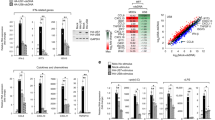
Abstract
Immune evasion and inhibition of apoptosis are required for successful virus infection. However, inhibition of apoptosis can increase antiviral immune responses, which can then clear viral infections. Here we show that human cytomegalovirus (HCMV)-encoded UL37 exon-1 protein (UL37x1) not only inhibits apoptosis but also suppresses the cGAS-STING immune pathway. Using co-immunoprecipitation assays, we found that UL37x1 binds to TBK1 to abrogate the TBK1-STING-IRF3 interaction. Although the anti-apoptosis function of UL37x1 increases immune signalling, the immunosuppressive role of UL37x1 counteracts this undesirable side-effect. Furthermore, we used mutational analyses to show that the loss of either immunosuppressive or anti-apoptotic function of UL37x1 significantly reduced HCMV replication in human primary foreskin fibroblasts and humanized mice by over twofold. Finally, loss of both functions resulted in over fourfold reduction of HCMV replication in the same cell type and mouse model, showing that both UL37x1 functions are crucial for HCMV infection. We conclude that this sophisticated mechanism enables HCMV to control innate immunity and apoptosis to ensure efficient infection.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the Article and its Supplementary Information. The nucleotide sequences of HCMV strains mentioned in this study are publicly available on NCBI GenBank AD169 (FJ527563.1) and Towne (GQ121041.1). Source data are provided with this paper.
References
Cohen, Y. & Stern-Ginossar, N. Manipulation of host pathways by human cytomegalovirus: insights from genome-wide studies. Semin. Immunopathol. 36, 651–658 (2014).
Wang, Y. Q. & Zhao, X. Y. Human cytomegalovirus primary infection and reactivation: insights from virion-carried molecules. Front. Microbiol. 11, 1511 (2020).
Liu, Y. et al. A cytomegalovirus peptide-specific antibody alters natural killer cell homeostasis and is shared in several autoimmune diseases. Cell Host Microbe 19, 400–408 (2016).
Manicklal, S., Emery, V. C., Lazzarotto, T., Boppana, S. B. & Gupta, R. K. The “silent” global burden of congenital cytomegalovirus. Clin. Microbiol. Rev. 26, 86–102 (2013).
Sun, L., Wu, J., Du, F., Chen, X. & Chen, Z. J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339, 786–791 (2013).
Zhong, B. et al. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity 29, 538–550 (2008).
Sun, W. et al. ERIS, an endoplasmic reticulum IFN stimulator, activates innate immune signaling through dimerization. Proc. Natl Acad. Sci. USA 106, 8653–8658 (2009).
Ishikawa, H. & Barber, G. N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455, 674–678 (2008).
Hopfner, K. P. & Hornung, V. Molecular mechanisms and cellular functions of cGAS-STING signalling. Nat. Rev. Mol. Cell Biol. 21, 501–521 (2020).
Zhang, X., Bai, X. C. & Chen, Z. J. Structures and mechanisms in the cGAS-STING innate immunity pathway. Immunity 53, 43–53 (2020).
Ma, Z., Ni, G. & Damania, B. Innate sensing of DNA virus genomes. Annu. Rev. Virol. 5, 341–362 (2018).
Wills, M. R., Poole, E., Lau, B., Krishna, B. & Sinclair, J. H. The immunology of human cytomegalovirus latency: could latent infection be cleared by novel immunotherapeutic strategies? Cell. Mol. Immunol. 12, 128–138 (2015).
Crough, T. & Khanna, R. Immunobiology of human cytomegalovirus: from bench to bedside. Clin. Microbiol. Rev. 22, 76–98 (2009).
Paijo, J. et al. cGAS senses human cytomegalovirus and induces type I interferon responses in human monocyte-derived cells. PLoS Pathog. 12, e1005546 (2016).
Fu, Y. Z. et al. Human cytomegalovirus tegument protein UL82 Inhibits STING-mediated signaling to evade antiviral immunity. Cell Host Microbe 21, 231–243 (2017).
Huang, Z. F. et al. Human cytomegalovirus protein UL31 inhibits DNA sensing of cGAS to mediate immune evasion. Cell Host Microbe 24, 69–80.e4 (2018).
Biolatti, M. et al. Human Cytomegalovirus Tegument Protein pp65 (pUL83) Dampens Type I Interferon Production by Inactivating the DNA Sensor cGAS without Affecting STING. J. Virol. 92, e01774-17 (2018).
Fu, Y. Z. et al. Human cytomegalovirus DNA polymerase subunit UL44 antagonizes antiviral immune responses by suppressing IRF3- and NF-κB-mediated transcription. J. Virol. 93, e00181-19 (2019).
Zou, H. M. et al. Human cytomegalovirus protein UL94 targets MITA to evade the antiviral immune response. J. Virol. 94, e00022-20 (2020).
Fu, Y. Z. et al. Human cytomegalovirus protein UL42 antagonizes cGAS/MITA-mediated innate antiviral response. PLoS Pathog. 15, e1007691 (2019).
Everett, H. & McFadden, G. Apoptosis: an innate immune response to virus infection. Trends Microbiol. 7, 160–165 (1999).
Orzalli, M. H. & Kagan, J. C. Apoptosis and necroptosis as host defense strategies to prevent viral infection. Trends Cell Biol. 27, 800–809 (2017).
Luo, B. et al. Erythropoeitin signaling in macrophages promotes dying cell clearance and immune tolerance. Immunity 44, 287–302 (2016).
McGaha, T. L. & Karlsson, M. C. Apoptotic cell responses in the splenic marginal zone: a paradigm for immunologic reactions to apoptotic antigens with implications for autoimmunity. Immunol. Rev. 269, 26–43 (2016).
White, M. J. et al. Apoptotic caspases suppress mtDNA-induced STING-mediated type I IFN production. Cell 159, 1549–1562 (2014).
Ning, X. et al. Apoptotic caspases suppress type I interferon production via the cleavage of cGAS, MAVS, and IRF3. Mol. Cell 74, 19–31.e7 (2019).
Peppenelli, M. A., Arend, K. C., Cojohari, O., Moorman, N. J. & Chan, G. C. Human cytomegalovirus stimulates the synthesis of select Akt-dependent antiapoptotic proteins during viral entry to promote survival of infected monocytes. J. Virol. 90, 3138–3147 (2016).
Reeves, M. B., Breidenstein, A. & Compton, T. Human cytomegalovirus activation of ERK and myeloid cell leukemia-1 protein correlates with survival of latently infected cells. Proc. Natl Acad. Sci. USA 109, 588–593 (2012).
Goldmacher, V. S. vMIA, a viral inhibitor of apoptosis targeting mitochondria. Biochimie 84, 177–185 (2002).
McCormick, A. L., Meiering, C. D., Smith, G. B. & Mocarski, E. S. Mitochondrial cell death suppressors carried by human and murine cytomegalovirus confer resistance to proteasome inhibitor-induced apoptosis. J. Virol. 79, 12205–12217 (2005).
Zhang, A., Hildreth, R. L. & Colberg-Poley, A. M. Human cytomegalovirus inhibits apoptosis by proteasome-mediated degradation of Bax at endoplasmic reticulum-mitochondrion contacts. J. Virol. 87, 5657–5668 (2013).
Skaletskaya, A. et al. A cytomegalovirus-encoded inhibitor of apoptosis that suppresses caspase-8 activation. Proc. Natl Acad. Sci. USA 98, 7829–7834 (2001).
Hayajneh, W. A. et al. The sequence and antiapoptotic functional domains of the human cytomegalovirus UL37 exon 1 immediate early protein are conserved in multiple primary strains. Virology 279, 233–240 (2001).
Zhao, B. et al. A conserved PLPLRT/SD motif of STING mediates the recruitment and activation of TBK1. Nature 569, 718–722 (2019).
Zhang, C. et al. Structural basis of STING binding with and phosphorylation by TBK1. Nature 567, 394–398 (2019).
Mavinakere, M. S. & Colberg-Poley, A. M. Dual targeting of the human cytomegalovirus UL37 exon 1 protein during permissive infection. J. Gen. Virol. 85, 323–329 (2004).
Griffante, G. et al. Human cytomegalovirus-induced host protein citrullination is crucial for viral replication. Nat. Commun. 12, 3910 (2021).
Mocarski, E. S. Jr. Biology and replication of cytomegalovirus. Transfus. Med. Rev. 2, 229–234 (1988).
Smith, M. S. et al. Granulocyte-colony stimulating factor reactivates human cytomegalovirus in a latently infected humanized mouse model. Cell Host Microbe 8, 284–291 (2010).
Bravo, F. J., Cardin, R. D. & Bernstein, D. I. A model of human cytomegalovirus infection in severe combined immunodeficient mice. Antivir. Res. 76, 104–110 (2007).
Biolatti, M. et al. Regulatory interaction between the cellular restriction factor IFI16 and viral pp65 (pUL83) modulates viral gene expression and IFI16 protein stability. J. Virol. 90, 8238–8250 (2016).
You, Y. et al. The suppression of apoptosis by alpha-herpesvirus. Cell Death Dis. 8, e2749 (2017).
Wang, Z. et al. A picorna-like virus suppresses the N-end rule pathway to inhibit apoptosis. eLife 6, e30590 (2017).
Pan, Y., Cheng, A., Wang, M., Yin, Z. & Jia, R. The dual regulation of apoptosis by flavivirus. Front. Microbiol. 12, 654494 (2021).
Lai, Y. et al. Regulation of apoptosis by enteroviruses. Front. Microbiol. 11, 1145 (2020).
Wasilenko, S. T., Meyers, A. F., Vander Helm, K. & Barry, M. Vaccinia virus infection disarms the mitochondrion-mediated pathway of the apoptotic cascade by modulating the permeability transition pore. J. Virol. 75, 11437–11448 (2001).
Shu, T. et al. SARS-Coronavirus-2 Nsp13 possesses NTPase and RNA helicase activities that can be inhibited by bismuth salts. Virol. Sin. 35, 321–329 (2020).
Lu, B. et al. Induction of INKIT by viral infection negatively regulates antiviral responses through inhibiting phosphorylation of p65 and IRF3. Cell Host Microbe 22, 86–98.e4 (2017).
Yu, D., Smith, G. A., Enquist, L. W. & Shenk, T. Construction of a self-excisable bacterial artificial chromosome containing the human cytomegalovirus genome and mutagenesis of the diploid TRL/IRL13 gene. J. Virol. 76, 2316–2328 (2002).
Tischer, B. K., Smith, G. A. & Osterrieder, N. En passant mutagenesis: a two step markerless red recombination system. Methods Mol. Biol. 634, 421–430 (2010).
Han, Y. et al. Feasibility study of mixing throat swab samples for severe acute respiratory syndrome coronavirus-2 screening. Virol. Sin. 35, 830–832 (2020).
Ren, Y. et al. The type I interferon-IRF7 axis mediates transcriptional expression of Usp25 gene. J. Biol. Chem. 291, 13206–13215 (2016).
Ren, Y. et al. The ORF3a protein of SARS-CoV-2 induces apoptosis in cells. Cell. Mol. Immunol. 17, 881–883 (2020).
Acknowledgements
We thank Y. Fu, Y. Wang and B. Zhong (Wuhan, China) for reagents.
This work was supported by the National Natural Science Foundation of China (NSFC) (31970169 to X.Z. and 32100106 to Y.R.), Strategic Priority Research Program of CAS (XDB29010300 to X.Z.), National Key R&D Program of China (2021YFC2300700 to X.Z.) and NSFC (U21A20423 to X.Z. and 81873964 to Y.Q.); a grant from the CAS Youth Innovation Promotion Association (2020332 to Y.Q.), the Hubei Province Natural Science Funds for Distinguished Young Scholars (2021CFA047 to Y.Q.) and the Young Top-notch Talent Cultivation Program of Hubei Province (Y.Q.).
Author information
Authors and Affiliations
Contributions
Y.R. performed most of the experiments. A.W., D.W., C.W., M.H., X.X. and L.J. performed specific experiments. Y.R. and X.Z. conceived the hypothesis. Y.R., W.Z., Y.Q. and X.Z. designed the experiments and analysed the data. Y.R., Y.Q. and X.Z. wrote the manuscript with inputs from all authors. X.Z. supervised the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Microbiology thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Sequence comparison of UL37x1 proteins.
The amino acid sequence alignment of UL37x1 proteins of different HCMV strains.
Extended Data Fig. 2 UL37x1 inhibits the DNA-triggered innate immune signaling in 293 T cells.
(a) Luciferase reporter assay analyzing IFNβ, ISRE, or NF-κB promoter activity was conducted in 293 T cells transfected with the plasmid of FLAG-cGAS plus FLAG-STING or an empty vector together with the indicated amounts of FLAG-UL37x1 expression plasmid for 24 h. The lower blots showed the expression levels of these transfected proteins. (b) 293 T cells were transfected with the plasmid of FLAG-cGAS plus FLAG-STING or an empty vector together with the indicated amounts of FLAG-UL37x1 expression plasmid for 24 h, followed by qPCR of the indicated antiviral genes. The lower blots showed the expression levels of these transfected proteins. (c) 293 T cells stably expressing STING were transfected with the plasmid of FLAG-UL37x1 or an empty vector. After 24 h, cells were untreated (mock) or transfected with indicated DNA ligands (HSV60, DNA90, or HSV120). At 3 and 6 h post transfection (h.p.t.), total RNA was extracted and subject to qPCR of indicated antiviral genes. Graphs show mean ± SD (n = 3, biologically independent experiments). Statistical significance was determined by two-way ANOVA. Immunoblots are representative of three independent experiments.
Extended Data Fig. 3 UL37x1 inhibits TBK1-mediated immune signaling.
(a) 293 T cells were transfected with the indicated plasmid in the presence or absence of the plasmid of FLAG-UL37x1. At 24 h.p.t., qRT-PCR was performed to measure the transcription of indicated antiviral genes. (b) HFFs were infected with HCMV for indicated time, followed by qPCR analysis of UL37x1. (c-d) HFFs were transfected with siRNA specifically for UL37x1 (UL37x1-siRNA#1, UL37x1-siRNA#2, or UL37x1-siRNA#3) or a control siRNA (Control). After 24 h, cells were infected with HCMV. At 6 h.p.i., qPCR was performed to measure the transcription of UL37x1 (c). At 12 h.p.i., cell lysates were subject to immunoblots with indicated antibodies (d). Immunoblots are representative of three independent experiments. Graphs show mean ± SD (n = 3, biologically independent experiments). Statistical significance was determined by two-way ANOVA in a, or one-way ANOVA in c.
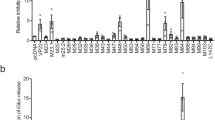
Extended Data Fig. 4 Identifying the key residues for UL37x1 inhibiting cGAS-STING axis.
(a-b) Upper, the schematic illustration of the UL37x1 truncations. Middle, 293 T cells were transfected with the plasmid of FLAG-cGAS plus FLAG-STING together with the plasmid of FLAG-UL37x1 or indicated truncation for 24 h, followed by qPCR analysis of IFNB1. The lower blots showed the expression levels of the indicated proteins. (c) 293 T cells were transfected with the plasmid of FLAG-UL37x1, FLAG-UL37x1△40-44, or FLAG-UL37x1K40R together with the plasmid of FLAG-TBK1, FLAG-IRF3-5D, or an empty vector. At 24 h.p.t., qPCR was performed to measure the transcription of indicated antiviral genes. (d) 293 T cells were transfected with the plasmid of HA-TBK1 together with the plasmid of FLAG-UL37x1 or FLAG-UL37x1△40-44. At 24 h.p.t., cell lysates were subject to immunoprecipitation with anti-FLAG antibody or IgG, followed by immunoblots with indicated antibodies. (e) HFFs stably expressing UL37x1, UL37x1K40R, or empty vector were transfected with HA-MAVS, Myc-TBK1. At 24 h.p.i., cell lysates were subject to immunoprecipitation with anti-HA antibody or IgG, followed by immunoblots with indicated antibodies. Immunoblots are representative of three independent experiments. (f) Luciferase reporter assay analyzing IFNβ, ISRE, or NF-κB promoter activity was conducted in 293 T cells transfected with or without the plasmid of FLAG-RIG-I CARD together with the plasmid of FLAG-UL37x1 or FLAG-UL37x1K40R for 24 h. (g) 293 T cells were transfected with or without the plasmid of FLAG-RIG-I CARD together with the plasmids of FLAG-UL37x1 or FLAG-UL37x1K40R for 24 h. Total RNAs were extracted and subject to qPCR of the indicated antiviral genes. Graphs show mean ± SD (n = 3, biologically independent experiments). Statistical significance was determined by two-way ANOVA.
Extended Data Fig. 5 The effects of UL37x1 on immune signaling and apoptosis.
(a) HFFs stably expressing UL37x1 or empty vector were infected with HCMV (MOI = 1). At 0, 6, and 12 h.p.i., qPCR was performed to measure the transcription of indicated antiviral genes. (b) HFFs stably expressing UL37x1 or empty vector were uninfected (mock) or infected with HCMV in the absence or presence of z-VAD-FMK. At 12 h.p.i., cells were collected and stained with Annexin V-FITC/PI for flow cytometry analysis (left, early apoptosis: Annexin V-FITC single positive, Q3; late apoptosis: Annexin V-FITC and PI double positive, Q2), and the percentage of apoptotic cells (Q2 + Q3) was measured (right). This gating strategy applies to all flow cytometry analyses in this study. (c) 293 T cells were transfected with the plasmid of FLAG-cGAS plus FLAG-STING or an empty vector together with or without the plasmid of FLAG-UL37x1. After 24 h, cells were collected and stained with Annexin V-FITC/PI for flow cytometry analysis, and the percentage of apoptotic cells was measured. (d) HFFs were transfected with HSV60, DNA90, or HSV120. After 12 h, cells were collected and stained with Annexin V-FITC/ PI for flow cytometry analysis, and the percentage of apoptotic cells was measured. (e-f) HFFs were transfected with UL37x1-siRNA#1, UL37x1-siRNA#2, UL37x1-siRNA#3 or control siRNA for 24 h, followed by infection with HCMV (MOI = 1). At 12 h.p.i., the mRNA levels of the indicated HCMV genes were measured by qPCR (e) and the protein level of HCMV-expressed reporter GFP was measured by immunoblot (f). (g) HFFs were transfected as in (b) for 24 h, followed by infection with HCMVTowne (MOI = 1). At 12 h.p.i., cell lysate was subject to immunoblot with anti-UL37x1 and anti-β-Actin. (h) HFFs were transfected with UL37x1-siRNA#1, UL37x1-siRNA#3 or control siRNA and infected with HCMVTowne (MOI = 1) in the absence or presence of z-VAD-FMK. At 12 h.p.i., qPCR was performed to measure the transcription of indicated antiviral genes. Immunoblots are representative of three independent experiments. Graphs show mean ± SD (n = 3 for a, e, and h, or n = 2, for b, c, and d biologically independent experiments). Statistical significance was determined by two-way ANOVA in a, b, c, d, and h, or one-way ANOVA in e.
Extended Data Fig. 6 The immunosuppressive and anti-apoptotic activities of UL37x1 are functionally separated.
(a) 293 T cells were transfected with the plasmid of FLAG-UL37x1 or FLAG-UL37x1△2-23. At 24 h.p.t., cells were stained with Mito Tracker Red (mitochondria staining reagent) at 37 °C. After 15 min, cells were fixed and stained with anti-FLAG and Alexa-488 conjugated anti-mouse IgG antibodies, followed by immunofluorescence microscopy. Scale bar, 10 μM. (b) 293 T cells were transfected with the plasmid of FLAG-UL37x1 or FLAG-UL37x1△2-23. At 24 h.p.t., cells were collected and the mitochondria was separated via gradient centrifugation. The indicated cell lysate fractions were subject to immunoblots with anti-FLAG, anti-β-Actin, and anti-GDH (glutamate dehydrogenase, used to mark mitochondria) antibodies. Immunoblots are representative of three independent experiments. (c) HFFs stably expressing UL37x1, UL37x1△2-23, or empty vector were uninfected or infected with HCMV (MOI = 1). At 12 h.p.i., cells were collected and stained with Annexin V-FITC/ PI for flow cytometry analysis, and the percentage of apoptotic cells was measured. (d) HFFs stably expressing FLAG-UL37x1, FLAG-UL37x1△40-44, FLAG-UL37x1K40R, or empty vector were uninfected or infected with HCMV (MOI = 1). At 12 h.p.i., cells were collected and stained with Annexin V-FITC/ PI for flow cytometry analysis, and the percentage of apoptotic cells was measured. Graphs show mean ± SD (n = 2, biologically independent experiments). Statistical significance was determined by two-way ANOVA.
Extended Data Fig. 7 A scheme for homologous recombination mediated genome editing of the UL37x1 gene locus.
(a, c and e) A scheme for homologous recombination mediated genome editing of the UL37x1 gene locus. (b, d and f) DNA sequence and reading frame of wild-type and edited UL37x1 alleles. (g) HFFs were infected with HCMVWT, HCMV△UL37x1, HCMV△2-23, or HCMVK40R. At 12 h.p.i., cell lysates were subject to immunoblots with anti-UL37x1 and anti-UL36 antibodies. Immunoblots are representative of two independent experiments.
Extended Data Fig. 8 Endogenous UL37x1 inhibits innate immunity and apoptosis.
(a) HFFs were infected with HCMVWT, HCMV△UL37x1, HCMV△2-23, or HCMVK40R (MOI = 1) in the presence of z-VAD-FMK. At 12 h.p.i., cells were collected and stained with Annexin V-FITC/PI for flow cytometry analysis, and the percentage of apoptotic cells was measured. (b-c) HFFs were infected with HCMVWT, HCMV△UL37x1, HCMV△2-23, or HCMVK40R (MOI = 1) in the presence of z-VAD-FMK. At 0, 6, and 12 h.p.i., total RNA were extracted and cell lysates were prepared, followed by qPCR to measure the transcription of indicated antiviral genes (b), and immunoprecipitation with anti-STING antibody followed by immunoblots with indicated antibodies (c), respectively. Immunoblots are representative of three independent experiments. (d-e) HFFs stably expressing UL37x1, UL37x1△2-23, UL37x1K40R, or empty vector were infected with HCMV△UL37x1 (MOI = 1). At 96 h.p.i., GFP signals were analyzed by fluorescence microscopy (d, left). The percentage of the infected cells (GFP positive) were measured and shown as the graph (d, right). The mRNA levels of the indicated HCMV genes in HFFs were measured by qPCR at 96 h.p.i (e). (f-g) STING-/- or cGAS-/- HFFs were infected with HCMVWT, HCMV△UL37x1, HCMV△2-23, or HCMVK40R. At 96 h.p.i., the mRNA levels of the indicated HCMV genes in HFFs were measured by qPCR. (h) HFFs were infected with HCMVWT, HCMV△UL37x1, HCMV△2-23, or HCMVK40R in the presence of z-VAD-FMK. At 96 h.p.i., the mRNA levels of the indicated HCMV genes in HFFs were measured by qPCR. Graphs show mean ± SD (n = 2 for a, or n = 3 for b and e-h, biologically independent experiments). Statistical significance was determined by one-way ANOVA in a and e-h, or two-way ANOVA in b.
Extended Data Fig. 9 Humanized mouse model for HCMV infection.
(a) The experimental workflow of in vivo HCMV infection experiment in humanized mice: NOD-scid IL2RGγcnull mice were engrafted with human CD34+ HPCs and then transfused with NHDFs infected with HCMVWT, HCMV△UL37x1, HCMV△2-23 or HCMVK40R (n = 6/group). Uninfected mice served as the negative control group (n = 6). At 28 days post infection, in each group, three mice were euthanized for tissue harvesting and three mice were treated with G-CSF and AMD-3100 for another 10 days and then euthanized for tissue harvesting and analysis. (b-c) NHDF cells were infected with wild-type or mutant HCMV. At 12 h.p.i., the mRNA levels of the indicated HCMV genes in NHDFs were measured by qPCR (b) and the protein level of HCMV-expressed reporter GFP was measured by immunoblot (c). Immunoblots are representative of three independent experiments. Graphs in b show mean ± SD (n = 3, biologically independent experiments). Statistical significance was determined by one-way ANOVA.
Extended Data Fig. 10 Model for the dual inhibition of innate immunity and apoptosis by HCMV UL37x1.
Left: Apoptotic caspases cleave key components of the cGAS-STING axis to inhibit innate immune signaling. Right: During HCMV infection, UL37x1 inhibits apoptosis, which enhances cGAS-STING immune signaling. On the other hand, UL37x1 can directly target to TBK1 to disrupt TBK1 interaction with STING as well as IRF3 recruitment to STING, thereby suppressing the cGAS-STING pathway. This immunosuppressing activity of UL37x1 counterbalances the immune enhancing “side-effect” of UL37x1’s anti-apoptotic activity.
Supplementary information
Source data
Source Data Fig. 1
Unprocessed western blots.
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Unprocessed western blots.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Unprocessed western blots.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Unprocessed western blots.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Unprocessed western blots.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 2
Unprocessed western blots.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Unprocessed western blots.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Unprocessed western blots.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Unprocessed western blots.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Unprocessed western blots.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Unprocessed western blots.
Source Data Extended Data Fig. 8
Unprocessed western blots.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Unprocessed western blots.
Source Data Extended Data Fig. 9
Statistical source data.
Rights and permissions
About this article
Cite this article
Ren, Y., Wang, A., Wu, D. et al. Dual inhibition of innate immunity and apoptosis by human cytomegalovirus protein UL37x1 enables efficient virus replication. Nat Microbiol 7, 1041–1053 (2022). https://doi.org/10.1038/s41564-022-01136-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-022-01136-6
This article is cited by
-
DNA damage response(DDR): a link between cellular senescence and human cytomegalovirus
Virology Journal (2023)
-
Mitochondrial DNA-triggered innate immune response: mechanisms and diseases
Cellular & Molecular Immunology (2023)
-
SARS-CoV-2 N protein enhances the anti-apoptotic activity of MCL-1 to promote viral replication
Signal Transduction and Targeted Therapy (2023)
-
Restructured membrane contacts rewire organelles for human cytomegalovirus infection
Nature Communications (2022)