Abstract
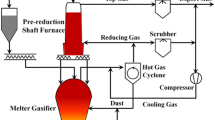
Shaft gas injection is considered helpful for realizing an oxygen blast furnace (OBF) to mitigate CO2 emissions substantially. This paper presents a systematic study of the shaft injection for a 430-m3 industrial OBF using a process model. The OBF is operated at 35, 50, or 100 pct oxygen enrichment. It is combined with the reducing gas injection through blast tuyeres to achieve a reasonable flame temperature. The effects of shaft gas injection rate, shaft gas injection position, and shaft gas injection temperature are studied with fixed hot metal temperature, bosh gas volume, and flame temperature. The results show that the fuel rate decreases as the oxygen enrichment increases. It also decreases with increasing shaft gas injection rate/temperature but increases at a higher injection position. All these changes slow down when the values of the three variables are relatively large. At a higher oxygen enrichment or lower shaft gas injection position/rate, the replacement ratio of coke by the shaft injected gas increases, indicating better utilization of shaft gas energy. However, the replacement ratio increases first to a maximum and then gradually decreases with increasing shaft gas injection temperature, identifying an optimum injection temperature. The inner flow and thermochemical behaviors of OBF are analyzed in detail. It shows that the fuel reduction by shaft injection is a collected effect of decreased carbon consumption by raceway combustion and direct reduction. The former contributor plays a dominating role, benefiting from the pre-heating effect. The latter contributor results from the indirect reduction enhancement because of the intensified reducing atmosphere and increased temperature. These pre-heating and pre-reduction roles are quantified to elucidate the impacts of the flow rate, position, and temperature of shaft injection.


















Similar content being viewed by others
Abbreviations
- \(a_{\rm FeO}\) :
-
The activity of molten wustite
- \(A\) :
-
Domain for plane integration, \({\rm m}^{2}\)
- \(A_{\rm c}\) :
-
Effective surface area of coke for reaction, \({\rm m}^{2}\)
- \(A_{1}\) :
-
Constant for describing burden distribution pattern, \({\rm m}^{ - 2}\)
- \(A_{2}\) :
-
Constant for describing burden distribution pattern, \({\rm m}^{ - 1}\)
- BF:
-
Blast furnace
- \(A_{3}\) :
-
Constant for describing burden distribution pattern
- \(c_{\rm p}\) :
-
Specific heat, \({\rm J} \, {\rm kg}^{ - 1} \, {\rm K}^{ - 1}\)
- \(C_{{{\rm SiO}_{2} }}\) :
-
Concentration of SiO2, \({\rm mol} \, {\rm m}^{ - 3}\)
- CZ:
-
Cohesive zone
- CBF:
-
Conventional blast furnace
- \(d\) :
-
Diameter of solid phase, m
- \(D\) :
-
Diffusion coefficient, \({\rm m}^{2} \, {\rm s}^{ - 1}\)
- \(D_{{\rm s}5}\) :
-
Intra-particle diffusion coefficient of H2 in reduced iron phase, \({\rm m}^{2} \, {\rm s}^{ - 1}\)
- \(E_{\rm f}\) :
-
Effectiveness factors of solution loss reaction by CO
- \(E_{\rm f}{^{\prime}}\) :
-
Effectiveness factors of water gas reaction
- \(E_{\rm gl}\) :
-
Volumetric enthalpy flux between gas and liquid, \({\rm W} \, {\rm m}^{ - 3}\)
- EBF:
-
Experimental blast furnace
- \(f_{\rm o}\) :
-
Fraction conversion of iron ore
- \({\mathbf{F}}\) :
-
Interaction force per unit volume, \({\rm kg} \, {\rm m}^{ - 2} \, {\rm s}^{ - 2}\)
- \({\mathbf{g}}\) :
-
Gravitational acceleration, \({\rm m} \, {\rm s}^{ - 2}\)
- \(h_{ij}\) :
-
Heat transfer coefficient, \({\rm W} \, {\rm m}^{ - 2} \, {\rm K}^{ - 1}\)
- \(H\) :
-
Enthalpy, \({\rm J} \, {\rm kg}^{ - 1}\)
- \(\Delta H\) :
-
Reaction heat, \({\rm J} \, {\rm mol}^{ - 1}\)
- HM:
-
Hot metal
- \(k\) :
-
Thermal conductivity, \({\rm W} \, {\rm m}^{ - 1} \, {\rm K}^{ - 1}\)
- \(k_{1}\) :
-
Rate constant of indirect reduction of iron ore by CO, \({\rm m} \, {\rm s}^{ - 1}\)
- \(k_{2}\) :
-
Rate constant of direct reduction of molten wustite, \({\rm mol} \, {\rm m}^{ - 2} \, {\rm s}^{ - 1}\)
- \(k_{3}\) :
-
Rate constant of solution loss reaction by CO, \({\rm m}^{3} \, {\rm kg}^{ - 1} \, {\rm s}^{ - 1}\)
- \(k_{5}\) :
-
Rate constant of indirect reduction of iron ore by H2, \({\rm m} \, {\rm s}^{ - 1}\)
- \(k_{6}\) :
-
Rate constant of water gas reaction, \({\rm m}^{3} \, {\rm kg}^{ - 1} \, {\rm s}^{ - 1}\)
- \(k_{8}\) :
-
Rate constant of silica reduction reaction in slag, \({\rm m} \, {\rm s}^{ - 1}\)
- \(k_{f}\) :
-
Gas-film mass transfer coefficient, \({\rm m} \, {\rm s}^{ - 1}\)
- \(k_{{\rm f}5}\) :
-
Gas-film mass transfer coefficient in indirect reduction of iron ore by H2, \({\rm m} \, {\rm s}^{ - 1}\)
- \(k_{{\rm f}6}\) :
-
Gas-film mass transfer coefficient water gas reaction, \({\rm m} \, {\rm s}^{ - 1}\)
- \(k_{\rm se}^{\rm e}\) :
-
Thermal conductivity of solid, \({\rm W} \, {\rm m}^{ - 1} \, {\rm K}^{ - 1}\)
- \(K_{1}\) :
-
Equilibrium constant of indirect reduction of iron ore by CO
- \(K_{5}\) :
-
Equilibrium constant of indirect reduction of iron ore by H2
- \(M_{i}\) :
-
Molar mass of \(i{{{\rm th}}}\) species in gas phase
- \(M_{\rm sm}\) :
-
Molar mass of \({\text{FeO}}\) or flux in solid phase, \({\text{kg}} \, {\text{mol}}^{ - 1}\)
- \(N_{\rm c}\) :
-
Number of coke in unit volume of bed, \({\rm m}^{ - 3}\)
- \(N_{\rm FeO}\) :
-
Corrected molar fraction of FeO in slag
- \(N_{\rm o}\) :
-
Number of iron oxide in unit volume of bed, \({\rm m}^{ - 3}\)
- \(Nu\) :
-
Nusselt number
- OBF:
-
Oxygen blast furnace
- \(p\) :
-
Pressure, \({\rm Pa}\)
- \(Pe_{\rm gx} ,Pe_{\rm gy}\) :
-
Peclet number in x or y direction
- \(Pr\) :
-
Prandtl number
- PCI:
-
Pulverized coal injection
- \(R\) :
-
Gas constant, \( 8.314 \,{\rm J} \, {\rm mol}^{ - 1} \, {\rm K}^{ - 1}\)
- \(r_{\rm throat}\) :
-
Radial distance in the throat, m
- \(R_{\rm k}^{*}\) :
-
Reaction rate for \(k^{{{\rm th}}}\) reaction, \({\rm mol} \, {\rm m}^{ - 3} \, {\rm s}^{ - 1}\)
- \(Re\) :
-
Reynolds number
- \(S\) :
-
Source term
- \(Sc\) :
-
Schmidt number
- \(Sh_{\rm r}^{ * }\) :
-
Normalized shrinkage ratio
- \(T\) :
-
Temperature, \({\rm K}\)
- \({\mathbf{u}}\) :
-
Interstitial velocity, \({\rm m} \, {\rm s}^{ - 1}\)
- \(\widetilde{u}\) :
-
Interstitial velocity vector, \({\rm m} \, {\rm s}^{ - 1}\)
- \(V_{\rm b}\) :
-
Bed volume, \({\rm m}^{3}\)
- \(V_{\rm ore}\) :
-
Volume fraction of ore
- \(V_{\rm coke}\) :
-
Volume fraction of coke
- \(Vol_{\rm cell}\) :
-
Volume of control volume, \({\rm m}^{3}\)
- \(y_{i}\) :
-
Mole fraction of \(i{{{\rm th}}}\) species in gas phase
- \(y_{\rm CO}^{*} ,y_{{{\rm H}_{2} }}^{*}\) :
-
Molar fraction of \({\text{CO}}\) and \({\text{H}}_{{2}}\) in equilibrium state for indirect reaction
- \(\alpha\) :
-
Specific surface area, \({\rm m}^{2} \, {\rm m}^{ - 3}\)
- \(\alpha_{\rm gs}\) :
-
Drag coefficient
- \(\beta\) :
-
Mass increase coefficient of fluid phase associated with reactions, \({\rm kg} \, {\rm mol}^{ - 1}\)
- \(\beta_{\rm gs}\) :
-
Drag coefficient
- \(\Gamma\) :
-
Diffusion coefficient
- \(\delta\) :
-
Distribution coefficient
- \(\varepsilon\) :
-
Volume fraction
- \(\eta\) :
-
Fractional acquisition of reaction heat
- \({\mathbf{\rm I}}\) :
-
Identity tensor
- \(\mu\) :
-
Viscosity, \({\rm kg} \, {\rm m}^{ - 1} \, {\rm s}^{ - 1}\)
- \(\xi_{\rm o} ,\xi_{\rm c}\) :
-
Local ore, coke volume fraction
- \(\rho\) :
-
Density, \({\rm kg} \, {\rm m}^{ - 3}\)
- \({\varvec{\tau}}\) :
-
Stress tensor, \({\rm Pa}\)
- \(\varphi\) :
-
General variable
- \(\omega\) :
-
Mass fraction
- \(\phi\) :
-
Average shape factor
- \({\rm e}\) :
-
Effective
- \({\rm g}\) :
-
Gas
- \({\rm l}\) :
-
Liquid
- \({\rm s}\) :
-
Solid
- \({\rm o}\) :
-
Ore
- \({\rm c}\) :
-
Coke
- \(i\) :
-
Identifier (g, s or l)
- \(i,m\) :
-
\(m{\rm th}\) Species in i phase
- \(j\) :
-
Identifier (g, s or l)
- \(k\) :
-
\(k{\rm th}\) Reaction
- \(n\) :
-
Direction (x or y)
- \(sm\) :
-
FeO or flux in solid phase
- \({\rm e}\) :
-
Effective
- \({\rm s}\) :
-
Solid
- \({\rm T}\) :
-
Transpose
References
W. Zhang, J. Dai, C.Z. Li, X.B. Yu, Z.L. Xue, and H. Saxén: Steel Res. Int., 2020, vol. 92, p. 2000326.
H. Yamaoka and Y. Kamei: ISIJ Int., 1992, vol. 32, pp. 701–08.
J. Stel, G. Louwerse, D. Sert, A. Hirsch, and N. Eklund: Steel Times International, 2013, vol. 37, pp. 26–29.
E. Karakaya, C. Nuur, and L. Assbring: J. Clean Prod., 2018, vol. 195, pp. 651–63.
S. Watakabe, K. Miyagawa, S. Matsuzaki, T. Inada, Y. Tomita, K. Saito, M. Osame, P. Sikström, L.S. Ökvist, and J. Wikstrom: ISIJ Int., 2013, vol. 53, pp. 2065–71.
M.A. Quader, S. Ahmed, R.A.R. Ghazilla, S. Ahmed, and M. Dahari: Renew Sust. Energy Rev., 2015, vol. 50, pp. 594–614.
L. Ren, S. Zhou, T.D. Peng, and X.M. Ou: Renew. Sust. Energy Rev., 2021, vol. 143, p. 110846.
H.Q. Nie, Z.Y. Li, S.B. Kuang, L.G. Yan, W.Q. Zhong, A.B. Yu, X.M. Mao, and H.F. Xu: Fuel, 2021, vol. 296, p. 120662.
A.K. Biswas: Principles of Blast Furnace Ironmaking: Theory and Practice, Cootha Publishing House, Australia, 1981, p. 302.
F. Fink: Steel Times, 1996, vol. 36, pp. 398–99.
M.S. Qin, Z.K. Gao, G.L. Wang, and Y.T. Zhang: Ironmak. Steelmak., 1988, vol. 15, pp. 287–92.
O. Yotaro, H. Hirohisa, M. Masahiro, M. Hiroyuki, and S. Hiroshi: Tetsu-to-Hagane, 1989, vol. 75, pp. 1278–85.
G.Q. Zuo and A. Hirsch: Rev. Metall., 2009, vol. 106, pp. 387–92.
Y.H. Qi, D.L. Yan, J.J. Gao, J.C. Zhang, and M.K. Li: Iron Steel, 2011, vol. 46, pp. 6–8.
X.B. Yu, Z.J. Hu, and Y.S. Shen: Fuel, 2021, vol. 302, 121092.
J.L. Zhang, G.W. Wang, J.G. Shao, and H.B. Zuo: J. Iron Steel Res. Int., 2014, vol. 21, pp. 151–58.
P. Jin, Z.Y. Jiang, C. Bao, Y.X. Lu, J.L. Zhang, and X.X. Zhang: Steel Res. Int., 2016, vol. 87, pp. 320–29.
P. Jin, Z.Y. Jiang, C. Bao, S.Y. Hao, and X.X. Zhang: Resour. Conserv. Recy., 2017, vol. 117, pp. 58–65.
W. Zhang, J.H. Zhang, and Z.L. Xue: Energy, 2017, vol. 121, pp. 135–46.
C.L. Li, Q.G. Xue, Y.L. Liu, Z.S. Dong, G. Wang, and J.S. Wang: Ironmak. Steelmak., 2017, vol. 46, pp. 761–70.
M. Helle and H. Saxén: ISIJ Int., 2015, vol. 55, pp. 2047–55.
X.F. She, X.W. An, J.S. Wang, Q.G. Xue, and L.T. Kong: J. Iron Steel Res. Int., 2017, vol. 24, pp. 608–16.
W. Zhang, J.H. Zhang, Z.L. Xue, Z.S. Zou, and Y.H. Qi: ISIJ Int., 2016, vol. 56, pp. 1358–67.
T. Miyashit, H. Nishio, T. Shimotsu, T. Yamada, and M. Ohtsuki: Trans. Iron Steel Inst. Jpn., 1973, vol. 13, pp. 1–10.
H. Yamaoka and Y. Kamei: ISIJ Int., 1992, vol. 32, pp. 709–15.
M.A. Tseitlin, S.E. Lazutkin, and G.M. Styopin: ISIJ Int., 1994, vol. 34, pp. 570–73.
S.B. Kuang, Z.Y. Li, and A.B. Yu: Steel Res Int, 2018, vol. 89, p. 1700071.
S. Natsui, S. Ueda, H. Nogami, J. Kano, R. Inoue, and T. Ariyama: ISIJ Int., 2011, vol. 51, pp. 51–58.
S. Natsui, S. Ueda, H. Nogami, J. Kano, R. Inoue, and T. Ariyama: ISIJ Int., 2011, vol. 51, pp. 1410–17.
Z.S. Dong, Q.G. Xue, H.B. Zu, X.F. She, J. Li, and J.S. Wang: ISIJ Int., 2016, vol. 56, pp. 1588–97.
Z.S. Dong, J.S. Wang, H.B. Zuo, X.F. She, and Q.G. Xue: Particuology, 2017, vol. 32, pp. 63–72.
Q.F. Hou, E. Dianyu, S.B. Kuang, Z.Y. Li, and A.B. Yu: Powder Technol., 2017, vol. 314, pp. 557–66.
Q.F. Hou, E. Dianyu, S.B. Kuang, Z.Y. Li, and A.B. Yu: Fuel Process. Technol., 2020, vol. 202, p. 106369.
Q.F. Hou, E. Dianyu, S.B. Kuang, Z.Y. Li, and A.B. Yu: Steel Res Int, 2020, vol. 91, p. 2000071.
X.F. Dong, A.B. Yu, J. Yagi, and P. Zulli: ISIJ Int., 2007, vol. 47, pp. 1553–70.
M.S. Chu, H. Nogami, and J. Yagi: ISIJ Int., 2004, vol. 44, pp. 2159–67.
M. Chu and J. Yagi: Steel Res. Int., 2010, vol. 81, pp. 1043–50.
H.T. Wang, M.S. Chu, T.L. Guo, W. Zhao, C. Feng, Z.G. Liu, and J. Tang: Steel Res. Int., 2016, vol. 87, pp. 539–49.
Z.Y. Li, S.B. Kuang, A.B. Yu, J.J. Gao, Y.H. Qi, D.L. Yan, Y.T. Li, and X.M. Mao: Metall. Mater. Trans. B, 2018, vol. 49, pp. 1995–2010.
L.Z. Liu, Z.Y. Jiang, X.R. Zhang, Y.X. Lu, J.K. He, J.S. Wang, and X.X. Zhang: Energy, 2018, vol. 163, pp. 144–50.
Z.G. Zhao, X.B. Yu, Y.S. Shen, Y.T. Li, H. Xu, and Z.J. Hu: Energy Fuel, 2020, vol. 34, pp. 15048–15060.
Z.L. Zhang, J.L. Meng, L. Guo, and Z.C. Guo: JOM, 2015, vol. 67, pp. 1936–44.
Z.L. Zhang, J.L. Meng, L. Guo, and Z.C. Guo: JOM, 2015, vol. 67, pp. 1945–55.
Z.L. Zhang, J.L. Meng, L. Guo, and Z.C. Guo: Metall. Mater. Trans. B, 2016, vol. 47, pp. 467–84.
Y. Ohno, M. Matsuura, H. Mitsufuji, and T. Furukawa: ISIJ Int., 1992, vol. 32, pp. 838–47.
T. Inada, K. Takatani, K. Takata, and T. Yamamoto: ISIJ Int., 2003, vol. 43, pp. 1143–50.
P.R. Austin, H. Nogami, and J. Yagi: ISIJ Int., 1997, vol. 37, pp. 748–55.
J.A. de Castro, A.J. da Silva, Y. Sasaki, and J. Yagi: ISIJ Int., 2011, vol. 51, pp. 748–58.
K. Yang, S. Choi, J. Chung, and J. Yagi: ISIJ Int., 2010, vol. 50, pp. 972–80.
X.F. Dong, A.B. Yu, S.J. Chew, and P. Zulli: Metall. Mater. Trans. B, 2010, vol. 41, pp. 330–49.
S.B. Kuang, Z.Y. Li, D.L. Yan, Y.H. Qi, and A.B. Yu: Miner. Eng., 2014, vol. 63, pp. 45–56.
S.J. Zhang, A.B. Yu, P. Zulli, B. Wright, and U. Tüzün: ISIJ Int., 1998, vol. 38, pp. 1311–19.
Z.Y. Li, S.B. Kuang, S.D. Liu, J.Q. Gan, A.B. Yu, Y.T. Li, and X.M. Mao: Powder Technol., 2019, vol. 353, pp. 385–97.
Z.Y. Zhou, A.B. Yu, and P. Zulli: Prog. Comput. Fluid Dyn., 2004, vol. 4, pp. 39–45.
Z.Y. Li, S.B. Kuang, D.L. Yan, Y.H. Qi, and A.B. Yu: Metall. Mater. Trans. B, 2017, vol. 48, pp. 602–18.
I. Muchi: Trans. Iron Steel Inst. Jpn., 1967, vol. 7, pp. 223–37.
L.L. Jiao, S.B. Kuang, L.L. Liu, A.B. Yu, Y.T. Li, X.M. Mao, and H. Xu: Metall. Mater. Trans. B, 2020, vol. 52, pp. 138–55.
L.L. Jiao, S.B. Kuang, A.B. Yu, Y.T. Li, X.M. Mao, and H. Xu: Metall. Mater. Trans. B, 2020, vol. 51, pp. 258–75.
L.L. Liu, B.Y. Guo, S.B. Kuang, and A.B. Yu: Metall. Mater. Trans. B, 2020, vol. 51, pp. 2211–29.
P.R. Austin, H. Nogami, and J. Yagi: ISIJ Int., 1998, vol. 38, pp. 239–45.
J.Z. Chen, T. Akiyama, H. Nogami, J. Yagi, and H. Takahashi: ISIJ Int., 1993, vol. 33, pp. 664–71.
L. L. Liu, S. B. Kuang, L. L. Jiao, B. Y. Guo and A. B. Yu: Fuel, 2021, p. 122832.
Y. Omori: Blast Furnace Phenomena and Modelling, Elsevier Applied Science, London, 1987.
M. Hatano and K. Kurita: Trans. Iron Steel Inst. Jpn., 1982, vol. 22, pp. 448–56.
T. Akiyama, R. Takahashi, and J. Yagi: ISIJ Int., 1993, vol. 33, pp. 703–10.
S. Ergun: Chem. Eng. Prog., 1952, vol. 48, pp. 89–94.
Acknowledgments
The authors are grateful to the Natural Science Foundation of China (Grant No. 52034003), the Australian Research Council, and Baowu for the financial support of this work.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nie, H., Yu, A., Jiao, L. et al. Numerical Investigation of Shaft Gas Injection Operation in Oxygen-Enriched Ironmaking Blast Furnace. Metall Mater Trans B 53, 2712–2734 (2022). https://doi.org/10.1007/s11663-022-02562-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-022-02562-x