Abstract
With the development of science and technology, various counterfeit and inferior products are emerging in endlessly, which makes people more and more important to the development of anti-counterfeiting technology. Materials capable of fluorescence imaging and biocompatibility are becoming popular in the field of anti-counterfeiting. Herein, we used carbon quantum dots, which had the characteristics of biocompatibility, non-toxicity, and high-efficiency luminescence, as the luminescence center, and introduced them into the carboxymethyl cellulose-based hydrogel to prepare a novel fluorescent cellulose-based hydrogel. The hydrogel displayed a bright blue color fluorescence under UV light, and Fe3+ could quench the fluorescence, thereby realizing information storage and anti-counterfeiting. Moreover, the stored information could be erased by ascorbic acid and reused many times. The results demonstrated that the fluorescent hydrogel had bright prospect for recyclable information storage and anti-counterfeiting.
Similar content being viewed by others
Introduction
In recent years, with the development of science and technology, people have gradually realized the importance of information confidentiality and authenticity. And the development of related anti-counterfeiting technologies has become more and more important (Wang et al. 2020; Fan et al. 2021; Ge et al. 2021). Luminescent materials, especially fluorescent materials such as organic dyes, conjugated polymer dots and inorganic quantum dots, have been applied in anti-counterfeiting applications. As a result of poor photostability, complex preparation processes, unsatisfactory biocompatibility and potential environmental hazards, these materials are unpractical for anti-counterfeiting applications in biological related fields (Pang et al. 2018). CDs, as a new class of fluorescent nanomaterials, have important application value in fluorescent anti-counterfeiting stem from the superiorities compared with the above-mentioned materials, such as excellent biocompatibility, easy preparation, strong photochemical stability and strong surface modification (Kong et al. 2015; Wu et al. 2017; Kalytchuk et al. 2018; Pang et al. 2018; Su et al. 2018; Gao et al. 2019; Liu et al. 2019; Yuan et al. 2019; Zhao et al. 2019; Naik et al. 2020). However, CDs are prone to aggregation quenching affecting its stability which can be maintained by introducing them into hydrogel system and using the electrostatic interaction between CDs and polymer chain segments (Naik et al. 2020; Zhang et al. 2021).
Compared with other polymer materials, carboxymethyl cellulose (CMC), due to biodegradability, biocompatibility, non-toxicity, and low cost (Hiroki et al. 2009; Wei and Periasamy 2011; Fan et al. 2014; Wang and Wang 2016; Kim et al. 2018; Teow et al. 2018; Ampaiwong et al. 2019; Tohamy et al. 2020), was widely used in the fields of agriculture and biomedicine as antibacterial materials (Yadollahi et al. 2015; Wang et al. 2021), tissue engineering and biosensors (Teti et al. 2015; Li et al. 2021; Siripongpreda et al. 2021; Zennifer et al. 2021), drug delivery systems (Kurdtabar et al. 2019; Sheng et al. 2021), and heavy metal removal adsorbents (Salama et al. 2015; Godiya et al. 2019). Based on the strong electrostatic interaction between –COOH of CMC and –NH2 functional group of CDs, CDs could be uniformly dispersed in the matrix, thereby preparing a carboxymethyl cellulose hydrogel with stable fluorescence properties. The fluorescent composite hydrogel formed by the combination of CDs and carboxymethyl cellulose hydrogel did not require complicated instruments and expensive equipment and had excellent properties such as low toxicity, good biocompatibility, and low cost. Exploring its application in the field of fluorescence anti-counterfeiting can further broaden the application range of carboxymethyl cellulose-based hydrogels.
Herein, we used the free radical polymerization method to introduce CDs into the hydrogel system to prepare the CDs/CMC/PAM fluorescent composite hydrogel. A series of characterization methods were used to explore the microscopic morphology, mechanical properties, rheological properties, thermal stability and fluorescence properties of the hydrogels, and the ion printing method was used to store the information on the fluorescent hydrogels for anti-counterfeiting identification.
Experimental section
Reagents and materials
Carboxymethylcellulose sodium (CMC-Na, AR grade), ethylenediamine (C2H8N2, AR grade), citric acid (C6H8O7, 99.5%), acrylamide (AM, AR grade), N,N′-methylenebisacrylamide (MBA, AR grade), ammonium persulfate (APS, 98%), iron(III) chloride hexahydrate (FeCl3·6H2O, AR), ascorbic acid (C6H8O6, AR) were provided by Aladdin Company (China).
Method
Synthesis of fluorescent CDs
CDs were synthesized according to the previously reported method (Zhu et al. 2013). 5 g of citric acid was dissolved in 20 mL deionized water at 293 K, stirred for 10 min, added 6 mL ethylenediamine solution to it, sonicated for 30 min, then placed the mixture in a PTFE-lined reactor (50 mL) at 180 °C for 5 h. After the reaction, they were naturally cooled to room temperature to obtain a dark brown solution. The obtained product was filtered once with a 0.22 μm filter to remove aggregated particles, followed by dialysis with deionized water changing every 2 h for 3 days in order to obtain transparent brown solution. The resulting solution was freeze-dried to give CDs.
Synthesis of fluorescent carboxymethyl cellulose-based hydrogel
The novel hydrogel was synthesized using free-radical polymerization method (Godiya et al. 2019). Initially, CDs were configured into solutions of different concentrations, and used ultrasonic treatment for 30 min to disperse them evenly. Then 0.5 g CMC was dissolved in the CDs solution to make the concentration of CMC at 5 wt%, and magnetically stirred for 2 h to make the CMC uniformly dispersed in the solution. Then AM (0.5 g), MBA (0.01 g, as a crosslinker) and KPS (0.01 g, as an initiator) were successively added with continuous stirring for 2 h with magnetic force. The hydrogel synthesis was carried out in a vacuum oven at 60 °C for 10 h. Thereafter, the as-prepared hydrogel was soaked in deionized water for 3 days to eliminate the residual reagents and the deionized water was repeatedly changed. The hydrogels were named CCP-3, CCP-5 and CCP-7 with the CDs content varying from 3 to 5 mg/mL and 7 mg/mL respectively. At the same time, the same method was used to prepare hydrogel without CDs and named CCP-0.
Characterization
The functional groups of CDs and hydrogels were analyzed using Fourier Transform Infrared Spectroscopy (FTIR, Nicolet IN 10™, Thermo Co., USA). The images of CDs were obtained with a transmission electron microscope (TEM, FEI Tecnai G2 F20, USA) and the TEM sample was prepared through a solution of CDs on a carbon membrane supported by a 200 mesh copper grid. The surface morphology of the prepared hydrogels was observed using a scanning electron microscope (SEM, Zeiss Sigma 300, Germany). The UV–Vis spectrum of CDs was measured with a UV–Vis spectrophotometer (UV-2600, Shimadzu Co., Japan) with a scanning region between 200 and 600 nm. Mechanical tests were carried out using an electronic universal material testing machine (Zwick 1.0, Germany) equipped with a 400 N mechanical sensor. Modulus testing (oscillation mode) was performed using an Anton Paar MCR302 rheometer. Hydrogel sheets with a diameter of 20 mm and a thickness of 2 mm were made, and frequency testing was performed with a fixed strain of 0.1%, frequency range of 0.1–10 Hz and constant test temperature of 25 °C. The thermal stability of the hydrogels was analyzed using a thermogravimetric analyzer model TGA5500.The fluorescence emission spectra (PL) was measured in standard quartz cuvettes with a slit width of 5 nm and a scan range from 250 to 390 nm at 20 nm intervals using an FLS1000 steady-state fluorescence spectrometer. The fluorescent hydrogel was cut to 0.5 × 0.5 cm2 with a thickness of 0.1 cm in size and the fluorescence spectrum (PL) was measured using an FLS1000 steady-state fluorescence spectrometer with a slit width of 5 nm to measure the best excitation peak and the fluorescence emission spectrum at the best excitation wavelength.
Results and discussion
Preparation of fluorescent hydrogel with CDs as fluorescent molecules
Figure 1 shows the synthesis of fluorescent hydrogel with CMC, CDs and PAM as the main components. The structural formula of CMC-Na refers to the article of French AD (French AD 2017). Citric acid was mixed with ethylenediamine and reacted hydrothermally in a reactor with a PTFE liner to produce uniformly sized CDs. The presence of –NH2 on the surface of CDs easily combined with H+ to form ammonium cations, and the molecular chain of carboxymethyl cellulose contained a large number of hydroxyl and carboxyl groups. Due to the strong electrostatic interaction between the two, CDs could be stably dispersed in carboxymethyl cellulose hydrogel (You et al. 2016). AM was polymerized into the CMC network by free radicals with the help of KPS and MBA to form a composite hydrogel with 3D network structure (Godiya et al. 2019). The CDs wrapped in 3D network structure not only gave the hydrogel new fluorescent properties, and as a kind of less than 10 nm in diameter class of spherical carbon nanoparticles they can enhance the mechanical properties of hydrogel and improve the poor mechanical properties of carboxymethyl cellulose hydrogel.
Characterization of fluorescent composite hydrogel
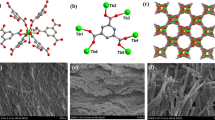
The morphology of the CDs was characterized by typical transmission electron microscopy (TEM). From the morphology measured by TEM, the prepared CDs are spherical with a diameter between 2.5 and 4.5 nm in Fig. 2a. At the same time, the high-resolution TEM image (Fig. 2b) shows that the CDs had well-resolved lattice stripes with a typical lattice spacing of 0.22 nm. For CDs, the effects of reaction time, temperature during the preparation process and the concentration of CDs on their fluorescence intensity were discussed later. Figure 2c shows the possible functional groups on the surface of CDs, which would be demonstrated later.
a TEM image b High-resolution TEM image c Simple diagram of CDs d UV spectrum and fluorescence excitation and emission spectra (Insets show photographs of CDs in aqueous solution under visible (left) and UV (right) light) e Fluorescence spectra of CDs under different excitation wavelengths f Fluorescence spectra of different reaction times g Fluorescence spectra at different reaction temperatures h Fluorescence spectra of different CDs concentrations i Fluorescence spectra under different PH
The UV/Vis spectrum and fluorescence spectrum of CDs are shown in Fig. 2d. It can be seen from the UV/Vis spectrum that CDs had two characteristic absorption peaks. The absorption band at 240 nm was presumed to be the transition of aromatic sp2 orbital π → π*, whereas the absorption peak at 340 nm was presumed to be the n → π* transition of –NH2 (Zhang et al. 2021). CDs exhibited blue fluorescence emission with the strongest emission intensity (460 nm) at an excitation of 360 nm. The color of CDs observed under visible light was yellow, while under UV light was blue (inset in Fig. 2d). Figure 2e shows the fluorescence emission spectra of CDs at excitation wavelengths of 250–390 nm. The fluorescence emission intensity reached the strongest when the excitation wavelength was 340 nm. At the same time, it can be seen that the CDs exhibited excitation-independent behavior, which may be attributed to the uniform surface structure and size of CDs (Xu et al. 2015).
The effects of reaction time, reaction temperature, concentration of CDs and PH value on the fluorescence response of the system are shown in Fig. 2f–i. The results showed that the fluorescence intensity of CDs increased with the reaction time and temperature; the fluorescence intensity increased with the CDs concentration; PH in the range of 5–9, the fluorescence intensity of CDs remained stable, and the highest of CDs was displayed when the PH was 7. On balance, we subsequently used CDs reacted at 180 °C for 5 h with a PH of 7 for our experiments.
The surface functional groups of CDs, CCP-0 and CCP-5 are characterized by FT-IR and the resulted spectra were plotted in Fig. 3a. In the FTIR analysis of CDs, the following were observed: stretching vibrations of C–OH at 3280 cm−1 and C–H at 2932 cm−1, stretching vibrations of C–N or C–O–C at 1292 cm−1, bending vibrations of N–H at 1561 cm−1, and the vibrational absorption band of C=O at 1655 cm−1 (Zhu et al. 2013; Zhang et al. 2017; Hu et al. 2021). The existence of these stretching bands indicated the existence of functional groups such as carboxyl and amide on the surface of CDs. In the CMC gel spectrum, some common peaks were observed at 3330 cm−1, 1592 cm−1, 1418 cm−1 and 1060 cm−1. These peaks were due to the O–H stretching vibration band, asymmetric COO–, symmetric COO– and C–O–C bending of CMC, respectively (Jeong et al. 2020; Tohamy et al. 2021). The characteristic peaks at 1650 cm−1 and 1595 cm−1 can be assigned to the carbonyl stretching vibration (amide I) and N–H bending vibration (amide II) of the amide group, respectively (Li et al. 2017; Godiya et al. 2019; Jeong et al. 2020). The peaks at 3343 cm−1 and 3171 cm−1 were attributed to C–OH stretching vibrations and the N–H symmetric stretching modes of –NH2 groups (Godiya et al. 2019). The peak at 1110 cm−1 came from the –CH–O–CH2 unit of the composite hydrogel. The absorption bands of N–H and O–H vibration of CCP-5 were shifted which indicated that a hydrogen bond was formed between the amino group of CDs and the carboxy group of CMC.
The compressive stress–strain curve of the hydrogels are shown in Fig. 3b. The maximum value of compressive strain was set at 70%, and no fracture occurred during compression, and it was able to recover the original state quickly after stress unloading. The compressive performance of CCP-0 hydrogel was the worst at 70% compressive strain, and the stress was only 120 kPa. With the increase of CDs concentration, the compressive performance of hydrogel gradually improved, and the compressive strength of fluorescent hydrogel reached the maximum value of 316 kPa when the CDs content was 7 mg/mL. The improved mechanical properties of CCP-n hydrogels compared to CCP-0 hydrogels were due to the strong electrostatic forces between CMC-PAM chains and CDs, as well as the role of CDs acting as physical cross-linkers within the hydrogel, which increased the number of physical cross-linking points within the hydrogel and enhanced the network structure of the hydrogel.
It can be seen from Fig. 3c and d that, compared to the hydrogel without the addition of CDs, the CCP-n hydrogel cross-section have a more regular pore channels and the basic structure is similar to that of CCP-0 hydrogel. This was due to the strong electrostatic force and hydrogen bonding between CDs and CMC-PAM segments increasing the cross-linking density of the hydrogel, resulting in the appearance of such relatively pore structure. The dense pore density created a more complete network structure that enhanced the mechanical properties of the hydrogel, consistent with the mechanical property tests described above.
In addition, tensile tests are performed on different hydrogels, as shown in Fig. 4a. Compared with CCP-0 hydrogel, the stress and elongation of CCP-n hydrogel were improved to 552% and 0.22 Mpa, respectively, which proved that the hydrogen bonding between CDs and polymer chains could improve the tensile properties of the composite hydrogels. And the tensile properties of CCP-0, CCP-3, CCP-5 and CCP-7 hydrogels were gradually improved, indicating that more hydrogen bonds were formed inside the hydrogels with the increase of CDs content, and a tighter cross-linked network was formed. Meanwhile, the swelling properties of the four hydrogels were tested, and it is obvious in Fig. 4b that the swelling ratios of CCP-0, CCP-3, CCP-5 and CCP-7 hydrogels were decreasing from 15.82 to 7.16, making known that the cross-link density of hydrogels was increasing, and hydrogels may have the potential to perform long-lasting operations in water.
The rheological behavior of the four hydrogels was investigated, and Fig. 4c, d show the relationship between oscillation frequency of hydrogels and the storage modulus G′ and loss modulus G″ at a fixed strain of 0.1%. Transversely, G′ is larger than G″ for all four hydrogels over the entire range of tested frequencies, indicating that all four hydrogels exhibit mainly solid-like behavior with better mechanical properties. When compared vertically, the dynamic modulus of hydrogels gradually increased with the increase of the amount of added CDs for both G′ and G″, and all of them increased with the increase of the oscillation frequency, which may be related to the hydrogen bond formed between the CDs and the CMC molecular chains.
As shown in Fig. 4e, the thermogravimetric curves of CCP-0 and CCP-5 hydrogels show a similar trend in the range of 25–600 °C. The initial decomposition temperature of CCP-5 hydrogel was slightly higher than that of CCP-0, probably because the addition of CDs slowed down the pyrolysis of the hydrogel system. 25–100 °C was the drying and water loss stage of hydrogels, in which the temperature gradually increases and the weight decreases more slowly. The thermal decomposition rate of hydrogels changed obviously at 100–250 °C, the weight of hydrogels remained unchanged, and the molecular chains in hydrogels gradually opened. At 250–450 °C, hydrogels lose most of their weight, and was the main stage of pyrolysis, in which CCP-0 hydrogel lose 33.03% and CCP-5 hydrogel lose 29.52% of their weight. At 450–600 °C, the weight of hydrogels gradually stabilized, and the maximum residual mass of CCP-5 hydrogel (57.43%) at 600 °C increased by 10.17% compared with CCP-0 hydrogel (47.26%), which proved that the addition of CDs enhanced the thermal stability of hydrogels.
Figure 5a shows the fluorescence intensity curve of the hydrogel with different concentrations of CDs. The higher the CDs concentration, the stronger the fluorescence intensity of the hydrogel, and the apex of the emission peak has nothing to do with the concentration of added CDs, which were consistent with the comparison result in Fig. 2h. Subsequently, by observing the change of fluorescence intensity before and after adding Fe3+ under visible light and UV light, the fluorescence quenching effect of Fe3+ on the prepared hydrogel was studied. As shown in Fig. 5, the introduction of Fe3+ significantly reduces the fluorescence intensity of the hydrogel as the result of the combining of Fe3+ and CDs. CCP-0 hydrogel exhibits no fluorescence effect both before and after immersion in Fe3+ (Fig. 5c, d), further indicating that the addition of CDs would impart fluorescent properties to the hydrogels. In addition, the color of different concentrations of CDs showed a certain difference, specifically, the yellow color gradually deepened with increasing concentration of CDs. The as-hydrogels also show the same pattern (Fig. 6), so it was speculated that the color change of the hydrogels was attributed to the concentration of the added CDs.
Realize 2D information storage by ion-printing
The introduction of Fe3+ into the prepared fluorescent hydrogel system using ion printing method obtained information storage with a fluorescent response. The filter paper cut into specific shape was immersed in the FeCl3 solution for 1 h to make the filter paper fully absorb the FeCl3 solution and placed it in oven to dry. The filter paper containing Fe3+ was applied to the surface of the hydrogel and separated from the hydrogel after 1 h. Through ion printing, information could be easily printed on the surface of the hydrogel (Fig. 7a). The color observed under visible light is different from that observed under 365 nm UV light to achieve the purpose of information storage and anti-counterfeiting (Fig. 7a and b).
Erasure of stored information based on fluorescence quenching-recovery
In order to reduce the energy consumption and environmental pollution caused by the massive use of paper that is not effectively utilized, many researchers have carried out research and development on it. Like other recyclable materials, our hydrogels could be recovered by simply immersing them in ascorbic acid solution, as shown in Fig. 8, the fluorescence of the hydrogel soaked in the ascorbic acid solution is restored, during which ascorbic acid binds to Fe3+, separating Fe3+ from the hydrogel system. Due to the quenching of fluorescence caused by Fe3+ binding with CDs in the system, the color of the ion-printing region became dark under UV light (365 nm), and became bright again after treatment with ascorbic acid solution. Since the binding ability of ascorbic acid and Fe3+ was stronger than that of Fe3+ and CDs, after treating the hydrogel with ascorbic acid solution, the Fe3+ in the hydrogel system would be detached, so the quenched fluorescence was restored. In Fig. 8, we store the “I love you” information in the hydrogel in turn, where:“I” stood for “I” “❤” stood for “love”, and “Y” stood for “you”. Cycling experiments showed that the fluorescent hydrogel had immense application potential in the field of information storage.
Mechanism analysis
The above studies showed that the fluorescent properties of hydrogels are endowed by CDs. Many researchers have explored the PL mechanism of CDs, but so far this issue is still controversial (Zhou et al. 2015; Raj and Balachandran 2020). The PL mechanisms that have been known include quantum confinement effect, surface state, molecular state, edge state, cross-linking enhanced emission effect and environmental effect (El-Shabasy et al. 2021; Yu et al. 2021), the first three of which are accepted by most scholars. Quantum Confinement Effect (QCE), commonly known as size effect, occurs when the size of the CDs is smaller than the exciton Bohr radius. Surface defect fluorescence is caused by radiative relaxation from the excited state to the ground state, and CDs surface defects lead to multicolor emission within the visible spectrum. The molecular state is entirely controlled by organic fluorophores bonded to the surface or interior of the core. The fluorophores are attached to the carbon skeleton on the surface or inside and can emit PL directly, which is common in CDs prepared using bottom-up methods. In this experiment, CDs were hydrothermally synthesized using small molecules CA and EDA, and the fluorescence mechanism was presumed to be the result of the combined action of surface state/molecular state and QCE according to the references (Zhu et al. 2013). The fluorescence of fluorescent hydrogels is derived from CDs, so the surface state/molecular state and QCE are also the main fluorescence generation mechanisms. The fluorescence behavior of CDs includes excitation wavelength-dependent fluorescence emission and excitation wavelength-independent fluorescence emission (Yu et al. 2021). The experiments show that the fluorescence of both the prepared CDs and fluorescent hydrogels exhibit good monochromaticity without excitation wavelength dependence. Fe3+ can undergo coordination or chelation reactions with a variety of functional groups on the surface of CDs (Xu et al. 2016; Gao et al. 2019). Due to this action, electrons in the excited state on CDs are transferred to the unfilled orbitals of iron ions, which leads to non-radiative electron/hole recombination (Xu et al. 2015, 2016), and eventually CDs produce fluorescence quenching, which in turn realizes information storage. Due to the strong reducibility of ascorbic acid, Fe3+ can be reduced to Fe2+ and then detached from the surface of CDs, so that the fluorescence properties of the hydrogel can be recovered and information can be erased.
Conclusions
This study showed the successful synthesis of a novel fluorescent cellulose-based hydrogel with CMC and CDs, as an information storage material with a fluorescent response. CDs, as a fluorescent material and nanomaterial, on the one hand gave the fluorescence characteristics of hydrogel and on the other hand improved the mechanical properties of hydrogel. Using ion printing method, Fe3+ combined with CDs to quench the fluorescence, thus storing the information in the hydrogel. The purpose of fluorescent anti-counterfeiting was achieved due to the different color of the information observed under visible and UV light. In addition, the fluorescence quenching behavior was eliminated by immersing the information-storing hydrogel in a solution of ascorbic acid. Through cyclic experiments, we believed that our hydrogel could be used as a reusable information storage and fluorescent anti-counterfeiting material.
References
Ampaiwong J, Rattanawaleedirojn P, Saengkiettiyut K, Rodthongkum N, Potiyaraj P, Soatthiyanon N (2019) Reduced graphene oxide/carboxymethyl cellulose nanocomposites: novel conductive films. J Nanosci Nanotechnol 19:3544–3550
El-Shabasy RM, Elsadek MF, Ahmed BM, Farahat MF, Mosleh KN, Taher MM (2021) Recent developments in carbon quantum dots: properties, fabrication techniques, and bio-applications. Processes 9:388
Fan LH, Peng M, Zhou XY, Wu H, Hu J, Xie WG et al (2014) Modification of carboxymethyl cellulose grafted with collagen peptide and its antioxidant activity. Carbohydr Polym 112:32–38
Fan XF, Gu LQ, Hu YL, Zhu Q (2021) Wearing an organic “coat” on nanocrystals of LaF3:Eu3+ to generate dynamic luminescence for optical anti-counterfeit. Adv Powder Technol 32:2645–2653
French AD (2017) Glucose, not cellobiose, is the repeating unit of cellulose and why that is important. Cellulose 24:4605–4609
Gao XX, Zhou X, Ma YF, Qian T, Wang CP, Chu FX (2019) Facile and cost-effective preparation of carbon quantum dots for Fe3+ ion and ascorbic acid detection in living cells based on the “on-off-on” fluorescence principle. Appl Surf Sci 469:911–916
Ge WY, Zhang PF, Zhang XM, Gao WX, Lu CH, Ge Y (2021) Amorphous alumina: a bright red matrix for flexible and transparent anti-counterfeiting. ACS Sustain Chem Eng 9:10220–10226
Godiya CB, Cheng X, Li DW, Chen Z, Lu XL (2019) Carboxymethyl cellulose/polyacrylamide composite hydrogel for cascaded treatment/reuse of heavy metal ions in wastewater. J Hazard Mater 364:28–38
Hiroki A, Tran HT, Nagasawa N, Yagi T, Tamada M (2009) Metal adsorption of carboxymethyl cellulose/carboxymethyl chitosan blend hydrogels prepared by Gamma irradiation. Radiat Phys Chem 78:1076–1080
Hu CS, Zhu YM, Zhao XF (2021) On-off-on nanosensors of carbon quantum dots derived from coal tar pitch for the detection of Cu2+, Fe3+, and L-ascorbic acid. Spectrochim Acta, Part A: Mol Biomol Spectrosc 250:119325
Jeong D, Kim C, Kim Y, Jung S (2020) Dual crosslinked carboxymethyl cellulose/polyacrylamide interpenetrating hydrogels with highly enhanced mechanical strength and superabsorbent properties. Eur Polym J 127:109586
Kalytchuk S, Wang Y, Polakova K, Zboril R (2018) Carbon dot fluorescence-lifetime-encoded anti-counterfeiting. ACS Appl Mater Interfaces 10:29902–29908
Kim GH, Im JN, Kim TH, Lee GD, Youk JH, Doh SJ (2018) Preparation and characterization of calcium carboxymethyl cellulose/chitosan blend nonwovens for hemostatic agents. Text Res J 88:1902–1911
Kong DP, Yan FY, Shi DC, Ye QH, Han ZY, Chen L et al (2015) Carbon dots: synthetic methods and applications as fluorescent probes for the detection of metal ions, inorganic anions and organic molecules. J Iran Chem Soc 12:1841–1857
Kurdtabar M, Baghestani G, Bardajee GR (2019) Development of a novel thermo-responsive hydrogel-coated gold nanorods as a drug delivery system. Gold Bull 52:9–17
Li N, Chen GX, Chen W, Huang JH, Tian JF, Wan XF et al (2017) Multivalent cations-triggered rapid shape memory sodium carboxymethyl cellulose/polyacrylamide hydrogels with tunable mechanical strength. Carbohydr Polym 178:159–165
Li J, Chen F, Lin X, Ding T (2021) Hydrogen-bonding-assisted toughening of hierarchical carboxymethyl cellulose hydrogels for biomechanical sensing. Carbohydr Polym 269:118252
Liu ML, Chen BB, Li CM, Huang CZ (2019) Carbon dots: synthesis, formation mechanism, fluorescence origin and sensing applications. Green Chem 21:449–471
Naik VM, Gunjal DB, Gore AH, Anbhule PV, Sohn D, Bhosale SV et al (2020) Nitrogen-doped carbon dot threads as a “turn-off” fluorescent probe for permanganate ions and its hydrogel hybrid as a naked eye sensor for gold(III) ions. Anal Bioanal Chem 412:2993–3003
Pang YY, Zhao RJ, Lu Y, Liu JY, Dong XP, Xi FN (2018) Facile preparation of N-doped graphene quantum dots as quick-dry fluorescent ink for anti-counterfeiting. New J Chem 42:17091–17095
Raj AM, Balachandran M (2020) Coal-based fluorescent zero-dimensional carbon nanomaterials: a short review. Energy Fuels 34:13291–13306
Salama A, Shukry N, El-Sakhawy M (2015) Carboxymethyl cellulose-g-poly(2-(dimethylamino) ethyl methacrylate) hydrogel as adsorbent for dye removal. Int J Biol Macromol 73:72–75
Sheng YS, Gao J, Yin ZZ, Kang J, Kong Y (2021) Dual-drug delivery system based on the hydrogels of alginate and sodium carboxymethyl cellulose for colorectal cancer treatment. Carbohydr Polym 269:118325
Siripongpreda T, Somchob B, Rodthongkum N, Hoven VP (2021) Bacterial cellulose-based re-swellable hydrogel: facile preparation and its potential application as colorimetric sensor of sweat pH and glucose. Carbohydr Polym 256:117506
Su AM, Zhong QM, Chen YY, Wang YL (2018) Preparation of carbon quantum dots from cigarette filters and its application for fluorescence detection of Sudan I. Anal Chim Acta 1023:115–120
Teow YH, Kam LM, Mohammad AW (2018) Synthesis of cellulose hydrogel for copper (II) ions adsorption. J Environ Chem Eng 6:4588–4597
Teti G, Salvatore V, Focaroli S, Durante S, Mazzotti A, Dicarlo M et al (2015) In vitro osteogenic and odontogenic differentiation of human dental pulp stem cells seeded on carboxymethyl cellulose-hydroxyapatite hybrid hydrogel. Front Physiol 6:297
Tohamy H-AS, Kamel S, El-Sakhawy M, Youssef MA, Abdallah AE, Anis B (2020) Thermal properties of graphene oxide prepared from different agricultural wastes. Egypt J Chem 63:3619–3629
Tohamy H-AS, El-Sakhawy M, Kamel S (2021) Carboxymethyl cellulose-grafted graphene oxide/polyethylene glycol for efficient Ni(II) adsorption. J Polym Environ 29:859–870
Wang LY, Wang MJ (2016) Removal of heavy metal ions by poly(vinyl alcohol) and carboxymethyl cellulose composite hydrogels prepared by a freeze-thaw method. ACS Sustain Chem Eng 4:2830–2837
Wang TL, Ji XY, Tao ZH, Zhou X, Hao Z, Wang XK et al (2020) Dual stimuli-responsive lanthanide-based phosphors for an advanced full-color anti-counterfeiting system. RSC Adv 10:15573–15578
Wang X, Qi J, Zhang W, Pu Y, Yang R, Wang P et al (2021) 3D-printed antioxidant antibacterial carboxymethyl cellulose/epsilon-polylysine hydrogel promoted skin wound repair. Int J Biol Macromol 187:91–104
Wei WY, Periasamy V (2011) Synthesis, structural and spectroscopic properties of encapsulated Chlorophyll-a thin film in carboxymethyl cellulose. J Porphyrins Phthalocyanines 15:122–130
Wu HF, Jiang JH, Gu XT, Tong CL (2017) Nitrogen and sulfur co-doped carbon quantum dots for highly selective and sensitive fluorescent detection of Fe(III) ions and L-cysteine. Microchim Acta 184:2291–2298
Xu Q, Pu P, Zhao JG, Dong CB, Gao C, Chen YS et al (2015) Preparation of highly photoluminescent sulfur-doped carbon dots for Fe(III) detection. J Mater Chem A 3:542–546
Xu Q, Wei J, Wang J, Liu Y, Li N, Chen Y et al (2016) Facile synthesis of copper doped carbon dots and their application as a “turn-off” fluorescent probe in the detection of Fe3+ ions. RSC Adv 6:28745–28750
Yadollahi M, Gholamali I, Namazi H, Aghazadeh M (2015) Synthesis and characterization of antibacterial carboxymethyl cellulose/ZnO nanocomposite hydrogels. Int J Biol Macromol 74:136–141
You Y, Zhang H, Liu Y, Lei B (2016) Transparent sunlight conversion film based on carboxymethyl cellulose and carbon dots. Carbohydr Polym 151:245–250
Yu J, Yong X, Tang Z, Yang B, Lu S (2021) Theoretical understanding of structure-property relationships in luminescence of carbon dots. J Phys Chem Lett 12:7671–7687
Yuan T, Meng T, He P, Shi YX, Li YC, Li XH et al (2019) Carbon quantum dots: an emerging material for optoelectronic applications. J Mater Chem C 7:6820–6835
Zennifer A, Senthilvelan P, Sethuraman S, Sundaramurthi D (2021) Key advances of carboxymethyl cellulose in tissue engineering & 3D bioprinting applications. Carbohydr Polym 256:117561
Zhang ZW, Yan K, Yang QL, Liu YH, Yan ZY, Chen JQ (2017) One-pot synthesis of fluorescent nitrogen-doped carbon dots with good biocompatibility for cell labeling. Luminescence 32:1488–1493
Zhang DD, Tian XT, Li HH, Zhao YP, Chen L (2021) Novel fluorescent hydrogel for the adsorption and detection of Fe (III). Colloids Surf A 608:122563
Zhao D, Ma WT, Wang R, Yang XZ, Li J, Qiu T et al (2019) The preparation of green fluorescence-emissioned carbon dots/poly(N-isopropylacrylamide) temperature-sensitive hydrogels and research on their properties. Polym 11:1171
Zhou Z, Shen Y, Li Y, Liu A, Liu S, Zhang Y (2015) Chemical cleavage of layered carbon nitride with enhanced photoluminescent performances and photoconduction. ACS Nano 9:12480–12487
Zhu SJ, Meng QN, Wang L, Zhang JH, Song YB, Jin H et al (2013) Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew Chem Int Ed 52:3953–3957
Funding
This work was sponsored by Special Fund for Beijing Forestry University and Beijing Common Construction Project [Grant No. 2016HXKFCLXY0015].
Author information
Authors and Affiliations
Contributions
HL: Conceptualization, Methodology, Software, Writing—Original draft preparation. SW: Data curation, Software. ZW: Visualization, Investigation. WM: Software, Validation. XH: Resources. JP: Writing-Reviewing and Editing. All authors commented on the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lv, H., Wang, S., Wang, Z. et al. Fluorescent cellulose-based hydrogel with carboxymethyl cellulose and carbon quantum dots for information storage and fluorescent anti-counterfeiting. Cellulose 29, 6193–6204 (2022). https://doi.org/10.1007/s10570-022-04643-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-022-04643-1