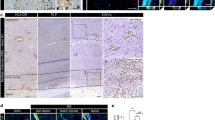
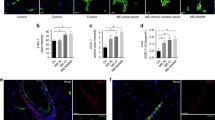
Abstract
Oncostatin M (OSM) is an IL-6 family member which exerts neuroprotective and remyelination-promoting effects after damage to the central nervous system (CNS). However, the role of OSM in neuro-inflammation is poorly understood. Here, we investigated OSM’s role in pathological events important for the neuro-inflammatory disorder multiple sclerosis (MS). We show that OSM receptor (OSMRβ) expression is increased on circulating lymphocytes of MS patients, indicating their elevated responsiveness to OSM signalling. In addition, OSM production by activated myeloid cells and astrocytes is increased in MS brain lesions. In experimental autoimmune encephalomyelitis (EAE), a preclinical model of MS, OSMRβ-deficient mice exhibit milder clinical symptoms, accompanied by diminished T helper 17 (Th17) cell infiltration into the CNS and reduced BBB leakage. In vitro, OSM reduces BBB integrity by downregulating the junctional molecules claudin-5 and VE-cadherin, while promoting secretion of the Th17-attracting chemokine CCL20 by inflamed BBB-endothelial cells and reactive astrocytes. Using flow cytometric fluorescence resonance energy transfer (FRET) quantification, we found that OSM-induced endothelial CCL20 promotes activation of lymphocyte function-associated antigen 1 (LFA-1) on Th17 cells. Moreover, CCL20 enhances Th17 cell adhesion to OSM-treated inflamed endothelial cells, which is at least in part ICAM-1 mediated. Together, these data identify an OSM-CCL20 axis, in which OSM contributes significantly to BBB impairment during neuro-inflammation by inducing permeability while recruiting Th17 cells via enhanced endothelial CCL20 secretion and integrin activation. Therefore, care should be taken when considering OSM as a therapeutic agent for treatment of neuro-inflammatory diseases such as MS.







Similar content being viewed by others
References
Abraham M, Karni A, Mausner-Fainberg K, Weiss ID, Peled A (2017) Natural and induced immunization against CCL20 ameliorate experimental autoimmune encephalitis and may confer protection against multiple sclerosis. Clin Immunol 183:316–324. https://doi.org/10.1016/j.clim.2017.09.018
Akgün K, Blankenburg J, Marggraf M, Haase R, Ziemssen T (2020) Event-driven immunoprofiling predicts return of disease activity in alemtuzumab-treated multiple sclerosis. Front Immunol 11:56. https://doi.org/10.3389/fimmu.2020.00056
Alcaide P, Maganto-Garcia E, Newton G, Travers R, Croce KJ, Bu DX et al (2012) Difference in Th1 and Th17 lymphocyte adhesion to endothelium. J Immunol 188(3):1421–1430. https://doi.org/10.4049/jimmunol.1101647
Alon R, Shulman Z (2011) Chemokine triggered integrin activation and actin remodeling events guiding lymphocyte migration across vascular barriers. Exp Cell Res 317(5):632–641. https://doi.org/10.1016/j.yexcr.2010.12.007
Ambrosini E, Remoli ME, Giacomini E, Rosicarelli B, Serafini B et al (2005) Astrocytes produce dendritic cell-attracting chemokines in vitro and in multiple sclerosis lesions. J Neuropathol Exp Neurol 64(8):706–715. https://doi.org/10.1097/01.jnen.0000173893.01929.fc
Benson K, Cramer S, Galla HJ (2013) Impedance-based cell monitoring: barrier properties and beyond. Fluids Barriers CNS 10(1):5. https://doi.org/10.1186/2045-8118-10-5
Broux B, Gowing E, Prat A (2015) Glial regulation of the blood-brain barrier in health and disease. Semin Immunopathol 37(6):577–590. https://doi.org/10.1007/s00281-015-0516-2
Broux B, Zandee S, Gowing E, Charabati M, Lécuyer MA, Tastet O et al (2020) Interleukin-26, preferentially produced by T(H)17 lymphocytes, regulates CNS barrier function. Neurol Neuroimmunol Neuroinflamm. https://doi.org/10.1212/nxi.0000000000000870
Chen SH, Benveniste EN (2004) Oncostatin M: a pleiotropic cytokine in the central nervous system. Cytokine Growth Factor Rev 15(5):379–391. https://doi.org/10.1016/j.cytogfr.2004.06.002
Chigaev A, Smagley Y, Haynes MK, Ursu O, Bologa CG, Halip L et al (2015) FRET detection of lymphocyte function-associated antigen-1 conformational extension. Mol Biol Cell 26(1):43–54. https://doi.org/10.1091/mbc.E14-06-1050
Deerhake ME, Danzaki K, Inoue M, Cardakli ED, Nonaka T, Aggarwal N et al (2021) Dectin-1 limits autoimmune neuroinflammation and promotes myeloid cell-astrocyte crosstalk via Card9-independent expression of Oncostatin M. Immunity 54(3):484–98.e8. https://doi.org/10.1016/j.immuni.2021.01.004
Dendrou CA, Fugger L, Friese MA (2015) Immunopathology of multiple sclerosis. Nat Rev Immunol 15(9):545–558. https://doi.org/10.1038/nri3871
Dhaeze T, Tremblay L, Lachance C, Peelen E, Zandee S, Grasmuck C et al (2019) CD70 defines a subset of proinflammatory and CNS-pathogenic T(H)1/T(H)17 lymphocytes and is overexpressed in multiple sclerosis. Cell Mol Immunol 16(7):652–665. https://doi.org/10.1038/s41423-018-0198-5
Drechsler J, Grötzinger J, Hermanns HM (2012) Characterization of the rat oncostatin M receptor complex which resembles the human, but differs from the murine cytokine receptor. PLoS ONE 7(8):e43155. https://doi.org/10.1371/journal.pone.0043155
El Sharkawi FZ, Ali SA, Hegazy MI, Atya HB (2019) The combined effect of IL-17F and CCL20 gene polymorphism in susceptibility to multiple sclerosis in Egypt. Gene 685:164–169. https://doi.org/10.1016/j.gene.2018.11.006
Ensoli F, Fiorelli V, Lugaresi A, Farina D, De Cristofaro M, Collacchi B et al (2002) Lymphomononuclear cells from multiple sclerosis patients spontaneously produce high levels of oncostatin M, tumor necrosis factors alpha and beta, and interferon gamma. Mult Scler 8(4):284–288. https://doi.org/10.1191/1352458502ms817oa
Fearon U, Mullan R, Markham T, Connolly M, Sullivan S, Poole AR et al (2006) Oncostatin M induces angiogenesis and cartilage degradation in rheumatoid arthritis synovial tissue and human cartilage cocultures. Arthritis Rheum 54(10):3152–3162. https://doi.org/10.1002/art.22161
Fitzhugh DJ, Naik S, Caughman SW, Hwang ST (2000) Cutting edge: C-C chemokine receptor 6 is essential for arrest of a subset of memory T cells on activated dermal microvascular endothelial cells under physiologic flow conditions in vitro. J Immunol 165(12):6677–6681. https://doi.org/10.4049/jimmunol.165.12.6677
Ghannam S, Dejou C, Pedretti N, Giot JP, Dorgham K, Boukhaddaoui H et al (2011) CCL20 and β-defensin-2 induce arrest of human Th17 cells on inflamed endothelium in vitro under flow conditions. J Immunol 186(3):1411–1420. https://doi.org/10.4049/jimmunol.1000597
Haghayegh Jahromi N, Marchetti L, Moalli F, Duc D, Basso C, Tardent H et al (2019) Intercellular adhesion molecule-1 (ICAM-1) and ICAM-2 differentially contribute to peripheral activation and CNS entry of autoaggressive Th1 and Th17 cells in experimental autoimmune encephalomyelitis. Front Immunol 10:3056. https://doi.org/10.3389/fimmu.2019.03056
Hanlon MM, Rakovich T, Cunningham CC, Ansboro S, Veale DJ, Fearon U et al (2019) STAT3 mediates the differential effects of Oncostatin M and TNFα on RA synovial fibroblast and endothelial cell function. Front Immunol 10:2056. https://doi.org/10.3389/fimmu.2019.02056
Haroon F, Drögemüller K, Händel U, Brunn A, Reinhold D, Nishanth G et al (2011) Gp130-dependent astrocytic survival is critical for the control of autoimmune central nervous system inflammation. J Immunol 186(11):6521–6531. https://doi.org/10.4049/jimmunol.1001135
Houben E, Hellings N, Broux B (2019) Oncostatin M, an underestimated player in the central nervous system. Front Immunol 10:1165. https://doi.org/10.3389/fimmu.2019.01165
Houben E, Janssens K, Hermans D, Vandooren J, Van den Haute C, Schepers M et al (2020) Oncostatin M-induced astrocytic tissue inhibitor of metalloproteinases-1 drives remyelination. Proc Natl Acad Sci U S A 117(9):5028–5038. https://doi.org/10.1073/pnas.1912910117
Huang J, Khademi M, Fugger L, Lindhe Ö, Novakova L, Axelsson M et al (2020) Inflammation-related plasma and CSF biomarkers for multiple sclerosis. Proc Natl Acad Sci U S A 117(23):12952–12960. https://doi.org/10.1073/pnas.1912839117
Ichihara M, Hara T, Kim H, Murate T, Miyajima A (1997) Oncostatin M and leukemia inhibitory factor do not use the same functional receptor in mice. Blood 90(1):165–173
Ishiwata I, Ishiwata C, Ishiwata E, Sato Y, Kiguchi K, Tachibana T et al (2005) Establishment and characterization of a human malignant choroids plexus papilloma cell line (HIBCPP). Hum Cell 18(1):67–72. https://doi.org/10.1111/j.1749-0774.2005.tb00059.x
Janssens K, Maheshwari A, Van den Haute C, Baekelandt V, Stinissen P, Hendriks JJ et al (2015) Oncostatin M protects against demyelination by inducing a protective microglial phenotype. Glia 63(10):1729–1737. https://doi.org/10.1002/glia.22840
Janssens K, Van den Haute C, Baekelandt V, Lucas S, van Horssen J, Somers V et al (2015) Leukemia inhibitory factor tips the immune balance towards regulatory T cells in multiple sclerosis. Brain Behav Immun 45:180–188. https://doi.org/10.1016/j.bbi.2014.11.010
Jones SA, Jenkins BJ (2018) Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat Rev Immunol 18(12):773–789. https://doi.org/10.1038/s41577-018-0066-7
Kerfoot SM, Raharjo E, Ho M, Kaur J, Serirom S, McCafferty DM et al (2001) Exclusive neutrophil recruitment with oncostatin M in a human system. Am J Pathol 159(4):1531–1539. https://doi.org/10.1016/s0002-9440(10)62538-2
Larochelle C, Cayrol R, Kebir H, Alvarez JI, Lécuyer MA, Ifergan I et al (2012) Melanoma cell adhesion molecule identifies encephalitogenic T lymphocytes and promotes their recruitment to the central nervous system. Brain 135(Pt 10):2906–2924. https://doi.org/10.1093/brain/aws212
Lassmann H, Bradl M (2017) Multiple sclerosis: experimental models and reality. Acta Neuropathol 133(2):223–244. https://doi.org/10.1007/s00401-016-1631-4
Lee DSW, Yam JY, Grasmuck C, Dasoveanu D, Michel L, Ward LA et al (2021) CCR6 expression on B cells is not required for clinical or pathological presentation of MOG protein-induced experimental autoimmune encephalomyelitis despite an altered germinal center response. J Immunol 207(6):1513–1521. https://doi.org/10.4049/jimmunol.2001413
Li R, Sun X, Shu Y, Wang Y, Xiao L, Wang Z et al (2017) Serum CCL20 and its association with SIRT1 activity in multiple sclerosis patients. J Neuroimmunol 313:56–60. https://doi.org/10.1016/j.jneuroim.2017.10.013
Lindberg RA, Juan TS, Welcher AA, Sun Y, Cupples R, Guthrie B et al (1998) Cloning and characterization of a specific receptor for mouse oncostatin M. Mol Cell Biol 18(6):3357–3367. https://doi.org/10.1128/mcb.18.6.3357
Liston A, Kohler RE, Townley S, Haylock-Jacobs S, Comerford I, Caon AC et al (2009) Inhibition of CCR6 function reduces the severity of experimental autoimmune encephalomyelitis via effects on the priming phase of the immune response. J Immunol 182(5):3121–3130. https://doi.org/10.4049/jimmunol.0713169
Lopes Pinheiro MA, Kooij G, Mizee MR, Kamermans A, Enzmann G, Lyck R et al (1862) (2016) Immune cell trafficking across the barriers of the central nervous system in multiple sclerosis and stroke. Biochim Biophys Acta 3:461–471. https://doi.org/10.1016/j.bbadis.2015.10.018
Ma Q, Shimaoka M, Lu C, Jing H, Carman CV, Springer TA (2002) Activation-induced conformational changes in the I domain region of lymphocyte function-associated antigen 1. J Biol Chem 277(12):10638–10641. https://doi.org/10.1074/jbc.M112417200
Maki W, Morales RE, Carroll VA, Telford WG, Knibbs RN, Stoolman LM et al (2002) CCR6 colocalizes with CD18 and enhances adhesion to activated endothelial cells in CCR6-transduced jurkat T cells. J Immunol 169(5):2346–2353. https://doi.org/10.4049/jimmunol.169.5.2346
Marchetti L, Engelhardt B (2020) Immune cell trafficking across the blood-brain barrier in the absence and presence of neuroinflammation. Vasc Biol 2(1):H1-h18. https://doi.org/10.1530/vb-19-0033
Meares GP, Ma X, Qin H, Benveniste EN (2012) Regulation of CCL20 expression in astrocytes by IL-6 and IL-17. Glia 60(5):771–781. https://doi.org/10.1002/glia.22307
Michel L, Grasmuck C, Charabati M, Lécuyer MA, Zandee S, Dhaeze T et al (2019) Activated leukocyte cell adhesion molecule regulates B lymphocyte migration across central nervous system barriers. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aaw0475
Moidunny S, Dias RB, Wesseling E, Sekino Y, Boddeke HW, Sebastião AM et al (2010) Interleukin-6-type cytokines in neuroprotection and neuromodulation: oncostatin M, but not leukemia inhibitory factor, requires neuronal adenosine A1 receptor function. J Neurochem 114(6):1667–1677. https://doi.org/10.1111/j.1471-4159.2010.06881.x
Mony JT, Khorooshi R, Owens T (2014) Chemokine receptor expression by inflammatory T cells in EAE. Front Cell Neurosci 8:187. https://doi.org/10.3389/fncel.2014.00187
Moser T, Akgün K, Proschmann U, Sellner J, Ziemssen T (2020) The role of TH17 cells in multiple sclerosis: therapeutic implications. Autoimmun Rev 19(10):102647. https://doi.org/10.1016/j.autrev.2020.102647
Murakami M, Kamimura D, Hirano T (2019) Pleiotropy and specificity: insights from the interleukin 6 family of cytokines. Immunity 50(4):812–831. https://doi.org/10.1016/j.immuni.2019.03.027
Nakamura K, Nonaka H, Saito H, Tanaka M, Miyajima A (2004) Hepatocyte proliferation and tissue remodeling is impaired after liver injury in oncostatin M receptor knockout mice. Hepatology 39(3):635–644. https://doi.org/10.1002/hep.20086
Profaci CP, Munji RN, Pulido RS, Daneman R (2020) The blood-brain barrier in health and disease: important unanswered questions. J Exp Med. https://doi.org/10.1084/jem.20190062
Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S et al (2009) C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol 10(5):514–523. https://doi.org/10.1038/ni.1716
Repovic P, Benveniste EN (2002) Prostaglandin E2 is a novel inducer of oncostatin-M expression in macrophages and microglia. J Neurosci 22(13):5334–5343. https://doi.org/10.1523/jneurosci.22-13-05334.2002
Rodríguez-Lorenzo S, Ferreira Francisco DM, Vos R, van Het Hof B, Rijnsburger M, Schroten H et al (2020) Altered secretory and neuroprotective function of the choroid plexus in progressive multiple sclerosis. Acta Neuropathol Commun 8(1):35. https://doi.org/10.1186/s40478-020-00903-y
Ruprecht K, Kuhlmann T, Seif F, Hummel V, Kruse N, Brück W et al (2001) Effects of oncostatin M on human cerebral endothelial cells and expression in inflammatory brain lesions. J Neuropathol Exp Neurol 60(11):1087–1098. https://doi.org/10.1093/jnen/60.11.1087
Sambrano J, Chigaev A, Nichani KS, Smagley Y, Sklar LA, Houston JP (2018) Evaluating integrin activation with time-resolved flow cytometry. J Biomed Opt 23(7):1–10. https://doi.org/10.1117/1.Jbo.23.7.075004
Slaets H, Nelissen S, Janssens K, Vidal PM, Lemmens E, Stinissen P et al (2014) Oncostatin M reduces lesion size and promotes functional recovery and neurite outgrowth after spinal cord injury. Mol Neurobiol 50(3):1142–1151. https://doi.org/10.1007/s12035-014-8795-5
Srinivasan B, Kolli AR, Esch MB, Abaci HE, Shuler ML, Hickman JJ (2015) TEER measurement techniques for in vitro barrier model systems. J Lab Autom 20(2):107–126. https://doi.org/10.1177/2211068214561025
Sugaya M, Fang L, Cardones AR, Kakinuma T, Jaber SH, Blauvelt A et al (2006) Oncostatin M enhances CCL21 expression by microvascular endothelial cells and increases the efficiency of dendritic cell trafficking to lymph nodes. J Immunol 177(11):7665–7672. https://doi.org/10.4049/jimmunol.177.11.7665
Takata F, Dohgu S, Matsumoto J, Machida T, Sakaguchi S, Kimura I et al (2018) Oncostatin M-induced blood-brain barrier impairment is due to prolonged activation of STAT3 signaling in vitro. J Cell Biochem 119(11):9055–9063. https://doi.org/10.1002/jcb.27162
Takata F, Dohgu S, Sakaguchi S, Sakai K, Yamanaka G, Iwao T et al (2019) Oncostatin-M-reactive pericytes aggravate blood-brain barrier dysfunction by activating JAK/STAT3 signaling in vitro. Neuroscience 422:12–20. https://doi.org/10.1016/j.neuroscience.2019.10.014
Takata F, Sumi N, Nishioku T, Harada E, Wakigawa T, Shuto H et al (2008) Oncostatin M induces functional and structural impairment of blood-brain barriers comprised of rat brain capillary endothelial cells. Neurosci Lett 441(2):163–166. https://doi.org/10.1016/j.neulet.2008.06.030
Tanaka M, Hirabayashi Y, Sekiguchi T, Inoue T, Katsuki M, Miyajima A (2003) Targeted disruption of oncostatin M receptor results in altered hematopoiesis. Blood 102(9):3154–3162. https://doi.org/10.1182/blood-2003-02-0367
Tenenbaum T, Steinmann U, Friedrich C, Berger J, Schwerk C, Schroten H (2013) Culture models to study leukocyte trafficking across the choroid plexus. Fluids Barriers CNS 10(1):1. https://doi.org/10.1186/2045-8118-10-1
Tinevez JY, Perry N, Schindelin J, Hoopes GM, Reynolds GD, Laplantine E et al (2017) TrackMate: an open and extensible platform for single-particle tracking. Methods 115:80–90. https://doi.org/10.1016/j.ymeth.2016.09.016
Ujlaky-Nagy L, Nagy P, Szöllősi J, Vereb G (2018) Flow cytometric FRET analysis of protein interactions. Methods Mol Biol 1678:393–419. https://doi.org/10.1007/978-1-4939-7346-0_17
van Keulen D, Pouwer MG, Pasterkamp G, van Gool AJ, Sollewijn Gelpke MD, Princen HMG et al (2018) Inflammatory cytokine oncostatin M induces endothelial activation in macro- and microvascular endothelial cells and in APOE*3Leiden.CETP mice. PLoS One 13(10):e0204911. https://doi.org/10.1371/journal.pone.0204911
Villares R, Cadenas V, Lozano M, Almonacid L, Zaballos A, Martínez AC et al (2009) CCR6 regulates EAE pathogenesis by controlling regulatory CD4+ T-cell recruitment to target tissues. Eur J Immunol 39(6):1671–1681. https://doi.org/10.1002/eji.200839123
Walling BL, Kim M (2018) LFA-1 in T cell migration and differentiation. Front Immunol 9:952. https://doi.org/10.3389/fimmu.2018.00952
Weiss TW, Samson AL, Niego B, Daniel PB, Medcalf RL (2006) Oncostatin M is a neuroprotective cytokine that inhibits excitotoxic injury in vitro and in vivo. Faseb j 20(13):2369–2371. https://doi.org/10.1096/fj.06-5850fje
Weksler B, Romero IA, Couraud PO (2013) The hCMEC/D3 cell line as a model of the human blood brain barrier. Fluids Barriers CNS 10(1):16. https://doi.org/10.1186/2045-8118-10-16
Wojkowska DW, Szpakowski P, Glabinski A (2017) Interleukin 17A promotes lymphocytes adhesion and induces CCL2 and CXCL1 release from brain endothelial cells. Int J Mol Sci. https://doi.org/10.3390/ijms18051000
Zhang X, Li J, Qin JJ, Cheng WL, Zhu X, Gong FH et al (2017) Oncostatin M receptor β deficiency attenuates atherogenesis by inhibiting JAK2/STAT3 signaling in macrophages. J Lipid Res 58(5):895–906. https://doi.org/10.1194/jlr.M074112
Acknowledgements
This work was financially supported by grants from the Research Foundation of Flanders (FWO Vlaanderen, G097318N), Bijzonder Onderzoeksfonds (BOF) UHasselt and the Fondation Charcot. The hCMEC/D3 cell line was provided by Tebu-bio (Le Perray-en-Yvelines, France). OSMRβ KO mice (B6.129S-Osmr <tm1Mtan >) were provided by the RIKEN BRC through the National Bio-Resource Project of MEXT, Japan. We would like to thank Lyne Bourbonnière for assistance in HBMEC culture, Dr. Antoine Fournier, Marc Charabati and Sam Duwé for technical assistance, and Britt Coenen, Athanasios Bethanis, Jules Teuwen, Ina Vantyghem and Kardelen Irem Isin for their practical help with experiments.
Author information
Authors and Affiliations
Contributions
Conceptualization: BB, NH; methodology: DH, EH, PB, HS, KJ, CH, BH, GD, SB, EG, ChH, LB, KFW, JF, SZ, RT, AP; formal analysis: DH, EH, CH, KJ, JF, SZ; writing-original draft: DH, EH, KJ; writing-review and editing: BB, NH, HS, GK, HEdV; visualization: DH; supervision: BB, NH. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
401_2022_2445_MOESM1_ESM.tif
Supplementary file1 Suppl. Fig. 1 OSM does not affect the functional properties of activated CD4+ T cells, CD8+ T cells and CD19+ B cells. CD4+ T cells, CD8+ T cells and B cells were isolated from PBMCs of healthy donors using magnetic selection (n=5). (a, d, g) T and B cell proliferation were analysed with flow cytometry after 6 days of stimulation with αCD3/28/2-coated beads or CpG2006, respectively, in the absence or presence of OSM (25 ng/ml) and anti-LIFR antibody (20 µg/ml). (b, c) Flow cytometric analysis of IFNγ and IL17 expression by CD4+ T memory cells cultured under non-skewing and Th1 or Th17 skewing conditions in the presence or absence of OSM. (e, f) Concentration of IL4, IL17, IFNγ, granzyme A, granzyme B and perforin in the conditioned medium of resting or stimulated CD8+ T cells, in the absence or presence of OSM and anti-LIFR antibody, measured using LegendPlex™ multiplex assay. (h, i) Flow cytometric analysis of the percentage CD80+ activated B cells and CD24-CD38+ plasmablasts, respectively, in the absence or presence of OSM and anti-LIFR antibody. Data are depicted as mean ± SEM. Statistical analysis was performed using Wilcoxon test, one-way ANOVA and two-way ANOVA using Tukey’s multiple comparisons test with *p≤0.05, **p≤0.01, ***p≤0.001. OSM, oncostatin M; LIFR, leukemia inhibitory factor; IFNγ, interferon gamma; IL, interleukin (TIF 642 KB)
401_2022_2445_MOESM2_ESM.tif
Supplementary file2 Suppl. Fig. 2 Less severe EAE in OSMRβ-deficient mice is not attributable to an altered peripheral immune cell response. WT and OSMRβ KO mice were injected with MOG35-55 in CFA and 40 ng/100 µl PTX (WT: n=30; OSMRβ KO: n=33; pooled data of 3 independent experiments). Mice were sacrificed at onset (13 dpi; WT: n=5; KO: n=5), peak (19 dpi; WT: n=9; KO: n=6) and chronic phase of EAE (50 dpi; WT: n=5; KO: n=5). (a) Sum of EAE scores were evaluated. (b) Lymphocyte proliferation in response to MOG or ConA as measured by CFSE incorporation in splenocytes from WT and OSMRβ KO mice, 10 days after EAE induction (n=3/genotype). (c) Flow cytometric analysis of the percentage of CD4+ T cells within the live cell population. White and grey bars depict WT and OSMRβ KO mice, respectively. (d) Gating strategy of the immune cell profile in the CNS at EAE peak. Single cells are gated, using the area and height of the forward scatter (FSC-A, FSC-H). Dead cells are excluded using Zombie NIR. Lymphocytes are gated based on forward and sideward scatter (FSC-A, SSC-A). Next, leukocytes are characterized based on CD45. T and B cells are distinguished based on CD3 and CD19, respectively. Within the CD3+ T cell gate, CD4+ T helper cells and CD8+ cytotoxic T cells are identified. Finally, IFNγ, IL17 and Foxp3 are used to gate Th1 (Q4), Th17 (Q1) and T regulatory cells, respectively. (e,f) Absolute numbers of infiltrating Th1 and Th17 cells, respectively, were calculated by multiplying the percentage cells within the lymphocyte gate by the total amount of CNS-infiltrating cells, counted by an automated cell counter. (g) Positive correlation between % Th17 cells and absolute number of Th17 cells in pooled WT and OSMR KO mice (EAE score > 0), using simple linear regression. (h-m) Flow cytometric analysis of the percentage of CD8+ T cells and CD19+ B cells in lymph nodes, spleen and CNS, respectively. Statistical analysis was performed using two-way ANOVA and Sidak’s multiple comparisons test. Data are depicted as mean ± SEM. EAE, experimental autoimmune encephalomyelitis; MOG, myelin oligodendrocyte glycoprotein; ConA, Concanavalin A; WT, wild type; OSMRβ KO, oncostatin M receptor knock-out; IFNγ, interferon gamma; IL17, interleukin 17; Th, T helper cell (TIF 1511 KB)
401_2022_2445_MOESM3_ESM.tif
Supplementary file3 Suppl. Fig. 3 OSM-induced effects on mouse BBB-ECs are abrogated in absence of OSMRβ signalling. Primary MBMECs isolated from OSMRβ KO mice (n=5) were treated with 25 ng/ml OSM in the presence/absence of 10 ng/ml TNF-α/IFN-γ for 48h. (a) TEER was measured manually. Flow cytometric analysis of (b) ICAM-1 and (c) VCAM-1 expression depicted as median fluorescence intensity (MFI). Statistical analysis was performed using one-way ANOVA with matched data and Šidák’s multiple comparisons test with *p≤0.05, **p≤0.01, ***p≤0.001, ***p≤0.0001. Data are depicted as mean ± SEM. MFI: median fluorescence intensity; OSM, oncostatin M; ICAM-1, intercellular cell adhesion molecule 1; VCAM-1, vascular cell adhesion molecule 1; TNFα, tumor necrosis factor alpha; IFNγ, interferon gamma; TEER, transendothelial electrical resistance (TIF 446 KB)
401_2022_2445_MOESM4_ESM.tif
Supplementary file4 Suppl. Fig. 4 ICAM-2 and ICAM-3 expression is not affected by OSM treatment. qPCR analysis of (a) ICAM-1, (b) ICAM-2 and (c) ICAM-3 OSMRβ mRNA hCMEC/D3 cells (n = 5) treated with 25 ng/ml OSM in the presence/absence of 10 ng/ml TNF-α/IFN-γ for 24h. Statistical analysis was performed using one-way ANOVA and Šidák’s multiple comparisons test with *p≤0.05, **p≤0.01, ***p≤0.001, ***p≤0.0001. Data are depicted as mean ± SEM. OSM, oncostatin M; TNFα, tumor necrosis factor alpha; IFNγ, interferon gamma; ICAM, intercellular cell adhesion molecule (TIF 457 KB)
Rights and permissions
About this article
Cite this article
Hermans, D., Houben, E., Baeten, P. et al. Oncostatin M triggers brain inflammation by compromising blood–brain barrier integrity. Acta Neuropathol 144, 259–281 (2022). https://doi.org/10.1007/s00401-022-02445-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-022-02445-0