363220040337
N. N. Leont’eva, V. A. Drozdov, and O. B. Bel’skaya, et al., Russ. J. Gen. Chem. 90, 509 (2020). https://doi.org/10.1134/S1070363220030275
I. A. Makaryan and I. V. Sedov, Russ. J. Gen. Chem. 91, 1912 (2021). https://doi.org/10.1134/S1070363221090371
D. A. Doinikov, A. S. Zavgorodnii, I. V. Kazakov, et al., Russ. J. Gen. Chem. 91, 1969 (2021). https://doi.org/10.1134/S1070363221100078
I. V. Postnikova, Russ. J. Gen. Chem. 91, 1218 (2021). https://doi.org/10.1134/S1070363221060360
ACKNOWLEDGMENTS
Analytical studies were carried out using the scientific equipment of the Center for Collective Use of the National Research Center “Kurchatov Institute”—IREA.
Funding
This work was supported by the Russian Science Foundation (project no. 19-13-00451).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by V. Avdeeva
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Buzanov, G.A., Stroganova, E.A., Bykov, A.Y. et al. Hydride Intercalation of Lithium into Ni3GaTe2. Russ. J. Inorg. Chem. 67, 616–621 (2022). https://doi.org/10.1134/S0036023622050035
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023622050035