Abstract
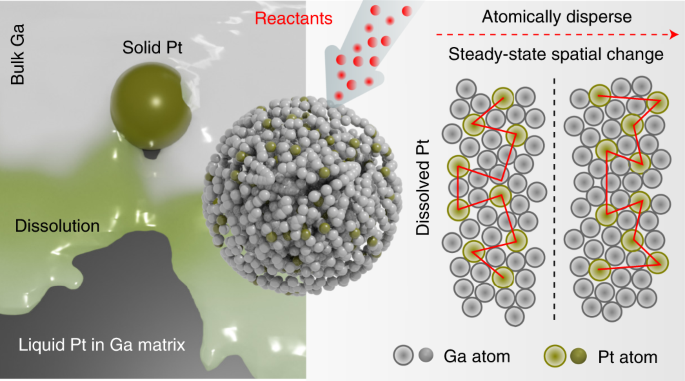
Insights into metal–matrix interactions in atomically dispersed catalytic systems are necessary to exploit the true catalytic activity of isolated metal atoms. Distinct from catalytic atoms spatially separated but immobile in a solid matrix, here we demonstrate that a trace amount of platinum naturally dissolved in liquid gallium can drive a range of catalytic reactions with enhanced kinetics at low temperature (318 to 343 K). Molecular simulations provide evidence that the platinum atoms remain in a liquid state in the gallium matrix without atomic segregation and activate the surrounding gallium atoms for catalysis. When used for electrochemical methanol oxidation, the surface platinum atoms in the gallium–platinum system exhibit an activity of \({\sim {2.8} \times {10^7}\,{{{\mathrm{mA}}}}\,{{{{\mathrm{mg}}}}_{{{{\mathrm{Pt}}}}}^{ - 1}}},\) three orders of magnitude higher than existing solid platinum catalysts. Such a liquid catalyst system, with a dynamic interface, sets a foundation for future exploration of high-throughput catalysis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Source data are provided with this paper.
References
Cheng, N. et al. Platinum single-atom and cluster catalysis of the hydrogen evolution reaction. Nat. Commun. 7, 13638 (2016).
Fang, S. et al. Uncovering near-free platinum single-atom dynamics during electrochemical hydrogen evolution reaction. Nat. Commun. 11, 1029 (2020).
Qiao, B. et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 3, 634–641 (2011).
Xu, H., Cheng, D., Cao, D. & Zeng, X. C. A universal principle for a rational design of single-atom electrocatalysts. Nat. Catal. 1, 339–348 (2018).
Xia, C. et al. General synthesis of single-atom catalysts with high metal loading using graphene quantum dots. Nat. Chem. 13, 887–894 (2021).
Wang, H. et al. Surpassing the single-atom catalytic activity limit through paired Pt–O–Pt ensemble built from isolated Pt1 atoms. Nat. Commun. 10, 3808 (2019).
Li, L. et al. Theoretical insights into single-atom catalysts. Chem. Soc. Rev. 49, 8156–8178 (2020).
Wang, A., Li, J. & Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2, 65–81 (2018).
Zhou, K. L. et al. Platinum single-atom catalyst coupled with transition metal/metal oxide heterostructure for accelerating alkaline hydrogen evolution reaction. Nat. Commun. 12, 3783 (2021).
Hannagan, R. T., Giannakakis, G., Flytzani-Stephanopoulos, M. & Sykes, E. C. H. Single-atom alloy catalysis. Chem. Rev. 120, 12044–12088 (2020).
Upham, D. C. et al. Catalytic molten metals for the direct conversion of methane to hydrogen and separable carbon. Science 358, 917–921 (2017).
Taccardi, N. et al. Gallium-rich Pd–Ga phases as supported liquid metal catalysts. Nat. Chem. 9, 862–867 (2017).
Palmer, C. et al. Dry reforming of methane catalysed by molten metal alloys. Nat. Catal. 3, 83–89 (2020).
Bauer, T. et al. Operando DRIFTS and DFT study of propane dehydrogenation over solid- and liquid-supported GaxPty catalysts. ACS Catal. 9, 2842–2853 (2019).
Wolf, M. et al. Capturing spatially resolved kinetic data and coking of Ga-Pt supported catalytically active liquid metal solutions during propane dehydrogenation in situ. Faraday Discuss. 229, 359–377 (2021).
Raman, N. et al. Highly effective propane dehydrogenation using Ga-Rh supported catalytically active liquid metal solutions. ACS Catal. 9, 9499–9507 (2019).
Liu, H. et al. Solid–liquid phase transition induced electrocatalytic switching from hydrogen evolution to highly selective CO2 reduction. Nat. Catal. 4, 202–211 (2021).
Esrafilzadeh, D. et al. Room temperature CO2 reduction to solid carbon species on liquid metals featuring atomically thin ceria interfaces. Nat. Commun. 10, 865 (2019).
Walbert, T. et al. In situ transmission electron microscopy analysis of thermally decaying polycrystalline platinum nanowires. ACS Nano 14, 11309–11318 (2020).
Daeneke, T. et al. Liquid metals: fundamentals and applications in chemistry. Chem. Soc. Rev. 47, 4073–4111 (2018).
Zavabeti, A. et al. A liquid metal reaction environment for the room-temperature synthesis of atomically thin metal oxides. Science 358, 332–335 (2017).
Lin, Y., Genzer, J. & Dickey, M. D. Attributes, fabrication and applications of gallium-based liquid metal particles. Adv. Sci. 7, 2000192 (2020).
Huang, W. et al. Highly active and durable methanol oxidation electrocatalyst based on the synergy of platinum–nickel hydroxide–graphene. Nat. Commun. 6, 10035 (2015).
Yatsenko, S. P., Rykova, L. N., Anikin, Y. A. & Dieva, É. N. The corrosion of group VIII metals in liquid gallium. Mater. Sci. 8, 310–313 (1974).
Grabau, M. et al. Surface enrichment of Pt in Ga2O3 films grown on liquid Pt/Ga alloys. Surf. Sci. 651, 16–21 (2016).
Tang, J. et al. Unique surface patterns emerging during solidification of liquid metal alloys. Nat. Nanotechnol. 16, 431–439 (2021).
Rahim, M. A. et al. Phenolic building blocks for the assembly of functional materials. Angew. Chem. Int. Ed. 58, 1904–1927 (2019).
Huang, L. et al. Single-atom nanozymes. Sci. Adv. 5, eaav5490 (2019).
Gao, L. et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2, 577–583 (2007).
Yang, J., Cohen Stuart, M. A. & Kamperman, M. Jack of all trades: versatile catechol crosslinking mechanisms. Chem. Soc. Rev. 43, 8271–8298 (2014).
Sileika, T. S. et al. Colorless multifunctional coatings inspired by polyphenols found in tea, chocolate and wine. Angew. Chem. Int. Ed. 52, 10766–10770 (2013).
Centurion, F. et al. Liquid metal-triggered assembly of phenolic nanocoatings with antioxidant and antibacterial properties. ACS Appl. Nano Mater. 4, 2987–2998 (2021).
Dickey, M. D. Emerging applications of liquid metals featuring surface oxides. ACS Appl. Mater. Interfaces 6, 18369–18379 (2014).
Bilodeau, R. A., Zemlyanov, D. Y. & Kramer, R. K. Liquid metal switches for environmentally responsive electronics. Adv. Mater. Interfaces 4, 1600913 (2017).
Pourbaix, M. Atlas Of Electrochemical Equilibria In Aqueous Solutions (National Association of Corrosion Engineers, 1974).
Mowry, S. & Ogren, P. J. Kinetics of methylene blue reduction by ascorbic acid. J. Chem. Educ. 76, 970 (1999).
Hao, Yu,E., Scott, K. & Reeve, R. W. A study of the anodic oxidation of methanol on Pt in alkaline solutions. J. Electroanal. Chem. 547, 17–24 (2003).
Tripković, A. V., Popović, K. D., Momčilović, J. D. & Draić, D. M. Kinetic and mechanistic study of methanol oxidation on a Pt(111) surface in alkaline media. J. Electroanal. Chem. 418, 9–20 (1996).
Zhang, Z. et al. Single-atom catalyst for high-performance methanol oxidation. Nat. Commun. 12, 5235 (2021).
Mallat, T. & Baiker, A. Oxidation of alcohols with molecular oxygen on solid catalysts. Chem. Rev. 104, 3037–3058 (2004).
Huheey, J. E., Keiter, E. A., Keiter, R. L. & Medhi, O. K. Inorganic Chemistry: Principles of Structure and Reactivity (Pearson Education, 2006).
Armbrüster, M., Schlögl, R. & Grin, Y. Intermetallic compounds in heterogeneous catalysis—a quickly developing field. Sci. Technol. Adv. Mater. 15, 034803 (2014).
Rodriguez, J. A. Interactions in bimetallic bonding: electronic and chemical properties of PdZn surfaces. J. Phys. Chem. 98, 5758–5764 (1994).
Lim, S.-C., Chan, C.-Y., Chen, K.-T. & Tuan, H.-Y. Synthesis of popcorn-shaped gallium-platinum (GaPt3) nanoparticles as highly efficient and stable electrocatalysts for hydrogen evolution reaction. Electrochim. Acta 297, 288–296 (2019).
Kumar, V. B. et al. Sonochemical formation of Ga-Pt intermetallic nanoparticles embedded in graphene and its potential use as an electrocatalyst. Electrochim. Acta 190, 659–667 (2016).
Ryu, J. & Surendranath, Y. Tracking electrical fields at the Pt/H2O interface during hydrogen catalysis. J. Am. Chem. Soc. 141, 15524–15531 (2019).
Wagner, A., Sahm, C. D. & Reisner, E. Towards molecular understanding of local chemical environment effects in electro- and photocatalytic CO2 reduction. Nat. Catal. 3, 775–786 (2020).
Zhang, M., Wang, M., Xu, B. & Ma, D. How to measure the reaction performance of heterogeneous catalytic reactions reliably. Joule 3, 2876–2883 (2019).
Acknowledgements
We thank the Australian Research Council (ARC) for a Laureate Fellowship grant (FL180100053) and Discovery Early Career Researcher Award (DE210101162) for the financial support of this study. We acknowledge the assistance of supercomputing resources from the National Computational Infrastructure (NCI), supported by the Australian Government, and assistance from Pawsey Supercomputer Centre. We also acknowledge the technical assistance from the Solid State & Elemental Analysis Unit (Mark Wainwright Analytical Centre, UNSW Sydney).
Author information
Authors and Affiliations
Contributions
M.A.R. and K.K.-Z. conceived the idea. M.A.R. and Jianbo Tang synthesized the catalysts. M.A.R. designed and performed the experiments with the help of Jianbo Tang, Junma Tang, F.C., Z.C., M.B., M.M., F.-M.A. and T.D. The molecular dynamic simulations were performed by A.J.C., N.M., C.F.M. and S.P.R. The DFT calculations were performed by P.V.K. The phase diagram was prepared by P.K. The draft manuscript was prepared with the help of R.B.K. and K.K.-Z. All the authors discussed the results and contributed to preparing the final draft of the Paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Wenyue Guo, Frédéric Jaouen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Figs. 1–19, Tables 1 and 2, Notes 1 and 2 and references.
Source data
Source Data Fig. 1
Source data for Fig. 1 including solubility data of Pt in liquid Ga, initial and final configurations of the AIMD simulations, and pairwise probability distribution of Pt atoms in Ga matrix in the bulk and interface
Source Data Fig. 2
Source data for Fig. 2 including absorbance at 500 nm for different catalysts over time (PG oxidation), and initial and final configurations of the AIMD simulations
Source Data Fig. 3f
DFT configurations for the methanol oxidation
Source Data Fig. 4a
Density of states calculation
Rights and permissions
About this article
Cite this article
Rahim, M.A., Tang, J., Christofferson, A.J. et al. Low-temperature liquid platinum catalyst. Nat. Chem. 14, 935–941 (2022). https://doi.org/10.1038/s41557-022-00965-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-022-00965-6
This article is cited by
-
Liquid metals for boosting stability of zeolite catalysts in the conversion of methanol to hydrocarbons
Nature Communications (2024)
-
Dynamicity of atoms in a liquid metal catalyst enables selective propylene synthesis
Nature Nanotechnology (2024)
-
Dynamic configurations of metallic atoms in the liquid state for selective propylene synthesis
Nature Nanotechnology (2024)
-
Adaptable graphitic C6N6-based copper single-atom catalyst for intelligent biosensing
Nature Communications (2023)
-
Current state and future prospects of liquid metal catalysis
Nature Catalysis (2023)



