Abstract
In two experiments using a Rapid Serial Visual Presentation (RSVP) we investigated how emotional and neutral faces (T1) modulate temporal attention for a following neutral face (T2). Typically, performance for T2 is spared when T2 immediately follows T1 (lag 1 sparing) but it is impaired when T2 is presented within 500 ms from T1 (Attentional Blink). Experiment 1 shows a shorter attentional blink following happy faces, relative to neutral and sad faces, which did not differ. Experiment 2 shows a lag 1 sparing only after happy T1s, but not after angry or neutral T1s, and a greater attentional blink following happy and angry T1-faces, compared to neutral T1-faces. Results indicate that happy faces exert different effects on temporal attention than negative (angry or sad) faces. Findings are discussed in terms of an interplay of resource depletion, due to emotional salience, and emotion-specific inhibitory mechanisms.
Similar content being viewed by others
Introduction
Attentional resources have limited capacity, and this limitation places severe constraints on the information consciously accessed at any given point in time (Peterson & Posner, 2012). Selection may depend on stimulus relevance to the current goals (i.e., endogenous attention), or on stimulus salience (i.e., exogenous attention, Peterson & Posner, 2012). Importantly, selection may also depend on emotional salience (Vuilleumier, 2005; Pourtois et al., 2013). There is some evidence that the specific emotional valence (i.e., positive vs negative) of a given stimulus may affect attention differently (e.g., Zinchenko et al., 2020). For instance, in the case of emotional faces—a salient stimulus for social interactions—negative faces hold attention, and delay attentional disengagement (e.g., Beloposky et al., 2011; Fox et al., 2000; Fox et al., 2002; Koster et al., 2004; Pool et al., 2016; Salemink et al., 2007), whereas positive faces yield flexible attention allocation (Olivers & Nieuwenhuis, 2006), and facilitate attention disengagement (Calvo & Nummenmaa, 2009). Additional evidence comes from interference studies, in which emotional targets are flanked by emotional distractors. For instance, Fenske and Eastwood (2003) found that, the interference by emotionally incongruent distractor-faces on sad target-faces was smaller, but the interference from emotionally incongruent distractor-faces on happy target-faces was greater compared to the interference on neutral target-faces. The authors interpreted this finding as due to positive target-faces facilitating attention to peripheral distractors, and to negative target-faces inhibiting attention to peripheral distractors. Similarly, Srivastava and Srinivasan (2010) showed that performance to peripheral targets was better following happy faces than following sad faces. Importantly, this effect was short-lived as it occurred only at short SOAs (i.e., 0 ms, 100 ms).
This evidence suggests that negative targets inhibit processing of concurrent information, whereas positive targets seem to facilitate it (see Olivers, & Meeter, 2008) but it is unclear whether this effect is limited to spatial attention, or it is a more general effect, affecting also stimuli presented in rapid temporal succession. The question is especially relevant for faces that change rapidly during social interactions and whose changes in emotional expression are short-lived (Ekman, 1999).
Two experiments investigated the effect of positive and negative faces on temporal attention using the Rapid Serial Visual Presentation (RSVP; Potter & Levy, 1969). The RSVP consists in presenting at a fixed, central location, a stream of stimuli in rapid temporal succession (e.g., 100 ms). Participants monitor the stream for two targets presented among distractors and their task is to report some targets' characteristics at the end of the stream. The time (i.e., lag) between the first (T1) and the second target (T2) is manipulated. Typically, performance for T2 shows two phenomena: The Attentional Blink and the lag 1 sparing. The attentional blink is a performance impairment when T2 is presented within 200–500 ms from T1 (Broadbent & Broadbent, 1987; Raymond et al., 1992). The lag 1 sparing refers to preserved performance when T2 immediately follows T1 (i.e., lag 1).
There are different theoretical accounts for these phenomena (see Dux & Marois, 2009 for a review): Traditionally, the attentional blink has been attributed to resource limitations due to T1 consolidation, which prevents T2 accessing working memory (e.g., two-stage model, Chun & Potter, 1995). The lag 1 sparing is attributed to the two targets sharing the same attentional episode. However, alternative accounts, emphasize the role of top-down attentional control mechanisms involved in target enhancement and/or distractor inhibition (Olivers & Meeter, 2008; Wyble et al., 2009) to prevent interference with T1 consolidation. Accordingly, the attentional blink is attributed to enhanced attention for T1, and delayed attention to (Nieuwenstein, 2006), or strategic inhibition of, the following items. Sparing occurs because T2 receives attentional enhancement along with T1.
The interplay of enhancement vs inhibitory mechanisms contributing to lag 1 sparing and attentional blink makes the RSVP particularly suitable for investigating whether emotional faces exert different effects on attention for information presented in close temporal proximity.
To date, previous RSVP findings show that emotional T1-faces modulate temporal selective attention by engendering a greater attentional blink compared to neutral faces (Bach et al., 2014; de Jong et al., 2010; Grynberg et al., 2013; Stebbins & Vanous, 2015; Stein et al., 2009; Zheng et al., 2015). Evidence of a larger attentional blink following negative T1s compared to neutral ones is well established, and it holds for angry, fearful, and painful T1-faces (Grynberg et al., 2013; Stein et al., 2009; Zheng et al., 2015). In contrast, only a few studies have used negative, positive, and neutral faces. More specifically, de Jong et al. (2010) used photos of angry, neutral or happy faces as T1, and letters as T2 presented at lag 1, 3, 5 or 7. Although results showed no lag 1 sparing regardless of T1 emotion, probably due to detrimental effect of a category switch between T1 and T2 at lag 1 (Visser et al., 1999), the attentional blink was greater after angry T1s than after neutral and happy T1s (see also Stebbins & Vanous, 2015). Maratos (2011) reported similar attentional blink effects using angry, happy, and neutral schematic faces, although they did not assess the lag 1 sparing.
These effects have been attributed to emotional stimuli prioritizing attention, leading to longer encoding and consolidation in working memory at the expenses of temporal close information. Indeed, neural evidence (Schwabe et al., 2011) shows enhanced brain activity in a cortical and subcortical network involved in higher processing of emotional T1s is associated with a greater impairment (e.g., attentional blink) for T2. However, this evidence seems to be specific to negative stimuli rather than to emotional stimuli in general. In fact, when emotional faces are presented as T2, usually negative, but not positive faces impair performance for the preceding T1 (a phenomenon known as “backward blink”; de Jong & Martens (2007)). Indeed, a recent study (Ray et al., 2020, Experiment 1) has reported that happy T1s yield a shorter attentional blink compared to angry T1s. Although, one could argue that as this effect occurred only when both T1 and T2 were positive faces and a control condition with neutral T2 was not included, it could be due to happy faces yielding a shorter attentional blink, to angry faces yielding a longer attentional blink or both. In fact, that there was no lag 1 sparing following both happy and angry T1-faces hints to the effect being due to angry faces accentuating the attentional blink. That is, if happy faces attenuated the attentional blink because they exerted less inhibition on following information, consumed less resources or both, then a lag 1 sparing should have been observed after happy faces but not after angry ones.
To assess whether positive and negative emotional faces have a different effect on attention for information that is presented in close temporal succession requires comparing the effects of positive and negative T1a on lag 1 sparing and the attentional blink. However, this has been rarely done in past studies using the RSVP with emotional faces and when they do, usually performance for T2 is compared at each lag (e.g., de Jong et al., 2010; Maratos, 2011) rather than assessed in terms of profiles of temporal selective attention (MacLean & Arnell, 2012). In fact, performance comparisons at each lag are informative of whether positive, negative, and neutral T1s pose different demands on attentional resources as they reflect the difficulty of the task performed on T1 (see Cousineau et al., 2006). More importantly, findings of better or worse performance at each lag cannot alone inform on the presence/absence of lag 1 sparing and /or attentional blink, which relies on performance changes across lags (e.g., MacLean & Arnell, 2012; Pecchinenda et al., 2020). Therefore, we report two experiments that investigated whether and how positive and negative faces compared to neutral ones, affect the lag 1 sparing and the attentional blink.
Experiment 1
Using a RSVP, we presented sad, happy, and neutral faces as T1 followed by neutral faces as T2. Target faces were presented among inverted distractor-faces and participants monitored the stream of stimuli to report the expression of T1 and the gender of T2. To assess whether T1 exerted different effects on attention toward T2, we report performance comparisons at each lag as they are informative of whether positive, negative, and neutral T1s pose different demands on attentional resources (i.e., they reflect the difficulty of the task performed on T1; see Cousineau et al., 2006). However, if sad and happy faces exert a stronger/weaker inhibition on the following information, then there should be differences in lag 1 sparing and the attentional blink following sad and happy T1s compared to neutral ones. Namely, if sad T1s exert greater inhibition on T2, then there should be no lag 1 sparing and a longer attentional blink following sad T1 compared to neutral T1s. In contrast, if happy T1s exert weaker inhibition on T2, than there should be lag 1 sparing and a shorter attentional blink following happy compared to neutral T1s. This will be assessed comparing performance across lags for each valence.
Method
Participants
Thirty participants (18 females, 12 males, age M = 23; SD = 6.57) completed the experiment in partial fulfilment of course credits. The sample size was calculated using G*Power software (Faul et al., 2009) based on the effects reported in previous studies investigating emotion modulation of the attentional blink (e.g., de Jong et al., 2010). This established that a sample of 30 participants was sufficient to detect a moderate-large effect size of f = 0.28 (α = 0.05, power = 0.85). Participants had normal or corrected to normal vision and were naïve to the experimental hypotheses.
Materials and apparatus
Eight different identities (4 females) displaying sad, happy, and neutral expressions (for a total of 24 stimuli) were selected from the Karolinska Directed Emotional Faces database (KDEF, Lundqvist et al., 1998) and were used as T1. Based on available validation data (Goeleven et al., 2008), we compared the mean of arousal level and correct identifications for the target emotion (i.e., emotion hit rates) of the selected T1-faces. The arousal level of happy (M = 3.85, SD = 0.32) and sad (M = 3.55, SD = 0.48) faces was higher than the arousal level of neutral faces (M = 2.42, SD = 0.21), t(7) = 9.78, p < 0.001, and t(7) = 7.53, p < 0.001, respectively. Importantly, the arousal levels of happy and sad emotional faces did not differ, t(7) = 1.47, p = 0.184, allowing to rule out an account in term of differences in arousal for any possible effects of sad and happy T1s on T2. In terms of correct identifications of the target emotion, hit rates for happy faces (M = 96.49, SD = 3.98) were higher than for neutral faces (M = 86.44, SD = 8.23), t(7) = 3.24, p = 0.014. Hit rates for sad faces (M = 88.06, SD = 8.84) did not differ from neutral faces, t(7) = 0.58, p = 0.58. Importantly, hit rates for sad and happy faces did not differ, t(7) = 2.26, p = 0.58. From the same database, an additional set of 24 different identities (12 females) with neutral emotion were selected and used as T2. Criteria for stimuli selection were: face/expression symmetry, clear forehead, no visible beard for male faces or make-up for female faces. The distractors were 32 different neutral faces (16 female) selected from FACES database (Ebner et al., 2010), and rotated by 180°. All stimuli were full-colour faces and were edited using Photoshop CS6 to remove skin markers, and to balance for colour (RGB value), luminance and contrast (29.2 cd/m2). Faces were adjusted to the vertical and cropped in an oval excluding ears and hair. Stimuli were presented on a Core™ i5 computer via a 21.5″ Dell P2210H (Analog) monitor (1600 × 900 pixels, 60 Hz). RSVP streams were presented using E-Prime software (Schneider et al., 2002) for Windows 7 Professional, which also recorded participants’ responses using a standard USB-keyboard.
Procedure
After participants had given informed consent, they sat in front of a computer in a dimly lit room. The task started with 15 practice trials followed by 600 experimental trials, divided in 5 blocks of 120 trials each. Each block consisted of 8 repetitions of the 15 conditions resulting from the factorial combination of T1-Valence (3: Happy, Sad, or Neutral) and lag (5 lags). In each block, T1 and T2 gender was balanced resulting in 4 possible combinations: female-female, female-male, male–female, and male-male. T1 and T2 identities were counterbalanced across the 5 blocks.
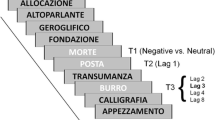
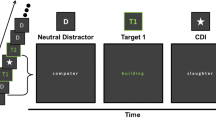
A single trial started with a fixation point (500 ms), followed by a stream of 18 stimuli: 16 upside-down distractors and 2 upright targets, all displayed at a rate of 83 ms (see Fig. 1). T1 could appear in position 4, 5, 6, 7 or 8 of the streams (i.e., preceded by 3, 4, 5, 6 or 7 distractors, respectively), whereas T2 was presented either at lag 1 (i.e., T2 immediately followed T1), lag 2 (i.e., T2 and T1 were separated by one distractor, etc.) lag 3, lag 4 or lag 8, relative to T1.
Participants started each trial by pressing the spacebar and were instructed to monitor each stream for two upright target-faces presented among rotated distractor-faces. Their task was to report at the end of each stream T1’s expression (neutral, happy, or sad) and T2’s gender (male or female) by pressing the designated, labelled buttons on the keyboard (1, 2, 3, and 4, 5, respectively).
Measures and data analyses
We first computed the percentage of correct T1 (pT1) and T2 identifications, conditional on correctly reporting T1, (pT2|T1) for all conditions. Next, both pT1 and pT2|T1 were analysed using an ANOVA for repeated measures with T1-Valence (3: Happy, Sad, Neutral) and Lag (5: lag 1, 2, 3, 4 and 8) as within-subject factors. A significant interaction was followed-up by: (a) relative comparisons across lags for each T1-Valence condition and the typical (2) absolute comparisons across the three T1-Valence at each lag. The first comparisons allowed us to establish the presence of lag 1 sparing, as well as the presence and magnitude of the attentional blink. More specifically, the lag 1 sparing is typically defined as performance accuracy at lag 1 exceeding the lowest level of accuracy by more than 5% in absolute term (Visser et al., 1999). The attentional blink is defined as the maximum performance impairment for T2 occurring within the attentional blink -window (i.e., lag 2 and lag 3) compared to performance outside this window (i.e., lag 8); (MacLean & Arnell, 2012). The magnitude of the attentional blink is then assessed by comparing the maximum performance impairment for T2 to the last lag of the attentional blink -window (in the present case, lag 4). For simplicity, only these hypotheses-based comparisons are reported. In addition, all comparisons were Bonferroni-corrected.
Results and discussion
T1 identification (pT1)
ANOVA results showed a significant main effect of T1-Valence, F (2, 58) = 4.21, p = 0.020, ηp2 = 0.127 and a significant main effect of Lag, F (4, 116) = 25.89, p < 0.001, ηp2 = 0.472. Importantly, the interaction between T1-Valence and Lag was also significant, F (8, 232) = 11.47, p < 0.001, ηp2 = 0.283. Accuracy for Sad T1s was lower at lag 1 than at all other lags, ps < 0.001. No other differences reached statistical significance. Pairwise comparisons across T1 valence showed that for Sad T1s, accuracy at lag 1 was lower compared to Happy and Neutral T1s, p = 00.4 and p < 0.001, respectively (see Table 1). No other differences reached statistical significance. To better understand this effect, we also analysed the identification errors by computing the overall percentage of misreporting Happy T1s as Neutral, against the overall percentage of misreporting Sad T1s as Neutral. This revealed that Sad T1s were more often misreported as Neutral T1s (M = 78.63, SE = 2.91), than as Happy T1s (M = 63.40, SE = 5.06), t (29) = 2.99, p = 0.006, pointing to sad faces being less distinguishable from the neutral faces when T1 and T2 were presented one after the other (i.e., at lag 1), that is, when the potential for masking is at its maximum.
T2|T1 identification (pT2|T1)
ANOVA results for pT2|T1 showed a significant main effect of T1-Valence, F (2, 58) = 3.92, p = 0.025, ηp2 = 0.119, and a significant main effect of Lag, F (4, 116) = 34.68, p < 0.001, ηp2 = 0.545. These effects were qualified by a significant interaction, F (8, 232) = 2.49, p = 0.013, ηp2 = 0.079 (see Fig. 2).
Pairwise comparisons across lags for pT2|T1-Neutral showed that performance at lag 2 (the worst across all lags) was lower than at lag 8, p < 0.001, indicating an attentional blink. The attentional blink was still present at lag 4 as it did not differ from lag 2, p = 0.135, and it was worse than at lag 8, p < 0.001. There was no lag 1 sparing as performance at lag 1 did not exceeded performance during the attentional blink by at least 5% (actual difference: 3.96%). For pT2|T1-Happy, the lowest performance was at lag 3 and it was lower than performance at lag 8, p < 0.001, indicating an attentional blink. The attentional blink recovered at lag 4, as performance was better than at lag 2, p = 0.034 and it did not differ from lag 8, p = 0.117. There was no lag 1 sparing, as performance at lag 1 fell short of exceeding performance during the attentional blink by at least 5% (actual difference: 4.61%). Finally, for pT2|T1-Sad, the lowest performance was at lag 2, and it was lower than performance at lag 8, p < 0.001, indicating an attentional blink. The attentional blink was still present at lag 4 as performance did not differ from lag 2, p = 0.123 and it was worse than at lag 8, p < 0.001. There was no lag 1 sparing as performance at lag 1 did not exceeded performance during the attentional blink by at least 5% (actual difference: 2.46%).
Pairwise comparisons across the three T1s showed that, at lag 8, pT2|T1-Neutral did not differ from pT2|T1-Sad, p = 0.667, but pT2|T1-Happy was lower than both pT2|T1-Neutral, p = 0.008, and pT2|T1-Sad, p < 0.001. There was no other difference among the three T1s at lag 1, 2, 3, and 4, all ps > 0.67 (see Table 2).
In summary, findings of Experiment 1 showed that when using sad, neutral, and happy faces as T1, there was no lag 1 sparing, albeit performance for T2 was less impaired after Happy-T1s. Importantly, the attentional blink after Neutral and Sad T1s occurred earlier (i.e., at lag 2) and lasted longer (i.e., over lag 3 and lag 4), then the attentional blink after Happy T1s (i.e., started at lag 3 and recovered at lag 4). This suggests that Happy T1-faces impair attention for the following information less than Sad and Neutral T1-faces, although this was at expenses of a full recovery at lag 8. However, the present findings are weakened by the unexpected low identification for sad T1-faces as they were often confused with neutral ones. This does not allow to rule out that the observed across lags differences following sad and happy T1-faces are due to differences in difficulty to identify the emotion of T1-faces.
Experiment 2
Our aim was to assess whether positive and negative T1s exert different effects on temporal selective attention by modulating the sparing and the attentional blink. That the task performed on T1 was more difficult for sad faces leaves open the alternative account that the shorter attentional blink observed after happy faces is due to this emotion being easier to identify (e.g., Chun & Potter, 1995) rather than to happy faces engendering a weaker inhibition on the following information, reducing the attentional blink (e.g., Olivers & Meeter, 2008). To further disentangle this effect, in Experiment 2, angry faces were used as T1s. Indeed, both happy and angry faces have distinctive perceptual features around the eyes and mouth regions (i.e., slightly open mouth), which should help distinguishing the emotional expressions from the neutral ones, regardless the valence. In addition, this time both emotions are approach-related (Carver & Harmon-Jones, 2009; Wilkowski & Meier, 2010) albeit they still differ on valence. This also allows to rule out that possible differences on the effects of positive and negative T1s could be due to avoidance motivation yielding greater inhibition toward following information and approach motivation enhancing attention to the following information. The predictions and comparisons are as of experiment 1.
Method
Participants
Thirty participants (17 females, 13 males, age M = 25.14; SD = 5.23), who had not taken part in Experiment 1, completed the experiment in partial fulfilment of course credits. They had normal or corrected to normal vision and were naïve to the experimental hypotheses.
Materials and apparatus
Twenty-four faces of eight different identities (four female) displaying angry, happy, or neutral expressions served as T1. As for the experiment 1, we compared arousal levels and hit rates for the selected faces used as T1, using published data (Goeleven et al., 2008). The arousal levels of happy (M = 3.70, SD = 0.35) and angry emotional faces (M = 4.03, SD = 0.85) did not differ, t(7) = 0.81, p = 0.44, but they were both higher than the arousal level of neutral faces (M = 2.51, SD = 0.15), t(7) = 7.90, p < 0.001, and t(7) = 5.03, p = 0.002, respectively. As for experiment 1, this allows to rule out that any possible effects of the emotional T1s be due to differences in arousal levels. Emotion hit rates for happy faces (M = 97.27, SD = 2.0) were higher than for neutral faces (M = 81.25, SD = 11.81), t(7) = 3.56, p = 0.009. Hit rates for angry faces (M = 80.47, SD = 26.31) were higher than for neutral faces, t(7) = 0.5807, p = 0.95. Importantly, hit rates for happy and angry face did not differ, t(7) = 1.91, p = 0.098. An additional set of 24 neutral faces (12 females) of different identities served as T2. All faces were selected from the Karolinska Directed Emotional Faces database (Lundqvist et al., 1998). In addition to the criteria used in Exp.1, faces were selected if the happy and angry expressions showed a slightly open mouth.
Procedure, experimental design, and data analyses
Procedure, Experimental Design, and Data Analyses were as in Experiment 1.
Results and discussion
T1 identification (pT1)
ANOVA results showed a main effect of T1 Valence, F (2, 58) = 11.21, p < 0.001, ηp2 = 0.279, and a main effect of Lag, F (4, 116) = 28.88, p < 0.001, ηp2 = 0.499. Importantly, the interaction was significant, F (8, 232) = 11.78, p < 0.001, ηp2 = 0.289 (see Table 3). Pairwise comparisons showed that for both Angry and Happy T1s, accuracy at lag 1 was lower than accuracy at all other lags, ps < 0.012 and ps < 0.001, respectively. No other comparison reached statistical significance. Pairwise comparisons at lag1 across the three types of T1s confirmed that performance for Neutral T1s was better than performance for Happy and Angry T1s, ps < 0.001. Importantly, this time accuracy for Happy T1s was lower than for Angry T1s, p = 0.014. Performance for Happy T1s at lag 2 was worse than for Neutral T1s also, p = 0.035. No other comparison reached statistical significance. As for Exp 1, an error analysis comparing the overall percentage of misreporting Happy T1s as Neutral, against the overall percentage of misreporting Angry T1s as Neutral, showed no differences, t(29) = 0.23, p = 0.82, suggesting that the lower identification accuracy for Happy T1 is not due to confusion between emotional and neutral faces. Finally, we compared overall identification performance for Angry (exp 2) and Sad (exp. 1) T1s. Results showed that pT1 for Angry faces (M = 90, SE = 1.6) was higher than pT1 for Sad faces (M = 83, SE = 3.7), t(4) = 3.17, p = 0.34. In contrast, pT1 for Happy faces did not differ across the two experiments (Exp. 1: M = 88, SE = 1.4; Exp. 2 M = 85, SE = 3.2), t(4) = 1.68, p = 1.7. To note, now that happy faces are more difficult to identify then angry faces, any possible difference in lag 1 and attentional blink following happy and angry T1s could not be due to happy faces posing less demands on attentional resources.
T2|T1 identification (pT2|T1)
ANOVA results for pT2|T1 showed a significant main effect of T1-Valence, F (2, 58) = 8.08, p < 0.001, ηp2 = 0.218, a significant main effect of Lag, F (4, 116) = 22.77, p < 0.001, ηp2 = 0.440, and a significant interaction, F (8, 232) = 2.11, p = 0.036, ηp2 = 0.068 (see Fig. 3 and Table 4).
Pairwise comparisons across lags showed that, for pT2|T1 Neutral, the lowest performance during the attentional blink-window was at lag 3, indicating an attentional blink as performance was lower than at lag 8, p < 0.001. Performance at lag 3 differed from lag 4, p < 0.001, indicating that the attentional blink had started to recover, although it was still significantly lower than at lag 8, p = 0.001. There was sparing as performance at lag 1 exceeded that of the attentional blink by at least 5% (actual difference: 9.58%). For pT2|T1 Happy, the lowest performance was at lag 2, indicating an attentional blink as performance was different from lag 8, p < 0.001. Performance at lag 2 differed from lag 4, p = 0.007, indicating that the attentional blink had started to recover, although it was still lower than at lag 8, p = 0.007. There was sparing as performance at lag 1 exceeded performance during the attentional blink by at least 5% (actual difference: 8.3%). For pT2|T1 Angry, the lowest performance during the attentional blink-window was at lag 2, indicating an attentional blink as performance differed from lag 8, p < 0.001. Performance at lag 4 was better than at lag 2, p < 0.009, indicating the attentional blink had started to recover, although it was still lower than at lag 8, p < 0.006. There was no sparing as performance at lag 1 did not exceeded that of the attentional blink by at least 5% (actual difference: 3.83%).
Pairwise comparisons across the three T1s showed no significant differences at lag 3 and at lag 8, all ps > 0.350. At lag 1, pT2|T1-Angry was worse than pT2|T1-Neutral, p = 0.007, whereas at lag 2 and 4 pT2|T1-Happy was worse than pT2|T1-Neutral, p = 0.009 and p = 0.015, respectively. No other comparison was statistically significant, all ps > 0.16.
The findings of Experiment 2 show that angry T1-faces were better identified than happy T1-faces consistent with the “anger superiority effect” (Maratos et al., 2008). Importantly, this result rules out that the lag 1 sparing and the shorter attentional blink observed after happy T1-faces is due to these stimuli being easier to identify and posing less demands on attentional resources. Importantly, although both angry and happy T1s impaired performance for T2 more than neutral T1s, there are clear differences in how happy and angry faces affect temporal selective attention for the following information. Only happy T1s, like neutral ones, yield a lag 1 sparing. However, whereas after neutral T1s there was an attentional blink at lag 3, happy T1s were followed by a longer attentional blink (over lag 2 and lag 3), which started to recover only at lag 4. In contrast, there was no lag 1 sparing after angry T1s and, similarly to what observed after happy T1s, the attentional blink started at lag 2 and continued over two lags. The pattern of temporal attention observed after happy T1-faces shares some characteristics with that observed after neutral T1s (i.e., the sparing at lag 1) and others with the pattern observed after angry T1s (i.e., an attentional blink starting earlier at lag 2 and lasting longer). That neutral and happy T1s engender lag 1 sparing but angry T1s do not is indicative of happy faces (like neutral faces) exerting weaker inhibition and angry faces exerting a stronger inhibition on the immediately following information. However, the present pattern also points to an interplay of attentional control mechanisms and resource depletion in yielding the lag 1 sparing and the attentional blink. The theoretical implications of these findings are discussed in more details in the General Discussion section below.
General discussion
In two experiments using the RSVP, we assessed whether negative and positive faces differently affect the two typical phenomena of temporal selective attention, the lag 1 sparing and the attentional blink. Findings from experiment 1 showed a similarly prolonged attention impairment following neutral and sad T1s. This finding was due to the difficulty in identifying sad T1-faces since sad faces were often misreported as neutral, especially when neutral T2 immediately followed at lag 1 and masking effects was at the highest. Although sad faces have been successfully used in past studies (i.e., Srivastava & Srinivasan, 2010), it may have helped that they were not used together with neutral faces. Indeed, identification for sad faces is usually lower than for other emotional faces even when presented at different spatial locations (Calvo & Nummenmaa, 2008). In addition, the RSVP by presenting two target-faces in close temporal proximity makes discriminability between stimuli low (see Müsch et al., 2012). Finally, it is also possible that when neutral faces are presented amid negative faces, the neutral faces appear more negative (e.g., Said et al., 2009; Tottenham et al., 2013), contributing to the poor discrimination between sad and neutral T1s. Alternatively, and regardless of these factors, it is also possible that sad faces simply fail to modulate temporal attention differently than neutral faces. As previous RSVP studies have used more intense painful faces, instead of sad ones, (Grynberg et al., 2013; Stein et al., 2009; Zheng et al., 2015), this is the first evidence on the effect of sad faces on temporal attention.
Importantly, Experiment 1 shows that there was no lag 1 sparing and a prolonged attentional blink after neutral and sad T1s. In contrast, the attentional blink after happy T1s started later (i.e., at lag 3) and was much shorter starting to recover at lag 4 and although there was no lag 1 sparing, performance at lag 1 was much better following happy T1s. This finding is in line with Ray et al. (2020) and suggests a reduced performance impairment after happy T1-faces. Unfortunately, the pattern observed following happy faces could not be solely attributed to positive faces exerting weaker inhibition on following information as happy faces were also easier to identify than negative faces. This means that resource depletion (e.g., Chun & Potter, 1995) could have contributed to the observed pattern. Experiment 2 helped clarifying this issue by using angry and happy faces as emotional T1s and neutral faces as control T1s and this time, findings provided clear evidence that positive and negative emotion differently modulate the lag 1 sparing and the attentional blink.
When using happy, angry, and neutral faces as T1, overall performance improved, albeit this time emotional faces were more difficult to identify than neutral faces, and this was especially true for happy faces. Whereas this may seem surprising, given that typically emotional faces are better detected than neutral ones (e.g., Pinkham et al., 2010; Schubö et al., 2006), it is not unusual when the RSVP task requires identifying and reporting the specific emotion (e.g., Grynberg et al., 2013; Stebbins & Vanous, 2015 but see de Jong et al., 2010). In addition, both happy and angry faces were chosen to have slightly open mouth and this meant that emotional expressions could not be simply identified by the presence/absence of low-level perceptual features. This, together with a threat-superiority effect typical for angry faces (e.g., Pinkham et al., 2010) may have contributed to the lower identification of happy T1. However, as our main interest was to assess the different effects of positive and negative faces on temporal selective attention pattern, that happy T1-faces were less easy to identify than neutral and angry T1-faces helps ruling out an account of the observed effects on T2 solely in terms of happy faces depleting less attentional resources.
Findings showed that happy and angry faces differently affected performance for the following target and this occurred in line with our prediction: neutral and happy T1s yielded a lag 1 sparing, which was absent for negative T1s. In addition, whereas the attentional blink after neutral faces was limited to lag 3, the attentional blink following both emotional faces started earlier, was longer and quantitatively greater than that following neutral T1s. This finding is at odd with evidence of a greater attentional blink only after angry faces but not after happy faces (de Jong et al., 2010), although there are important methodological differences between the two studies that could account for this difference. In our study, stimuli were presented more rapidly (i.e., 83 ms vs 120 ms) and T2 was another salient stimulus—a face—rather than a letter (for similar effects of T1-faces on T2-letters see Robinson et al., 2017). Indeed, past studies show when both targets are faces, angry and happy T1s equally impair performance for T2 at early lags (e.g., Bach et al., 2014; Ray et al., 2020).
Although it has been argued that emotional stimuli by depleting resources give a greater attentional blink, it becomes evident that the profiles of temporal attention observed following happy and angry T1-faces are difficult to describe solely in terms of resource depletion (Chun & Potter, 1995) as if this were the case, both happy and angry T1s should have eliminated the lag 1 sparing and engendered a longer attentional blink. Similarly, whereas the lag 1 sparing after neutral T1s could be attributed to these stimuli being easier to identify and/or attracting/prioritizing less attentional resources than emotional T1s, this is not the case for happy T1-faces. Rather, the present findings seem better explained by an interplay of resource depletion and attentional control mechanism related to enhancement/inhibition of following targets (Olivers & Meeter, 2008; Raymond et al., 1992). Accordingly, angry faces exert stronger inhibition on the following targets to prevent interference on T1, thus eliminating the lag 1 sparing and prolonging the attentional blink (Olivers & Meeter, 2008). By contrast, happy faces exert weaker inhibition on the following target, engendering lag 1 sparing when the two targets shared the same attentional episode. However, resource depletion did play a role since emotional salience increased the duration of the attentional blink. The combined contribution of resource depletion and top-down control mechanisms is in line with recent hybrid models of the attentional blink (episodic Simultaneous Type/Serial Token model, Wyble et al., 2009), which incorporate the suppression strategy to prevent interference on T1 consolidation, with the capacity limitations of stage 2.
Finally, one could argue that the weaker/stronger inhibition is due to differences in motivation intensity between angry and happy faces. Indeed, it has been suggested that emotions with high motivation intensity, such as anger, narrow attention, whether low motivated emotions, such as happiness and sadness, broaden it (Gable & Harmon-Jones, 2010b). However, although the motivation intensity hypothesis may contribute to explaining the findings of Experiment 2, it fails to explain those of Experiment 1 as both sad and happy faces have low motivation intensity. The more parsimonious explanation for the findings of both experiments reported here is that happy and angry—but not sad faces—affect temporal selective attention in terms of exerting weaker and stronger inhibition on following information.
The present evidence also extends previous finding of specific effects of negative-disgusted faces (Vermeulen et al., 2009) and of negative-fearful faces (Pecchinenda et al., 2020) on temporal attention. Disgusted faces exerted a stronger inhibition toward T2 (Vermeulen et al., 2009), whereas fearful faces exerted a weaker inhibition toward the immediately following T2, and these effects occurred only when disgusted faces were used as primes before a RSVP stream (Vermeulen et al., 2009) or when hybrid T1-faces, consisting of a neutral expression at high spatial frequency superimposed onto the fearful expression at a low spatial frequency (Pecchinenda et al., 2020). These effects have been interpreted as fear increasing (i.e., to better detect the source of threat) and disgust diminishing (i.e., to reduce sensory exposure to something noxious) sensory intake from the environment.
The present findings show that weaker/stronger inhibition on following information is due to happy vs angry faces rather than to positive vs negative faces, as negative faces may have different effect depending on the specific emotion. Indeed, happy faces exert a weaker inhibition on following information and allow flexibility (Positive Affect Hypothesis; Olivers & Nieuwenhuis, 2006) and angry faces exert stronger inhibition on the following information. The effect of positive emotions on temporal attention is also consistent with the function of broadening attention and the action repertoire, which helps building resources (Fredrickson & Branigan, 2005; Gokce et al., 2021).
Limitations and conclusion
As a final note, we would like to point out that it could be argued that angry T1-faces, because of their higher arousal, yield greater inhibition on following information, eliminating the lag 1 sparing and impairing performance across earlier lags. However, as the selected happy and angry T1-faces did not differ on arousal levels, such an account is not applicable. Importantly, the present finding is in line with Ray et al. (2020) who also have provided some evidence that valence, rather than arousal, differentially modulates temporal selective attention. Unfortunately, there are no valence ratings for the selected stimuli and a direct comparison in terms of valence is not possible. However, the comparisons based on the available identification performance for the emotional categories (Goeleven et al., 2008) shows that the stimuli used as T1 were correctly categorized based on emotion categories (i.e., happiness, sadness, anger) above chance level (> 80%). Therefore, the present findings cannot be attributed to T1s’ arousal levels but to the specific positive (happy) and negative (sad and angry) emotion category.
As this is the first time that different temporal profiles of selective attention are reported with sad/angry, happy, and neutral T1s over neutral T2s, the present findings may prompt future research to assess the generalizability of the observed effects to other emotional stimuli without increasing task difficulty.
References
Bach, D. R., Schmidt-Daffy, M., & Dolan, R. J. (2014). Facial expression influences face identity recognition during the attentional blink. Emotion, 14(6), 1007. https://doi.org/10.1037/a0037945
Belopolsky, A. V., Devue, C., & Theeuwes, J. (2011). Angry faces hold the eyes. Visual Cognition, 19, 27–36. https://doi.org/10.1080/13506285.2010.536186
Broadbent, D. E., & Broadbent, M. H. P. (1987). From detection to identification: Response to multiple targets in rapid serial visual presentation. Perception & Psychophysics, 42, 105–113. https://doi.org/10.3758/BF03210498
Calvo, M. G., & Nummenmaa, L. (2008). Detection of emotional faces: Salient physical features guide effective visual search. Journal of Experimental Psychology: General, 137(3), 471. https://doi.org/10.1037/a0012771
Calvo, M. G., & Nummenmaa, L. (2009). Eye-movement assessment of the time course in facial expression recognition: Neurophysiological implications. Cognitive, Affective, & Behavioral Neuroscience, 9(4), 398–411. https://doi.org/10.3758/cabn.9.4.398
Carver, C. S., & Harmon-Jones, E. (2009). Anger is an approach- related affect: Evidence and implications. Psychological Bulletin, 135, 183–204. https://doi.org/10.1037/a0013965
Chun, M. M., & Potter, M. C. (1995). A two-stage model for multiple target detection in rapid serial visual presentation. Journal of Experimental Psychology: Human Perception & Performance, 21, 109–127. https://doi.org/10.1037/0096-1523.21.1.109
Cousineau, D., Charbonneau, D., & Jolicœur, P. (2006). Parameterizing the attentional blink effect. Canadian Journal of Experimental Psychology/revue Canadienne De Psychologie Expérimentale, 60(3), 175. https://doi.org/10.1037/cjep2006017
de Jong, P. J., Koster, E. H. W., van Wees, R., & Martens, A. M. (2010). Angry facial expressions hamper subsequent target identification. Emotion, 10, 727–732. https://doi.org/10.1037/a0019353
de Jong, P. J., & Martens, S. (2007). Detection of emotional expressions in rapidly changing facial displays in high-and low-socially anxious women. Behaviour Research and Therapy, 45(6), 1285–1294. https://doi.org/10.1016/j.brat.2006.10.003
Dux, P. E., & Marois, R. (2009). How humans search for targets through time: A review of data and theory from the attentional blink. Attention, Perception & Psychophysics, 71(8), 1683–1700. https://doi.org/10.3758/APP.71.8.1683
Ebner, N. C., Riediger, M., & Lindenberger, U. (2010). FACES-A database of facial expressions in young, middle-age, and older woman and man: Development and validation. Behaviour Research Methods, 42(1), 351–362. https://doi.org/10.3758/BRM.42.1.351
Ekman, P. (1999). Facial expressions. Handbook of Cognition and Emotion, 16(301), e320.
Faul, F., Erdfelder, E., Buchner, A., & Lang, A. G. (2009). Statistical power analyses using G* Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods, 41(4), 1149–1160.
Fenske, M. J., & Eastwood, J. D. (2003). Modulation of focused attention by faces expressing emotion: Evidence from flanker task. Emotion, 3, 327–343. https://doi.org/10.1037/1528-3542.3.4.327
Fox, E., Lester, V., Russo, R., Bowles, R. J., & Dutton, K. (2000). Facial expressions of emotion: Are angry faces detected more efficiently? Cognition & Emotion, 14, 61–92. https://doi.org/10.1080/026999300378996
Fox, E., Russo, R., & Dutton, K. (2002). Attentional bias for threat: Evidence for delayed disengagement from emotional faces. Cognition and Emotion, 16(3), 355–379. https://doi.org/10.1080/02699930143000527
Fredrickson, B. L., & Branigan, C. (2005). Positive emotions broaden the scope of attention and thought-action repertoires. Cognition & Emotion, 19(3), 313–332. https://doi.org/10.1080/02699930441000238
Gable, P., & Harmon-Jones, E. (2010a). The motivational dimensional model of affect: Implications for breadth of attention, memory, and cognitive categorisation. Cognition and Emotion, 24(2), 322–337. https://doi.org/10.1080/02699930903378305
Gable, P., & Harmon-Jones, E. (2010b). The blues broaden, but the nasty narrows: Attentional consequences of negative affects low and high in motivational intensity. Psychological Science, 21(2), 211–215. https://doi.org/10.1177/0956797609359622
Goeleven, E., De Raedt, R., Leyman, L., & Verschuere, B. (2008). The Karolinska directed emotional faces: A validation study. Cognition and Emotion, 22(6), 1094–1118. https://doi.org/10.1080/02699930701626582
Gokce, A., Zinchenko, A., Annac, E., Conci, M., & Geyer, T. (2021). Affective modulation of working memory maintenance. Advances in Cognitive Psychology, 2, 107–116. https://doi.org/10.5709/acp-0321-7
Grynberg, D., Vermeulen, N., & Luminet, O. (2013). Amplification of attentional blink by distress-related facial expression: Relationship with alexithymia and affectivity. International Journal of Psychology, 49(5), 371–380. https://doi.org/10.1002/ijop.12006
Koster, E. H. W., Crombez, G., Verschuere, B., & De Houwer, J. (2004). Selective attention to threat in the dot-probe paradigm: Differentiating vigilance and difficulty to disengage. Behavioural Research and Therapy, 42, 1183–1192. https://doi.org/10.1016/j.brat.2003.08.001
Lundqvist, D., Flykt, A., & Öhman, A. (1998). The karolinska directed emotional faces (KDEF). CD ROM from Department of Clinical Neuroscience, Psychology section, Karolinska Institute. ISBN 91–630–7164–9.
MacLean, M. H., & Arnell, K. M. (2012). A conceptual and methodological framework for measuring and modulating the attentional blink. Attention, Perception & Psychophysics, 74, 1080–1097. https://doi.org/10.3758/s13414-012-0338-4
Maratos, F. A. (2011). Temporal processing of emotional stimuli: The capture and release of attention by angry faces. Emotion, 11(5), 1242–1247. https://doi.org/10.1037/a0024279
Maratos, F.A., Mogg, K., & Bradley, B.P. (2008). Identification of angry faces in the attentional blink. Cognition and Emotion, 22(7), 1340–1352. https://doi.org/10.1080/02699930701774218
Müsch, K., Engel, A. K., & Schneider, T. R. (2012). On the blink: The importance of target-distractor similarity in eliciting an attentional blink with faces. PLoS ONE, 7(7), e41257. https://doi.org/10.1371/journal.pone.0041257
Nieuwenstein, M. R. (2006). Top-down controlled, delayed selection in the attentional blink. Journal of Experimental Psychology: Human Perception & Performance, 31, 973–985. https://doi.org/10.1037/0096-1523.32.4.973
Olivers, C. N. L., & Meeter, M. (2008). A boost and bounce theory of temporal attention. Psychological Review, 115(4), 836–863. https://doi.org/10.1037/a0013395
Olivers, C. N., & Nieuwenhuis, S. (2006). The beneficial effects of additional task load, positive affect, and instruction on the attentional blink. Journal of Experimental Psychology: Human Perception and Performance, 32(2), 364. https://doi.org/10.1037/0096-1523.32.2.364
Pecchinenda, A., Monachesi, B., & Laeng, B. (2020). Fearful expressions of rapidly presented hybrid-faces modulate the lag 1 sparing in the attentional blink. Acta Psychologica, 209, 103124. https://doi.org/10.1016/j.actpsy.2020.103124
Petersen, S. E., & Posner, M. I. (2012). The attention system of the human brain: 20 years after. Annual Review of Neuroscience, 35, 73–89. https://doi.org/10.1146/annurev-neuro-062111-150525
Pinkham, A. E., Griffin, M., Baron, R., Sasson, N. J., & Gur, R. C. (2010). The face in the crowd effect: Anger superiority when using real faces and multiple identities. Emotion, 10(1), 141–146. https://doi.org/10.1037/a0017387
Pool, E., Brosch, T., Delplanque, S., & Sander, D. (2016). Attentional bias for positive emotional stimuli: A meta-analytic investigation. Psychological Bulletin, 142(1), 79–106. https://doi.org/10.1037/bul0000026
Potter, M. C., & Levy, E. I. (1969). Recognition memory for a rapid sequence of pictures. Journal of Experimental Psychology, 81, 10–15. https://doi.org/10.1037/h0027470
Pourtois, G., Schettino, A., & Vuilleumier, P. (2013). Brain mechanisms for emotional influences on perception and attention: What is magic and what is not. Biological Psychology, 92(3), 492–512. https://doi.org/10.1016/j.biopsycho.2012.02.007
Ray, S. B., Mishra, M. V., & Srinivasan, N. (2020). Attentional blink with emotional faces depends on emotional expressions: A relative positive valence advantage. Cognition and Emotion, 34(6), 1226–1245. https://doi.org/10.1080/02699931.2020.1736517
Raymond, J. E., Shapiro, K. L., & Arnell, K. M. (1992). Temporary suppression of visual processing in an RSVP task: An attentional blink? Journal of Experimental Psychology: Human Perception and Performance, 18(3), 849–860. https://doi.org/10.1037/0096-1523.18.3.849
Robinson, A. K., Plaut, D. C., & Behrmann, M. (2017). Word and face processing engage overlapping distributed networks: Evidence from RSVP and EEG investigations. Journal of Experimental Psychology: General, 146(7), 943. https://doi.org/10.1037/xge0000302
Said, C. P., Sebe, N., & Todorov, A. (2009). Structural resemblance to emotional expressions predicts evaluation of emotionally neutral faces. Emotion, 9(2), 260. https://doi.org/10.1037/a0014681
Salemink, E., van de Hout, M. A., & Kindt, M. (2007). Selective attention and threat: Quick orienting versus slow disengagement and two versions of the dot probe task. Behaviour Research and Therapy, 45, 607–615. https://doi.org/10.1016/j.brat.2006.04.004
Schneider, W., Eschman, A., & Zuccolotto, A. (2002). E-Prime: user’s guide. Psychology Software Incorporated.
Schubö, A., Gendolla, G., Meinecke, C., & Abele, A. E. (2006). Detecting emotional faces and features in a visual search task paradigm: Are faces special? Emotion, 6, 246–256. https://doi.org/10.1037/1528-3542.6.2.246
Schwabe, L., Merz, C. J., Walter, B., Vaitl, D., Wolf, O. T., & Stark, R. (2011). Emotional modulation of the attentional blink: The neural structures involved in capturing and holding attention. Neuropsychologia, 49, 416–425. https://doi.org/10.1016/j.neuropsychologia.2010.12.037
Srivastava, P., & Srinivasan, N. (2010). Time course of visual attention with emotional faces. Attention, Perception, & Psychophysics, 72(2), 369–377. https://doi.org/10.3758/APP.72.2.369
Stebbins, H. E., & Vanous, J. B. (2015). The influence of stimulus sex and emotional expression on the attentional blink. Emotion, 15(4), 511. https://doi.org/10.1037/emo0000082
Stein, T., Zwickel, J., Ritter, J., Kitzmantel, M., & Schneider, W. X. (2009). The effect of fearful faces on the attentional blink is task dependent. Psychonomic Bulletin & Review, 16(1), 104–109. https://doi.org/10.1016/j.cognition.2011.01.008
Tottenham, N., Phuong, J., Flannery, J., Gabard-Durnam, L., & Goff, B. (2013). A negativity bias for ambiguous facial-expression valence during childhood: Converging evidence from behavior and facial corrugator muscle responses. Emotion, 13(1), 92. https://doi.org/10.1037/a0029431
Vermeulen, N., Godefroid, J., & Mermillod, M. (2009). Emotional modulation of attention: Fear increases but disgust reduces the attentional blink. PLoS ONE, 4(11), e7924. https://doi.org/10.1371/journal.pone.0007924
Visser, T. A. W., Bischop, W. F., & Di Lollo, V. (1999). Attentional switching in spatial and nonspatial domains: Evidence from the attentional blink. Psychological Bulletin, 125, 458–469. https://doi.org/10.1037/0033-2909.125.4.458
Vuilleumier, P. (2005). How brains beware: Neural mechanisms of emotional attention. Trends in Cognitive Sciences, 9(12), 585–594. https://doi.org/10.1016/j.tics.2005.10.011
Wilkowski, B. M., & Meier, B. P. (2010). Bring it on: Angry facial expression potentiate approach-motivated motor behaviour. Journal of Personality and Social Psychology, 98(2), 201–210. https://doi.org/10.1037/a0017992
Wyble, B., Bowman, H., & Nieuwenstein, M. (2009). The attentional blink provides episodic distinctiveness: Sparing at a cost. The Journal of Experimental Psychology: Human Perception and Performance, 35(3), 787–807. https://doi.org/10.1037/a0013902
Zheng, C., Wang, J. Y., & Luo, F. (2015). Painful faces-induced attentional blink modulated by top–down and bottom–up mechanisms. Frontiers in Psychology, 6, 695. https://doi.org/10.3389/fpsyg.2015.00695
Zinchenko, A., Geyer, T., Müller, H. J., & Conci, M. (2020). Affective modulation of memory-based guidance in visual search: Dissociative role of positive and negative emotions. Emotion, 20(7), 1301. https://doi.org/10.1037/emo0000602
Funding
Open access funding provided by Università degli Studi di Roma La Sapienza within the CRUI-CARE Agreement. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. BM was supported by a PhD studentship from Sapienza University and the present work was part her PhD thesis in Behavioural Neuroscience. Ministero dell’Istruzione, dell’Università e della Ricerca (Grant no. PhD studentship in Behavioural Neuroscience and RM120172B77EE5F8). A.P. is funded by grant RM120172B77EE5F8 and grant RG12117A86626C87 from the Ministero Italiano Università e Ricerca.
Author information
Authors and Affiliations
Contributions
BM and AP designed the experiment. BM prepared the experimental stimuli and oversaw data collection. BM and AP conducted data analyses and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
The study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Departmental Ethics Committee.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Monachesi, B., Pecchinenda, A. Weaker inhibition after happy faces: evidence from an attentional blink task with emotional and neutral faces. Motiv Emot 46, 535–545 (2022). https://doi.org/10.1007/s11031-022-09950-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11031-022-09950-5