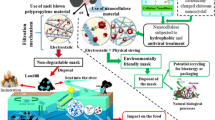
Abstract
Particulate matter (PM) pollution and SARS-CoV-2 (COVID-19) have brought severe threats to public health. High level of PM serves as a carrier of COVID-19 which is a global pandemic. This study fabricated filter membrane for face mask using bacterial cellulose and fingerroot extract (BC–FT) via immersion technique. The surface area, pore volume and pore size of BC were analyzed by Brunauer–Emmett–Teller. The physiochemical properties of the membrane were analyzed by scanning electron microscopy, Fourier transform infrared spectroscopy and X-ray diffractometer. The crystallinity decreased from 63.7% in pure BC to 52.4% in BC–FT filter membrane. Young’s modulus increased from 1277.02 MPa in pure BC to 2251.17 MPa in BC–FT filter membrane. The filter membrane showed excellent PM 0.1 removal efficiency of 99.83% and antimicrobial activity against Staphylococcus aureus and Escherichia coli. The fabricated membrane is excellent to prevent inhalation of PM2.5 and COVID-19 respiratory droplet.
Graphic abstract

Similar content being viewed by others
Introduction
Air pollution has become a major concern in the world due to the various harmful health effects in humans and animals (Manisalidis et al. 2020). One of the main pollutants that poses serious health threat is particulate matter. Particulate matter (PM) “is a heterogeneous mixture of suspended liquid droplets and solid particles in the air with various size, shape, and chemical features” (Maleki et al. 2021). PM contains various substances including metals, elemental and organic carbon, sulfates, and nitrates (Maleki et al. 2021). There are two kinds of PM namely PM 10 and PM 2.5. The PM 10 is an inhalable particle with diameter less than or equal to 10 μm, while PM 2.5 is a pollutant that has diameter less than or equal to 2.5 μm (Zhang et al. 2019). The PM 2.5 is more dangerous than PM 10 because it can invade the deepest parts of the lungs and easily reach the bloodstream due to the small size of the particles (Manisalidis et al. 2020). Some reported health effects for inhaling PM 2.5 include respiratory and cardiovascular morbidity such as chronic obstructive pulmonary disease (COPD), Ischemic heart disease, mortality from cardiovascular, respiratory diseases and lung cancer (Huang et al. 2019; Manisalidis et al. 2020). Over 7 million people die every year due to air pollution (Nations 2019), with about 103.1 million disability-adjusted life-years (DALYs) (Choi et al. 2021).
The emergence of novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) also known as COVID-19, has taken a heavy toll on global health. The rapid spread of the disease has resulted in global shortage of personal protective equipment, with high demand for face mask (Choi et al. 2021; Organization 2020). COVID-19 is a respiratory disease that damages lungs, body tissues and other organs (Aydin et al. 2021). The virus can be transmitted from an infected person through the following routes; surface/contact, respiratory droplet, and aerosol (Anand and Mayya 2020). The minimum size of a respiratory droplets that can contain COVID-19 is approximately 0.1 to 10 μm (Anand and Mayya 2020; Lee 2020), that is considered as one of the most dangerous routes of transmission. The droplets and aerosols can get into the human body (respiratory system) by inhalation and through the mouth. Earlier studies have shown that the PM concentration is associated with the “transmission dynamics and quick global spread of COVID-19” (Coccia 2020; Maleki et al. 2021). The RNA of COVID-19 can be present on PM in atmospheric condition and high levels of PM. PM concentration could be an indicator for a high potential COVID-19 infection (Setti et al. 2020). Wearing of face mask is one of the highly recommended preventive measures to curtail COVID-19 infection by reducing risk of direct and indirect viral exposure (Zangmeister et al. 2020).
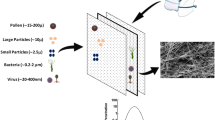
The most important part of face mask is the filter membrane. The filtration efficiency of face mask is affected by fiber diameter of the filter membrane. Fiber diameter at the nanometer scale is more effective than the one at the micrometer scale, due to large surface area for capturing particles. The filtration efficiency of polyacrylonitrile (PAN) filter membrane with diameter of 0.3 μm was reported to have lower efficiency (90.0%) when compared to 77 nm fiber diameter filter membrane with 99.26% filtration efficiency (Cao et al. 2019). The removal efficiency of filter membrane with fiber diameter of 590 ± 180 nm had better filtration efficiency (92.00 ± 1.00%) than that of commercial mask (64.00 ± 1.50%) with fiber diameter of 3.9 ± 1.6 μm (Tan et al. 2019). One major challenge of these filter membranes is that they are made from synthetic fibers that are difficult to degrade. The PM pollutants couple with the recent global Covid-19 pandemic have contributed to the rise in waste from used face mask. About 468.9 tons of medical wastes including face masks are generated every day (Sangkham 2020), most of which are non-biodegradable and contain toxic chemicals that poses environmental and health problems (Sullivan et al. 2021). Elimination methods such as incineration may lead to global warming. Herein, this study used biodegradable bacterial cellulose, which is skin friendly, non-allergic, eco-friendly and sustainable as a filter membrane for face mask.
Bacterial cellulose (BC) is a biodegradable natural cellulose with diameter of fibers ranging 20 to 100 nm (Manoukian et al. 2019). It is also known as microbial cellulose produced by several types of acetic acid bacteria, including Komagataeibacter xylinus. BC has specific properties such as high water-holding capacity due to its hydrophilic nature, high tensile strength, smaller fiber diameter with thickness of 3–4 nm and relatively less expensive to produce. The fibers are orderly arranged with high surface area, non-toxic and biocompatible (Manoukian et al. 2019; Torgbo and Sukyai 2018). Using BC as filter membrane for face mask will result in better filtration efficiency than commercial membrane due to high surface area, nano fiber diameter and high hydrophilicity.
Heat and humidity build up from breathing when wearing face mask. This results in optimal conditions for the growth of microorganisms. Also, when a patient wearing face mask coughs or sneezes it causes an accumulation of microorganisms on the mask. Similarly, the person wearing the mask near a patient (for instance Covid-19 patient) who sneeze may contract pathogens from the patient. Bacterial and fungal contamination has been reported on the inside and outside areas of used face masks, with higher contamination on outside areas (Luksamijarulkul et al. 2014). Therefore, this research used fingerroot extract to address this problem. Fingerroot (Boesenbergia rotunda) belong to the Zingiberaceae family and it is a traditional medicinal plant native to Southeast Asia and Indo-China (Ongwisespaiboon and Jiraungkoorskul 2017). Fingerroot also serves as food ingredient in daily food intake due to its biological and nutritional properties. Its phytochemical components include essential oils, flavonoids, boesenbergin, krachaizin, panduratin A, panduratin B and pinostrobin (Ongwisespaiboon and Jiraungkoorskul 2017). The phytochemical components are known to possess several biological activities including antibacterial, antiviral and wound healing properties. Studies have shown good antibacterial activity against microorganisms that are commonly involved in hospital-acquired infections such as Staphylococcus aureus and Escherichia coli and against acne-inducing bacteria such as Propionibacterium acnes (Mazlan et al. 2016; Rahman et al. 2016; Zainin et al. 2013). A study has also shown that its phytochemical component exerts inhibitory effect against COVID-19 (Kanjanasirirat et al. 2020). In this study, an eco-friendly and inexpensive standard green solvent dimethyl sulfoxide (DMSO) was used to fabricate the fingerroot extract and BC filter membrane. DMSO is nontoxic and recyclable when compared to traditional solvents. Therefore, it has been used by previous studies to fabricate membrane (Xie et al. 2019).
The recent challenges of PM and COVID-19 pandemic have caused rising global demand for face masks and shortage of the raw materials for their production. The rising environmental challenges with used face mask, require fabrication of efficient and eco-friendly filter membrane. This study fabricated filter membrane for face mask with high removal efficiency using sustainable and biodegradable BC and fingerroot extract as an antimicrobial agent. The results presented can answer the question on whether BC can be used as filter membrane of face mask for efficient removal of PMs.
Experimental section
Materials and chemicals
Komagataeibacter xylinus (TISTR No.975) was purchased from the Thailand Institute of Scientific and Technological Research, Pathum Thani, Thailand. Coconut water was purchased from the local market. Fingerroot extract powder was purchased from AP operations Co., Ltd. (Thailand). Acetic acid (CH3COOH), ammonium sulfate ((NH4)2SO4) and sodium hydroxide (NaOH), were purchased from Ajax Finechem Pty., Ltd. (New Zealand). Dimethyl sulphoxide (CH3SOCH3) was purchased from Loba Chemie Pvt. Ltd. (India). All other chemicals were of analytical grade and used without further purification.
Preparation of BC
The BC was prepared following a previous method (Torgbo and Sukyai 2019) with modifications. Basically, the culture medium was prepared by mixing 90 mL of coconut water with 5% (w/v) sucrose and 2.5% (w/v) ammonium sulfate, followed by sterilization at 100 °C. After sterilization, the medium was cooled to room temperature and the pH was adjusted to 4.5 with 5% (v/v) glacial acetic acid and 10% (v/v) of inoculum (Komagataeibacter xylinus) was inoculated. Microbial cultures were incubated at room temperature for 7 days under static condition. After incubation, the BC pellicles were collected and boiled in deionized (DI) water to remove the residual medium and other impurities. BC pellicles were then boiled in 1% (w/v) NaOH solution for 1 h to remove the cells and boiled in DI water until the pH of the water became neutral (pH 7). The purified BC was frozen at − 20 °C for at least 24 h before lyophilized.
Determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
The MIC and MBC of fingerroot extract were determined in 96-well microplates using broth microdilution method (Sunthornvarabhas et al. 2020). Mueller–Hinton broth was used as diluent for Staphylococcus aureus and Escherichia coli. The positive control composed of diluent and nutrient broth, while the negative control contained only bacteria strains. Microplates were inoculated at 37 °C for 24 h. To determine the MBC, 10 µL of bacterial suspension was removed from each well after overnight growth, (before adding the resazurin working solution) and spread onto Mueller–Hinton agar prior to incubation at 37 °C for 24 h. The MBC was defined as the lowest concentration of fingerroot extract at which 99.9% of the inoculated microorganisms were killed (Sukatta et al. 2021).
Preparation of BC with fingerroot extract filter membrane
The fingerroot extract (3% w/v) was dissolved in 100 mL dimethyl sulfoxide (DMSO). The freeze-dried BC (7 cm × 11 cm) was soaked in the fingerroot solution and shaken at 200 rpm using mechanical shaker at room temperature for 24 h. Thereafter, the BC membrane was rinsed with deionized water to remove unbound particles as well as excess DMSO and frozen at − 20 °C for 24 h before freeze dried. The freeze-dried BC with fingerroot extract filter membrane (BC–FT filter membrane) was pressed with manually designed (5 cm × 10 cm, with 0.5 cm interval between needles) 3D sterilized needle grid to create pores. The amount of extract in BC–FT membrane was determined by the difference in weight of BC before and after addition of the extract (BC–FT). The process is summarized in Fig. 1.
Characterization
Scanning electron microscopy
Surface morphologies of pure BC and BC–FT filter membrane were examined using a scanning electron microscope (FEI Quanta 450, Czech Republic) at an accelerating voltage of 15–20 kV.
Porous size and porosity measurement by Brunauer–Emmett–Teller (BET)
The surface area, pore volume and pore size of pure BC membrane were analyzed by nitrogen adsorption and desorption at 200 °C. The various parameters were calculated using Micromeritics ASAP 2020 V3.00 software, Brunauer–Emmett–Teller (BET) equation and Barrett–Joyner–Halenda (BJH) method.
Fourier transform infrared (FTIR) spectroscopy
The chemical interaction and functional groups of pure BC and BC–FT filter membrane were analyzed using Fourier transform infrared spectrometer (Bruker model Tensor 27, USA) at room temperature. Pure BC and BC–FT filter membrane were analyzed by ATR mode. The fingerroot extract was blended with potassium bromide (KBr) powder and compressed to form a disc and analyzed by KBr method. The spectra were recorded in the transmittance mode with a resolution of 4 cm−1 in the range from 500 to 4000 cm−1.
X-ray diffraction (XRD)
The crystallinity and characteristic finger print of pure BC and fingerroot extract in BC–FT filter membrane were measured using an x-ray diffractometer (Bruker D8 Advance, Germany) using Cu-Kα radiation (λ = 1.54 Å) at 40 kV. The samples were scanned in 2θ angles from 5° to 50° with a scanning rate of 5°/min. Briefly, the sample was mounted in deep well sample holder made of polymethyl methacrylate (PMMA), packed well by glass slide and put into the sample stage. In the diffractometer, X-rays produced by the tube passed through primary optical component and irradiated the sample in the air environment, which was diffracted by the sample phases to pass through secondary optical component containing anti-scatter slit to ensure that no air scatter reach the detector. The individual crystalline peaks were extracted by peak-fitting process from the diffraction intensity profiles, with a maximum intensity ˃8,000 counts. A peak fitting program (OriginPro; www.originlab.com) was used, assuming pseudo-Voigt function for each peak and a broad peak ascribed to the amorphous contribution. After subtracting the background to ensure a stable baseline fitting and reduce fitting error, the pattern was deconvoluted into crystalline and amorphous contribution. The crystallinity index (CrI) was calculated by dividing the total area of main peaks of crystalline cellulose by the total peaks area (all crystalline plus amorphous peaks) according to Eq. 1.
Mechanical property measurements
The tensile stress and Young’s modulus of pure BC and the filter membrane were determined according to ASTM D882-02 standard using universal testing machine (Shimadzu model AGS5kN, Japan) fitted with a 500 N load cell, with crosshead speed of 20 mm/min and 20 mm distance between clamps. The samples were cut into 10 × 50 mm strips with replicates. The results were presented as an averaged for each sample.
Removal efficiency measurement
The removal efficiency of BC filter membrane and commercial membrane were determined by filtering polystyrene latex particles with diameter of 0.1 μm according to ASTM F2299-03 standard using TSI’s Automated Filter Tester 3160. The BC filter membrane was cut spherically with diameter of 5.02 cm as the middle layer in commercial outer and inner layers of face mask before been tested. The efficiency (η) was calculated using Eq. 2 (Tan et al. 2019):
where Cupstream is the concentration of particulate matter (PM0.1) taken before filtration and Cdownstream is the concentration of particulate matter taken after filtration.
Agar disc diffusion
The antimicrobial activity of BC with fingerroot was examined using the agar disc diffusion method by Chollakup et al. (2020) with modification. Briefly, bacteria were incubated on the nutrient agar plates at 35 °C for 24 h and then inoculated into NaCl (0.85% w/v) to obtain the inoculum suspension with the turbidity equivalent to McFarland No. 0.5 (108 CFU/mL). Staphylococcus aureus and Escherichia coli were inoculated on Mueller–Hinton agar. After that, the BC–FT filter membrane was cut into a circle (1 cm diameter) and placed on inoculated agar plates. The plates were then incubated at 37 °C for 24 h.
Statistical analysis
The data collected were subjected to one-way analysis of variance (ANOVA) using GenStat software 12th edition. The experiments were performed with n = 3 replicates and data presented as the average of three replicates. Bonferroni test was used to evaluate the differences between groups; p value < 0.05 was considered significant.
Results and discussion
Brunauer–Emmett–Teller (BET) analysis of BC membrane
The surface area, pore volume and pore size of pure BC dried by freeze drying and hydraulic press were analyzed by Brunauer–Emmett–Teller (BET) equation and Barrett–Joyner–Halenda (BJH) method, to determine which drying process is most appropriate. The result in Table 1 shows the surface area, pore volume and pore size of pure BC dried by freeze dry method is higher than hydraulic press. The freeze-dried BC (BC-FD) has surface area, pore volume and pore size of 69.915 m2/g, 0.270 cm3/g and 13.984 nm, respectively. This implies that freeze drying method is more appropriate for drying BC to fabricate the filter membrane. Higher surface area means higher area to capture PM, while higher pore size and pore volume means higher breathability, because air can pass through the pores with ease than the one with less pore size and volume. Nonetheless, BC dried by both methods have pore size in nano scale which is good for fabricating filter membrane, as PM cannot pass through it Zhang et al. (2020).
Morphology of pure BC and BC–FT filter membrane
The surface properties of pure BC and BC–FT filter membrane were observed using SEM. The SEM images of the sample are shown in Fig. 2. The pure BC shows characteristic morphology of cellulose with porous and interwoven fibrils networks (Tsai et al. 2018). After immersion in fingerroot solution, BC–FT filter membrane shows some changes in the surface architecture and porous networks. The cellulose fibrils (Fig. 2b) were covered by small particles of fingerroot extract. The amount of fingerroot extract on the BC membrane was estimated as 162.9 mg (5.43% of fingerroot extract). This confirmed successful impregnation of fingerroot extract into BC membrane. The porous network of pure BC provides an ideal environment for penetration of the extract under ex-situ shaking and attachment via physical and chemical interaction. This justifies the fabrication of low-cost filter membrane from BC and plant-based bioactive material for face mask.
FTIR analysis of pure BC and BC–FT filter membrane
The chemical functional groups of pure BC and BC–FT filter membrane were characterized using FTIR spectroscopy. FTIR spectra of pure BC and BC–FT filter membrane in Fig. 3 shows that, the BC–FT have same peak with pure BC. The O–H stretching vibration of hydroxyl group of cellulose was found at 3348 cm−1. The peaks at 2895 cm−1, 1639 cm−1, 1526 cm−1 and 1160 cm−1 represented the stretching vibration of C–H group, stretching vibration of C=C group, symmetric bending of CH2 and antisymmetric bridge stretching of 1,4-β-d-glucoside, respectively (Wang et al. 2017). The new absorption peaks observe in BC–FT at 1015 cm−1, 984 cm−1 and 950 cm−1 referred to C–H bending vibration from isoprenoids in fingerroot (Thummajitsakul and Silprasit 2021). The BC–FT shows stretching vibration in peak intensity of the various absorbance that are presence in pure BC. The stretched peak intensity and appearance of new peaks indicated the chemical interactions and bonding of fingerroot extract in BC matrices.
X-ray diffractogram of pure BC and BC–FT filter membrane
The crystallinity and characteristic fingerprints of pure BC and BC–FT filter membrane were measured using an X-ray diffractometer. Figure 4 shows the diffractogram of modeled individual peaks and fitted peaks of BC and BC–FT with their amorphous contributions (dotted lines). There were at least three peaks separated from the diffraction intensity profiles. The distinct peaks at 2θ = 14.6°, 16.9° and 22.7° represents the diffraction planes of (100), (010) and (110), respectively, corresponding to the Miller indices of diffraction crystallographic planes of Iα cellulose (French 2014). The diffractogram presents slight difference in the crystallinity degree of the two samples. The sharp diffraction peaks with high intensity in pure BC indicate more organized crystals and higher crystallinity than in BC–FT. The loading of fingerroot extract results in a significant reduction of crystallinity from 63.7% of pure BC to 52.4% in the BC–FT membrane. The stiffness of the fibrils and ribbons of cellulose is imparted by its crystallinity. Higher crystallinity means stronger polymer chain, less flexibility and higher thermal stability. The lesser crystalline peak areas and CrI of BC–FT shows that, the BC–FT filter membrane will easily degrade when disposed of than pure BC (Torgbo and Sukyai 2020). The addition of extract to BC possibly caused decreased in crystallinity due to the amorphous nature of the extract. The lower crystallinity was associated with easier and faster degradation process due to the transformation of crystallinity region into amorphous region (Torgbo and Sukyai 2020).
Mechanical properties of pure BC and BC–FT filter membrane
The mechanical properties of pure BC and BC–FT filter membrane were measured using tensile test. The results show highly significant difference between the samples. The addition of fingerroot extract to the BC resulted in an increased tensile strength of 243.21 MPa which is higher than pure BC of 159.74 MPa as shown in Table 2. Young’s modulus of pure BC and BC–FT filter membrane were 1277.02 MPa and 2251.17 MPa, respectively. It could be deduced that fingerroot extract improved the mechanical properties of BC by binding to the matrices. Figure 5 shows the representative stress-strain curves of pure BC and BC–FT filter membrane. The percentage elongation of pure BC and BC–FT filter membrane were 13.85% and 11.90%, which means that the elasticity of pure BC is higher than BC–FT. The reinforced nanoparticles from fingerroot led to stiffening of BC matrix which restricted the mobility of BC molecular chains (Usawattanakul et al. 2021), with subsequent reduction in elasticity of the BC–FT filter membrane. For the commercial membrane, the tensile machine could not detect the breaking point because it can only withstand a maximum force between 1.8 and 2.0 N, which is really low compared to 500 N used for the BC membranes.
Removal efficiency of BC–FT filter membrane
The removal efficiency of commercial membrane and BC–FT filter membrane was determined by filtering polystyrene latex (PSL) particles with diameter of 0.1 μm according to ASTM F2299-03 standard. In Fig. 6, the BC–FT filter membrane shows excellent removal efficiency of 99.83 ± 0.38% which is significantly higher than the pure BC and commercial membrane of 95.37 ± 0.014% and 93.23 ± 0.23%. The commercial membrane is made of polypropylene, a widely used polymer for synthetic fibers production. The polypropylene is mostly obtained through polymerization of propylene with or without other alpha olefin monomers, at high temperature cracking of petroleum hydrocarbons and propane. It is resistant to environmental stress, thus contributing to it longevity and environmental pollution (Koerner and Koerner 2018). The excellent performance of the fabricated filter membrane may be due to the fiber diameter of BC and incorporation of the plant extract. The BC have diameter in nano scale, while the commercial membrane fibers have diameter in micro scale. The nano scale fibers have more surface area than micro scale diameter fibers. It has been acknowledged that the removal efficiency of filter membrane depends on the surface area. Thus, the removal efficiency in general, increased with increases in surface area (Chen et al. 2019). The plant derived compounds are reported to have very strong chelating activity of various ions, and can chelate compounds containing carbonyl, hydroxyls and catechol group (Makarov et al. 2014). These mechanisms explain the ability of fingerroot extract to enhance adsorption of PSL particles onto the surface of BC–FT membrane. Also, BC–FT filter membrane is made up of abundant O–H group which may induce interactions between the PSL particles and BC fibers (Zhang et al. 2019). The BC–FT filter membrane shows higher removal efficiency than those reported earlier with different materials (Al-Attabi et al. 2018; Cao et al. 2019; Chen et al. 2019; Tan et al. 2019). The high efficiency of the filter membrane in filtering 0.1 μm particles means it can effectively filter respiratory droplets which are noted for the transmission of Covid-19.
Antimicrobial activity of fingerroot extract and BC–FT filter membrane
The antibacterial activity of the extract was investigated to determine the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC). The result in Table 3 shows the extract exhibited strong inhibitory and bactericidal activities on S. aureus with the MIC and MBC values of < 0.05 mg/mL and < 0.05 mg/mL, respectively. However, higher concentration was required against E. coli with MIC and MBC values of 6.4 mg/mL and 12.8 mg/mL, respectively. The differences in the sensitivity between the bacteria strains may be due to the variation in their cell wall structure. E. coli is gram negative bacteria with thin peptidoglycan cell wall and outer membrane that contain lipopolysaccharide. It also has periplasmic space between cytoplasmic membrane and thin outer membrane where lactamase enzyme is secreted as resistance mechanism against antimicrobial agent (Elisha et al. 2017), compared with S. aureus (gram positive bacteria) which cell wall has only peptidoglycan layer. Thus, it requires higher concentration of the extract to inhibit E. coli than S. aureus. The antimicrobial activity of BC–FT filter membrane was determined and the result shows no inhibition zone (clear zone) around the BC–FT disc. However, it killed the bacteria cells that were in close contact with the disc (clear zone under the disc as shown in Fig. S1). The inability of the test sample to show clear zone does not discount the effectiveness of the fingerroot extract in the membrane. It could probably be as a result of some limitations of agar disc diffusion test. The test requires migration of antimicrobial agent into the nutrient agar. The inability of the active compound to migrate/diffuse into the agar and lack of proclivity or incompatibility of antimicrobial agent with the agar, could cause none visual zone. One of the major phytochemicals in B. rotunda with antimicrobial activity is essential oils, which is hydrophobic in nature (Ait-Ouazzou et al. 2011), this could not easily migrate in the media to cause clear zone inhibition (Leontiev et al. 2018). The active compounds in the filter membrane caused death of cells that came in contact with the membrane by interacting directly with the bacterial cell membrane, enzymes and proteins, disrupted the integrity of the membrane and cell wall, and inhibit the biosynthesis of amino acids (Li et al. 2019; Mostafa et al. 2018).
Conclusions
In this work, novel BC–FT filter membrane for highly efficient air filtration with antimicrobial properties was successfully fabricated. BC–FT filter membrane is highly effective in removing PM0.1 with 99.83% removal efficiency, which is better than that of commercial membrane. Fingerroot extract used for the filter membrane inhibited and killed S. aureus and E. coli. at minimum concentration of 0.05 and 12.8 mg/mL, respectively. BC–FT filter membrane also killed the tested bacteria strains attached to the filter membrane. The membrane demonstrated good mechanical properties and low crystallinity index. The BC–FT can be applied as a filter membrane for face mask to curtail the hazards of PM2.5 and COVID-19 infection by reducing risk of direct and indirect exposure to viral particles in respiratory droplets.
Code availability
Not applicable.
References
Ait-Ouazzou A, Cherrat L, Espina L, Lorán S, Rota C, Pagán R (2011) The antimicrobial activity of hydrophobic essential oil constituents acting alone or in combined processes of food preservation. Innov Food Sci Emerg Technol 12(3):320–329. https://doi.org/10.1016/j.ifset.2011.04.004
Al-Attabi R, Dumée LF, Kong L, Schütz JA, Morsi Y (2018) High efficiency poly (acrylonitrile) electrospun nanofiber membranes for airborne nanomaterials filtration. Adv Eng Mater 20(1):1700572
Anand S, Mayya YS (2020) Size distribution of virus laden droplets from expiratory ejecta of infected subjects. Sci Rep 10(1):21174. https://doi.org/10.1038/s41598-020-78110-x
Aydin A, Cebi G, Demirtas ZE, Erkus H, Kucukay A, Ok M, Sakalli L, Alpdagtas S, Gunduz O, Ustundag CB (2021) Combating COVID-19 with tissue engineering: a review. Emerg Mater 4(1):329–349. https://doi.org/10.1007/s42247-020-00138-6
Cao M, Gu F, Rao C, Fu J, Zhao P (2019) Improving the electrospinning process of fabricating nanofibrous membranes to filter PM2.5. Sci Total Environ 666:1011–1021. https://doi.org/10.1016/j.scitotenv.2019.02.207
Chen KN, Sari FNI, Ting JM (2019) Multifunctional TiO2/polyacrylonitrile nanofibers for high efficiency PM2.5 capture, UV filter, and anti-bacteria activity. Appl Surf Sci 493:157–164. https://doi.org/10.1016/j.apsusc.2019.07.020
Choi S, Jeon H, Jang M, Kim H, Shin G, Koo JM, Lee M, Sung HK, Eom Y, Yang HS, Jegal J (2021) Biodegradable, efficient, and breathable multi-use face mask filter. Adv Sci 8(6):2003155
Chollakup R, Pongburoos S, Boonsong W, Khanoonkon N, Kongsin K, Sothornvit R, Sukyai P, Sukatta U, Harnkarnsujarit N (2020) Antioxidant and antibacterial activities of cassava starch and whey protein blend films containing rambutan peel extract and cinnamon oil for active packaging. LWT 130:109573. https://doi.org/10.1016/j.lwt.2020.109573
Coccia M (2020) Factors determining the diffusion of COVID-19 and suggested strategy to prevent future accelerated viral infectivity similar to COVID. Sci Total Environ 729:138474
Elisha IL, Botha FS, McGaw LJ, Eloff JN (2017) The antibacterial activity of extracts of nine plant species with good activity against Escherichia coli against five other bacteria and cytotoxicity of extracts. BMC Complement Altern Med 17(1):133. https://doi.org/10.1186/s12906-017-1645-z
French AD (2014) Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 21(2):885–896. https://doi.org/10.1007/s10570-013-0030-4
Huang Y, Bao M, Xiao J, Qiu Z, Wu K (2019) Effects of PM2.5 on cardio-pulmonary function injury in open manganese mine workers. Int J Environ Res Public Health 16(11), 2017
Kanjanasirirat P, Suksatu A, Manopwisedjaroen S, Munyoo B, Tuchinda P, Jearawuttanakul K, Seemakhan S, Charoensutthivarakul S, Wongtrakoongate, Rangkasenee N, Pitiporn S, Waranuch N, Chabang N, Khemawoot P, Sa-ngiamsuntorn K, Pewkliang Y, Thongsri P, Chutipongtanate S, Hongeng S, Borwornpinyo S, Thitithanyanont A (2020) High-content screening of Thai medicinal plants reveals Boesenbergia rotunda extract and its component Panduratin A as anti-SARS-CoV-2 agents. Sci Rep 10(1):1–12
Koerner GR, Koerner RM (2018) 7.3 - Polymeric Geomembrane Components in Landfill Liners. In: Cossu R, Stegmann R (eds) Solid Waste Landfilling. Elsevier, pp 313–341
Lee BU (2020) Minimum Sizes of respiratory particles carrying SARS-CoV-2 and the Possibility of aerosol generation. Int J Environ Res Public Health 17(19):6960. https://doi.org/10.3390/ijerph17196960
Leontiev R, Hohaus N, Jacob C, Gruhlke MCH, Slusarenko AJ (2018) A comparison of the antibacterial and antifungal activities of thiosulfinate analogues of allicin. Sci Rep 8(1):6763. https://doi.org/10.1038/s41598-018-25154-9
Li Y, Liu T, Liu Y, Tan Z, Ju Y, Yang Y, Dong W (2019) Antimicrobial activity, membrane interaction and stability of the D-amino acid substituted analogs of antimicrobial peptide W3R6. J Photochem Photobiol B Biol 200:111645. https://doi.org/10.1016/j.jphotobiol.2019.111645
Luksamijarulkul P, Aiempradit N, Vatanasomboon P (2014) Microbial contamination on used surgical masks among hospital personnel and microbial air quality in their working wards: a hospital in Bangkok. Oman Med J 29(5):346
Makarov VV, Love AJ, Sinitsyna OV, Makarova SS, Yaminsky IV, Taliansky ME, Kalinina NO (2014) “Green” nanotechnologies: synthesis of metal nanoparticles using plants. Acta Nat 6(1):35–44
Maleki M, Anvari E, Hopke PK, Noorimotlagh Z, Mirzaee SA (2021) An updated systematic review on the association between atmospheric particulate matter pollution and prevalence of SARS-CoV-2. Environ Res 195:110898–110898. https://doi.org/10.1016/j.envres.2021.110898
Manisalidis I, Stavropoulou E, Stavropoulos A, Bezirtzoglou E (2020) Environmental and health impacts of air pollution: a review. Front Public Health 8:14–14. https://doi.org/10.3389/fpubh.2020.00014
Manoukian OS, Sardashti N, Stedman T, Gailiunas K, Ojha A, Penalosa A, Mancuso C, Hobert M, Kumbar SG (2019) Biomaterials for tissue engineering and regenerative medicine. In: Narayan R (ed) Encyclopedia of biomedical engineering. Elsevier, Oxford, pp 462–482
Mazlan RR, Zakaria M, Rukayadi Y (2016) Antimicrobial activity of fingerroot [Boesenbergia rotunda (L.) Mansf. A.] extract against Streptococcus mutans and Streptococcus sobrinus. J Pure Appl Microbiol 10(3):1755–1762
Mostafa AA, Al-Askar AA, Almaary KS, Dawoud TM, Sholkamy EN, Bakri MM (2018) Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J Biol Sci 25(2):361–366. https://doi.org/10.1016/j.sjbs.2017.02.004
Nations U (2019) Stressing air pollution kills 7 million people annually, secretary-general & nbsp; Urges Governments to Build Green Economy, in Message for World Environment Day
Ongwisespaiboon O, Jiraungkoorskul W (2017) Fingerroot, Boesenbergia rotunda and its aphrodisiac activity. Pharmacogn Rev 11(21):27
Organization WH (2020) Shortage of personal protective equipment endangering health workers worldwide. In
Rahman MAH, Yan LK, Rukayadi Y (2016) Antibacterial activity of fingerroot (Boesenbergia rotunda) extract against acne-inducing bacteria. Res J Pharm Biol Chem Sci 7(6):2158–2164
Sangkham S (2020) Face mask and medical waste disposal during the novel COVID-19 pandemic in Asia. Case Stud Chem Environ Eng 2:100052
Setti L, Passarini F, De Gennaro G, Barbieri P, Perrone MG, Borelli M, Palmisani J, Di Gilio A, Torboli V, Fontana F, Clemente L, Pallavicini A, Ruscio M, Piscitelli P, Miani A (2020) SARS-Cov-2RNA found on particulate matter of Bergamo in Northern Italy: first evidence. Environ Res 188:109754. https://doi.org/10.1016/j.envres.2020.109754
Sukatta U, Rugthaworn P, Khanoonkon N, Anongjanya P, Kongsin K, Sukyai P, Harnkarnsujarit N, Sothornvit R, Chollakup R (2021) Rambutan (Nephelium lappaceum) peel extract: antimicrobial and antioxidant activities and its application as a bioactive compound in whey protein isolate film. Songklanakarin J Sci Technol 43(1):160–168
Sullivan GL, Delgado-Gallardo J, Watson TM, Sarp S (2021) An investigation into the leaching of micro and nano particles and chemical pollutants from disposable face masks—linked to the COVID-19 pandemic. Water Res 196:117033. https://doi.org/10.1016/j.watres.2021.117033
Sunthornvarabhas J, Rungthaworn P, Sukatta U, Juntratip N, Sriroth K (2020) Antimicrobial tendency of bagasse lignin extracts by raman peak intensity. Sugar Tech 22(4):697–705. https://doi.org/10.1007/s12355-019-00778-x
Tan NPB, Paclijan SS, Ali HNM, Hallazgo CMJS, Lopez CJF, Ebora YC (2019) Solution blow spinning (SBS) nanofibers for composite air filter masks. ACS Appl Nano Mater 2(4):2475–2483
Thummajitsakul S, Silprasit K (2021) Classification of some boesenbergia and alpinia extracts and their medicinal products based on chemical composition, antioxidant activity, and concentration of some heavy metals. Songklanakarin J Sci Technol 43(1):160–168
Torgbo S, Sukyai P (2018) Bacterial cellulose-based scaffold materials for bone tissue engineering. Appl Mater Today 11:34–49. https://doi.org/10.1016/j.apmt.2018.01.004
Torgbo S, Sukyai P (2019) Fabrication of microporous bacterial cellulose embedded with magnetite and hydroxyapatite nanocomposite scaffold for bone tissue engineering. Mater Chem Phys 237:121868. https://doi.org/10.1016/j.matchemphys.2019.121868
Torgbo S, Sukyai P (2020) Biodegradation and thermal stability of bacterial cellulose as biomaterial: the relevance in biomedical applications. Polym Degrad Stab 179:109232
Tsai YH, Yang YN, Ho YC, Tsai ML, Mi FL (2018) Drug release and antioxidant/antibacterial activities of silymarin-zein nanoparticle/bacterial cellulose nanofiber composite films. Carbohydr Polym 180:286–296. https://doi.org/10.1016/j.carbpol.2017.09.100
Usawattanakul N, Torgbo S, Sukyai P, Khantayanuwong S, Puangsin B, Srichola P (2021) Development of Nanocomposite film comprising of polyvinyl alcohol (PVA) incorporated with bacterial cellulose nanocrystals and magnetite nanoparticles. Polymers 13(11):1778
Wang SS, Han YH, Ye YX, Shi XX, Xiang P, Chen DL, Li M (2017) Physicochemical characterization of high-quality bacterial cellulose produced by Komagataeibacter sp. strain W1 and identification of the associated genes in bacterial cellulose production. RSC Adv 7(71):45145–45155
Xie W, Li T, Chen C, Wu H, Liang S, Chang H, Liu B, Drioli E, Wang Q, Crittenden JC (2019) Using the green solvent dimethyl sulfoxide to replace traditional solvents partly and fabricating PVC/PVC-g-PEGMA blended ultrafiltration membranes with high permeability and rejection. Ind Eng Chem Res 58(16):6413–6423. https://doi.org/10.1021/acs.iecr.9b00370
Zainin N, Lau K, Zakaria M, Son R, Razis AA, Rukayadi Y (2013) Antibacterial activity of Boesenbergia rotunda (L.) Mansf. A. extract against Escherichia coli. Int Food Res J 20(6):3319
Zangmeister CD, Radney JG, Vicenzi EP, Weaver JL (2020) Filtration efficiencies of nanoscale aerosol by cloth mask materials used to slow the spread of SARS-CoV-2. ACS Nano 14(7):9188–9200. https://doi.org/10.1021/acsnano.0c05025
Zhang C, Yao L, Yang Z, Kong ESW, Zhu X, Zhang Y (2019) Graphene oxide-modified polyacrylonitrile nanofibrous membranes for efficient air filtration. ACS Appl Nano Mater 2(6):3916–3924
Zhang GH, Zhu QH, Zhang L, Yong F, Zhang Z, Wang SL, Wang Y, He L, Tao GH (2020) High-performance particulate matter including nanoscale particle removal by a self-powered air filter. Nat Commun 11(1):1653. https://doi.org/10.1038/s41467-020-15502-7
Acknowledgements
This research is supported by Kasetsart University Research and Development Institute, grant number FF(KU) 25.64, under the Development of Advance Research Competence System for Competitiveness in Agriculture and Food. The authors are grateful to the Department of Biotechnology, Faculty of Agro-Industry, Kasetsart University for all laboratory equipment, instruments and chemicals.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human/animal rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jonsirivilai, B., Torgbo, S. & Sukyai, P. Multifunctional filter membrane for face mask using bacterial cellulose for highly efficient particulate matter removal. Cellulose 29, 6205–6218 (2022). https://doi.org/10.1007/s10570-022-04641-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-022-04641-3