Abstract
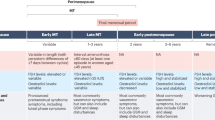
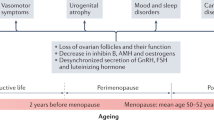
Every woman who lives past midlife will experience menopause, which, by definition, is complete cessation of ovarian function. This process might occur spontaneously (natural menopause) or be iatrogenic (secondary menopause), and can be further classified as ‘early’ if it occurs before the age of 45 years and ‘premature’ if it occurs before the age of 40 years. Globally, the mean age of natural menopause is 48.8 years, with remarkably little geographic variation. A woman’s age at menopause influences health outcomes in later life. Early menopause is associated with a reduced risk of breast cancer, but increased risks of premature osteoporosis, cardiovascular disease and premature death. The cardinal symptoms of menopause, and adverse health sequelae, are due to loss of ovarian oestrogen production. Consequently, menopausal hormone therapy (MHT) that includes oestrogen or an oestrogenic compound ameliorates menopausal symptoms, while preventing menopause-associated bone loss and cardiometabolic changes. Importantly, comprehensive care of postmenopausal women involves lifestyle optimization (attention to nutrition and physical activity, reducing alcohol consumption and not smoking) and treating other established chronic disease risk factors. This Review offers a commentary specifically on the contemporary use of MHT and novel pharmaceutical alternatives to manage menopausal symptoms.
Key points
-
Most women experience menopause between the ages of 45 and 55 years, and 75% will experience oestrogen deficiency symptoms.
-
Menopausal hormone therapy (MHT), including tibolone, remains the most effective treatment for menopausal symptoms.
-
MHT considerably lowers the risk of hip, vertebral and other osteoporosis-related fracture in women with normal bone density, osteopenia and osteoporosis; fracture prevention is a primary indication for its use.
-
Urogenital atrophy symptoms are common and should be treated, with several effective hormonal and non-hormonal treatments available.
-
Several novel therapies for the treatment of menopausal symptoms are in development; however, the new non-hormonal therapies are specific for vasomotor symptoms and, unlike oestrogen therapy, will not prevent bone loss or cardiometabolic disease risk.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Harlow, S. D. et al. Executive summary of the Stages of Reproductive Aging Workshop +10: addressing the unfinished agenda of staging reproductive aging. Climacteric 15, 105–114 (2012). This is, internationally, considered the primary reference for the physiology of the menopause transition.
Davis, S. R. et al. Menopause. Nat. Rev. Dis. Primers 1, 15004 (2015).
Worsley, R., Bell, R. J., Gartoulla, P. & Davis, S. R. Low use of effective and safe therapies for moderate to severe menopausal symptoms: a cross-sectional community study of Australian women. Menopause 23, 11–17 (2015).
Sneader, W. Drug Discovery: The Evolution of Modern Medicines (Wiley, 1989).
Doisy, E. A., Veler, C. & Thayer, S. The preparation of the crystalline ovarian hormone from the urine of pregnant women. J. Biol. Chem. 86, 499–509 (1930).
Medvei, V. C. The History of Clinical Endocrinology: A Comprehensive Account of Endocrinology from Earliest Times to Present Day (Parthenon, 1993).
Schmidt-Gollwitzer, K. Estrogen/hormone replacement therapy present and past. Gynecol. Endocrinol. 15 (Suppl. 4), 11–16 (2001).
Speroff, L., Glass, R. J. & Kase, N. G. Clinical Gynecological Endocrinology and Infertility (Lippincott, Williams and Wilkins, 1999).
Allen, W. & Wintersteiner, O. Crystalline progestin. Science 80, 190–193 (1934).
Butenandt, A. & Westphal, U. Zur isolierung und charakterisierung des corpus-luteum-hormons. Ber. Dtsch. Chem. Ges. 67, 1440–1442 (1934).
Hartmann, M. & Wettstein, A. Ein krystallisiertes Hormon aus Corpus-luteum. Helv. Chim. Acta 17, 878–882 (1934).
Slotta, K., Ruschig, H. & Fels, E. Reindarstellung der Hormone aus dem Corpus-luteum. Ber. Dtsch. Chem. Ges. 67, 1270–1273 (1934).
Goldzieher, W. in Menopause: Biology and Pathobiology (eds Lobo, R., Kesley, J. & Marcus, R.) 397–404 (Academic, 2000).
Mack, T. M. et al. Estrogens and endometrial cancer in a retirement community. N. Engl. J. Med. 294, 1262–1267 (1976).
Ziel, H. K. & Finkle, W. D. Increased risk of endometrial carcinoma among users of conjugated estrogens. N. Engl. J. Med. 293, 1167–1170 (1975).
Smith, D., Prentice, R., Thompson, D. & Herrmann, W. Association of exogenous estrogen and endometrial carcinoma. N. Engl. J. Med. 293, 1164–1167 (1975).
Whitehead, M., Townsend, P. T., Pryse-Davies, J., Ryder, T. A. & King, R. J. Effects of estrogens and progestins on the biochemistry and morphology of the postmenopausal endometrium. N. Engl. J. Med. 305, 1599–1605 (1981).
Rossouw, J. et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative Randomised Controlled Trial. JAMA 288, 321–333 (2002).
Baber, R. J. et al. 2016 IMS recommendations on women’s midlife health and menopause hormone therapy. Climacteric 19, 109–150 (2016). This document summarizes current internationally recognised guidelines for management of menopause and menopausal hormone therapy use.
Gartoulla, P., Worsley, R., Bell, R. J. & Davis, S. R. Moderate to severe vasomotor and sexual symptoms remain problematic for women aged 60 to 65 years. Menopause 22, 694–701 (2015). This paper provides clarity around the prevalence and duration of menopausal VMS, and risk factors for VMS in an unselected sample.
Islam, M. R., Gartoulla, P., Bell, R. J., Fradkin, P. & Davis, S. R. Prevalence of menopausal symptoms in Asian midlife women: a systematic review. Climacteric 18, 157–176 (2015).
Avis, N. E. et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern. Med. 175, 531–539 (2015).
Duffy, O. K., Iversen, L., Aucott, L. & Hannaford, P. C. Factors associated with resilience or vulnerability to hot flushes and night sweats during the menopausal transition. Menopause 20, 383–392 (2013).
Islam, R. M., Bell, R. J., Billah, B., Hossain, M. B. & Davis, S. R. Prevalence and severity of vasomotor symptoms and joint pain in women at midlife in Bangladesh: a population-based survey. Menopause 23, 731–739 (2016).
Gartoulla, P., Bell, R. J., Worsley, R. & Davis, S. R. Moderate-severely bothersome vasomotor symptoms are associated with lowered psychological general wellbeing in women at midlife. Maturitas 81, 487–492 (2015).
Gartoulla, P., Bell, R. J., Worsley, R. & Davis, S. R. Menopausal vasomotor symptoms are associated with poor self-assessed work ability. Maturitas 87, 33–39 (2016). One of the few studies that have evaluated the impact of VMS on work ability in a large, unselected sample of women.
Geukes, M., van Aalst, M. P., Nauta, M. C. & Oosterhof, H. The impact of menopausal symptoms on work ability. Menopause 19, 278–282 (2012).
Zervas, I. M. et al. Additive effect of depressed mood and vasomotor symptoms on postmenopausal insomnia. Menopause 16, 837–842 (2009).
Brunner, R. L. et al. Effects of conjugated equine estrogen on health-related quality of life in postmenopausal women with hysterectomy: results from the Women’s Health Initiative Randomized Clinical Trial. Arch. Intern. Med. 165, 1976–1986 (2005).
Worsley, R., Bell, R. J., Gartoulla, P., Robinson, P. J. & Davis, S. R. Moderate-severe vasomotor symptoms are associated with moderate-severe depressive symptoms. J. Womens Health 26, 712–718 (2017).
Freeman, E. W. et al. The role of anxiety and hormonal changes in menopausal hot flashes. Menopause 12, 258–266 (2005).
Sturdee, D. W. & Panay, N. Recommendations for the management of postmenopausal vaginal atrophy. Climacteric 13, 509–522 (2010).
Bachmann, G. A. & Nevadunsky, N. S. Diagnosis and treatment of atrophic vaginitis. Am. Fam. Phys. 61, 3090–3096 (2000).
North American Menopause Society. The role of local vaginal estrogen for treatment of vaginal atrophy in postmenopausal women: position statement of the North American Menopause Society. Menopause 14, 357–369 (2007).
Barlow, D. H. et al. Urogenital ageing and its effect on sexual health in older British women. Br. J. Obstet. Gynaecol. 104, 87–91 (1997).
Pastore, L. M., Carter, R. A., Hulka, B. S. & Wells, E. Self-reported urogenital symptoms in postmenopausal women: Women’s Health Initiative. Maturitas 49, 292–303 (2004).
Weber, M. T., Rubin, L. H., Schroeder, R., Steffenella, T. & Maki, P. M. Cognitive profiles in perimenopause: hormonal and menopausal symptom correlates. Climacteric 24, 401–407 (2021).
Weber, M. T., Mapstone, M., Staskiewicz, J. & Maki, P. M. Reconciling subjective memory complaints with objective memory performance in the menopausal transition. Menopause 19, 735–741 (2012).
Weber, M. T., Maki, P. M. & McDermott, M. P. Cognition and mood in perimenopause: a systematic review and meta-analysis. J. Steroid Biochem. Mol. Biol. 142, 90–98 (2014).
Sternfeld, B. et al. Physical activity and changes in weight and waist circumference in midlife women: findings from the Study of Women’s Health Across the Nation. Am. J. Epidemiol. 160, 912–922 (2004).
Davis, S. R. et al. Understanding weight gain at menopause. Climacteric 15, 419–429 (2012). This white paper of the International Menopause Society provides a comprehensive review of this issue.
Abdulnour, J. et al. The effect of the menopausal transition on body composition and cardiometabolic risk factors: a Montreal-Ottawa New Emerging Team group study. Menopause 19, 760–767 (2012).
Ho, S. C., Wu, S., Chan, S. G. & Sham, A. Menopausal transition and changes of body composition: a prospective study in Chinese perimenopausal women. Int. J. Obes. 34, 1265–1274 (2010).
Sowers, M. et al. Changes in body composition in women over six years at midlife: ovarian and chronological aging. J. Clin. Endocrinol. Metab. 92, 895–901 (2007).
Nakamura, T. et al. Estrogen prevents bone loss via estrogen receptor α and induction of Fas ligand in osteoclasts. Cell 130, 811–823 (2007).
Khosla, S. & Monroe, D. G. Regulation of bone metabolism by sex steroids. Cold Spring Harb. Perspect. Med. 8, a031211 (2018).
Charatcharoenwitthaya, N., Khosla, S., Atkinson, E. J., McCready, L. K. & Riggs, B. L. Effect of blockade of TNF-α and interleukin-1 action on bone resorption in early postmenopausal women. J. Bone Miner. Res. 22, 724–729 (2007).
Sowers, M. R. et al. Amount of bone loss in relation to time around the final menstrual period and follicle-stimulating hormone staging of the transmenopause. J. Clin. Endocrinol. Metab. 95, 2155–2162 (2010).
de Villiers, T. J. & Stevenson, J. C. The WHI: the effect of hormone replacement therapy on fracture prevention. Climacteric 15, 263–266 (2012).
National Institute for Health and Care Excellence. Menopause: diagnosis and management (NICE, 2015).
Zhu, L., Jiang, X., Sun, Y. & Shu, W. Effect of hormone therapy on the risk of bone fractures: a systematic review and meta-analysis of randomized controlled trials. Menopause 23, 461–470 (2016).
Eastell, R. et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society* Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 104, 1595–1622 (2019). This article provides an excellent evidence-based summary of management of postmenopausal osteoporosis.
Mandrup, C. M. et al. Effects of menopause and high-intensity training on insulin sensitivity and muscle metabolism. Menopause 25, 165–175 (2018).
Leeners, B., Geary, N., Tobler, P. N. & Asarian, L. Ovarian hormones and obesity. Hum. Reprod. Update 23, 300–321 (2017).
Krause, W. C. et al. Oestrogen engages brain MC4R signalling to drive physical activity in female mice. Nature 599, 131–135 (2021).
Hevener, A. L., Zhou, Z., Moore, T. M., Drew, B. G. & Ribas, V. The impact of ERα action on muscle metabolism and insulin sensitivity–strong enough for a man, made for a woman. Mol. Metab. 15, 20–34 (2018).
Zhu, L. et al. Estrogens prevent metabolic dysfunctions induced by circadian disruptions in female mice. Endocrinology 156, 2114–2123 (2015).
Darling, G. M., Johns, J. A., McCloud, P. I. & Davis, S. R. Estrogen and progestin compared with simvastatin for hypercholesterolemia in postmenopausal women. N. Engl. J. Med. 337, 595–601 (1997).
Leiberman, E. H. et al. Estrogen improves endothelium-dependent, flow-mediated vasodilation in postmenopausal women. Ann. Intern. Med. 121, 936–941 (1994).
Hulley, S. et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA 280, 605–613 (1998).
Salpeter, S. R. et al. Meta-analysis: effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes. Metab. 8, 538–554 (2006).
Vinogradova, Y., Coupland, C. & Hippisley-Cox, J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ 364, k4810 (2019).
Margolis, K. L. et al. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women’s Health Initiative Hormone Trial. Diabetologia 47, 1175–1187 (2004).
Xu, B. et al. Estrogens promote misfolded proinsulin degradation to protect insulin production and delay diabetes. Cell Rep. 24, 181–196 (2018).
Pereira, R. I. et al. Timing of estradiol treatment after menopause may determine benefit or harm to insulin action. J. Clin. Endocrinol. Metab. 100, 4456–4462 (2015).
Herrington, D. et al. Effects of estrogen replacement on the progression of coronary-artery atherosclerosis. N. Engl. J. Med. 343, 522–529 (2000).
Clarkson, T. B. Estrogen effects on arteries vary with stage of reproductive life and extent of subclinical atherosclerosis progression. Menopause 14, 373–384 (2007).
Williams, J. K. et al. Regression of atherosclerosis in female monkeys. Arterioscler. Thromb. Vasc. Biol. 15, 827–836 (1995).
Stampfer, M. J. & Colditz, G. A. Estrogen replacement and coronary heart disease: a quantitative assessment of the epidemiologic evidence. Prev. Med. 20, 47–63 (1991).
Grodstein, F. et al. Postmenopausal hormone therapy and mortality. N. Engl. J. Med. 336, 1769–1775 (1997).
Boardman, H. M. et al. Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst. Rev. 3, CD002229 (2015).
Marjoribanks, J., Farquhar, C., Roberts, H., Lethaby, A. & Lee, J. Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst. Rev. 1, CD004143 (2017).
Miller, V. M. et al. The Kronos Early Estrogen Prevention Study (KEEPS): what have we learned? Menopause 26, 1071–1084 (2019).
Hodis, H. N. et al. Vascular effects of early versus late postmenopausal treatment with estradiol. N. Engl. J. Med. 374, 1221–1231 (2016).
Henderson, V. W. Cognitive changes after menopause: influence of estrogen. Clin. Obstet. Gynecol. 51, 618–626 (2008).
Rocca, W. A. et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology 69, 1074–1083 (2007).
Kim, Y. J. & Brinton, R. D. Precision hormone therapy: identification of positive responders. Climacteric 24, 350–358 (2021).
Yaffe, K., Sawaya, G., Leiderburg, I. & Grady, D. Estrogen therapy in postmenopausal women: effects on cognitive function and dementia. JAMA 279, 688 (1998).
Shumaker, S. A. et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women. The Women’s Health Initiative Memory Study: a randomised controlled trial. JAMA 289, 2651–2662 (2003).
Espeland, M. A. et al. Long-term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50 to 55 years. JAMA Intern. Med. 173, 1429–1436 (2013).
Gleason, C. E. et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS-cognitive and affective study. PLoS Med. 12, e1001833 (2015).
Henderson, V. W. et al. Cognitive effects of estradiol after menopause: a randomized trial of the timing hypothesis. Neurology 87, 699–708 (2016).
Rocca, W. A. et al. Association of premenopausal bilateral oophorectomy with cognitive performance and risk of mild cognitive impairment. JAMA Netw. Open 4, e2131448 (2021).
Maki, P. M. et al. Guidelines for the evaluation and treatment of perimenopausal depression: summary and recommendations. Menopause 25, 1069–1085 (2018). This is an important summary document of perimenopausal depression and management.
Nanda, K., Bastian, L. A., Hasselblad, V. & Simel, D. L. Hormone replacement therapy and the risk of colorectal cancer: a meta-analysis. Obstet. Gynecol. 93, 880–888 (1999).
Hebert-Croteau, N. A meta-analysis of hormone replacement therapy and colon cancer in women. Cancer Epidemiol. Biomark. Prev. 7, 653–659 (1998).
Grodstein, F., Newcomb, P. A. & Stampfer, M. J. Postmenopausal hormone therapy and the risk of colorectal cancer: a review and meta-analysis. Am. J. Med. 106, 574–582 (1999).
Golezar, S., Ramezani Tehrani, F., Khazaei, S., Ebadi, A. & Keshavarz, Z. The global prevalence of primary ovarian insufficiency and early menopause: a meta-analysis. Climacteric 22, 403–411 (2019).
Shuster, L. T., Rhodes, D. J., Gostout, B. S., Grossardt, B. R. & Rocca, W. A. Premature menopause or early menopause: long-term health consequences. Maturitas 65, 161–166 (2010).
Muka, T. et al. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality: a systematic review and meta-analysis. JAMA Cardiol. 1, 767–776 (2016).
Anagnostis, P. et al. Early menopause and premature ovarian insufficiency are associated with increased risk of type 2 diabetes: a systematic review and meta-analysis. Eur. J. Endocrinol. 180, 41–50 (2019).
European Society of Human Reproduction and Embryology. Guideline on the management of premature ovarian insufficiency (ESHRE, 2015). This is an important document for clinicians treating women with premature ovarian insufficiency.
Lambrinoudaki, I. et al. Premature ovarian insufficiency: a toolkit for the primary care physician. Climacteric 24, 425–437 (2021).
Huber, D., Seitz, S., Kast, K., Emons, G. & Ortmann, O. Hormone replacement therapy in BRCA mutation carriers and risk of ovarian, endometrial, and breast cancer: a systematic review. J. Cancer Res. Clin. Oncol. 147, 2035–2045 (2021).
Judd, H. L. et al. Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA 275, 370–375 (1996).
Simpson, E. R. & Davis, S. R. Minireview: aromatase and the regulation of estrogen biosynthesis–some new perspectives. Endocrinology 142, 4589–4594 (2001).
Lobo, R. Absorption and metabolic effects of different types of estrogens and progestogens. Obstet. Gynecol. Clin. North Am. 14, 143–167 (1987).
Slater, C. et al. Markedly elevated levels of estrone sulfate after long term oral, but not transdermal, administration of estradiol in postmenopausal women. Menopause 8, 200–203 (2001).
Kuhl, H. Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric 8, 3–63 (2005).
Wheatley, S., Bell, R. J., Stuckey, B. G., Robinson, P. J. & Davis, S. R. Clinical audit of estradiol implant therapy: long duration of action and implications in non-hysterectomised women. Maturitas 94, 84–86 (2016).
Liu, J. H. The role of progestogens in menopausal hormone therapy. Clin. Obstet. Gynecol. 64, 772–783 (2021).
Tempfer, C. B., Hilal, Z., Kern, P., Juhasz-Boess, I. & Rezniczek, G. A. Menopausal hormone therapy and risk of endometrial cancer: a systematic review. Cancers 12, 2195 (2020).
Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet 394, 1159–1168 (2019).
Vinogradova, Y., Coupland, C. & Hippisley-Cox, J. Use of hormone replacement therapy and risk of breast cancer: nested case-control studies using the QResearch and CPRD databases. BMJ 371, m3873 (2020).
Lyytinen, H. & Ylikorkala, O. Hormone therapy and risk for breast cancer in Finnish postmenopausal women [Finnish]. Duodecim 127, 235–242 (2011).
Fournier, A. et al. Use of different postmenopausal hormone therapies and risk of histology- and hormone receptor-defined invasive breast cancer. J. Clin. Oncol. 26, 1260–1268 (2008).
Anderson, G. L. et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA 291, 1701–1712 (2004).
Chlebowski, R. T. et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA 324, 369–380 (2020).
Depypere, H. & Inki, P. The levonorgestrel-releasing intrauterine system for endometrial protection during estrogen replacement therapy: a clinical review. Climacteric 18, 470–482 (2015).
Lundstrom, E., Virijevic, I. & Soderqvist, G. Progestogen addition with low-dose levonorgestrel intrauterine system in menopausal hormone treatment gives less normal breast tissue proliferation than oral norethisterone acetate or medroxyprogesterone acetate. Horm. Mol. Biol. Clin. Investig. https://doi.org/10.1515/hmbci-2019-0051 (2020).
Palacios, S., Colli, E. & Regidor, P. A. Efficacy and cardiovascular safety of the new estrogen-free contraceptive pill containing 4 mg drospirenone alone in a 24/4 regime. BMC Womens Health 20, 218 (2020).
Palacios, S. et al. Oestrogen-free oral contraception with a 4 mg drospirenone-only pill: new data and a review of the literature. Eur. J. Contracept. Reprod. Health Care 25, 221–227 (2020).
Kloosterboer, H. Tibolone: a steroid with tissue-specific mode of action. J. Steroid Biochem. Mol. Biol. 76, 231–238 (2001).
Markiewicz, L. & Gurpide, E. In vitro evaluation of estrogenic, estrogen antagonistic and progestagenic effects of a steroidal drug (Org OD-14) and its metabolites on human endometrium. J. Steroid Biochem. 35, 535–541 (1990).
Nijland, E. A. et al. Tibolone and transdermal E2/NETA for the treatment of female sexual dysfunction in naturally menopausal women: results of a randomized active-controlled trial. J. Sex. Med. 5, 646–656 (2008).
Archer, D. F. et al. Endometrial effects of tibolone. J. Clin. Endocrinol. Metab. 92, 911–918 (2007).
Cummings, S. R. et al. The effects of tibolone in older postmenopausal women. N. Engl. J. Med. 359, 697–708 (2008).
Renoux, C., Dell’Aniello, S. & Suissa, S. Hormone replacement therapy and the risk of venous thromboembolism: a population-based study. J. Thromb. Haemost. 8, 979–986 (2010).
Formoso, G. et al. Short-term and long-term effects of tibolone in postmenopausal women. Cochrane Database Syst. Rev. 10, CD008536 (2016).
Lv, C. et al. The effect of tibolone treatment on lipid profile in women: a systematic review and dose-response meta-analysis of randomized controlled trials. Pharmacol. Res. 169, 105612 (2021).
Egarter, C. et al. Tibolone versus conjugated estrogens and sequential progestogen in the treatment of climacteric complaints. Maturitas 23, 55–62 (1996).
Delmas, P. D. et al. Effects of tibolone and raloxifene on bone mineral density in osteopenic postmenopausal women. Osteoporos. Int. 19, 1153–1160 (2008).
Pickar, J. H., Boucher, M. & Morgenstern, D. Tissue selective estrogen complex (TSEC): a review. Menopause 25, 1033–1045 (2018).
Pinkerton, J. V. et al. Evaluation of the efficacy and safety of bazedoxifene/conjugated estrogens for secondary outcomes including vasomotor symptoms in postmenopausal women by years since menopause in the selective estrogens, menopause and response to therapy (SMART) trials. J. Womens Health 23, 18–28 (2014).
Pinkerton, J. V. Tissue-selective estrogen complex for menopausal hormone therapy. Clin. Obstet. Gynecol. 61, 463–469 (2018).
Yue, W. et al. Effect of a tissue selective estrogen complex on breast cancer: role of unique properties of conjugated equine estrogen. Int. J. Cancer 143, 1259–1268 (2018).
Pinkerton, J. V., Komm, B. S. & Mirkin, S. Tissue selective estrogen complex combinations with bazedoxifene/conjugated estrogens as a model. Climacteric 16, 618–628 (2013).
Kingsberg, S. A., Krychman, M., Graham, S., Bernick, B. & Mirkin, S. The Women’s EMPOWER survey: identifying women’s perceptions on vulvar and vaginal atrophy and its treatment. J. Sex. Med. 14, 413–424 (2017).
Herbert, D. et al. Australian women’s understanding of menopause and its consequences: a qualitative study. Climacteric 23, 622–628 (2020).
Santen, R. J. Vaginal administration of estradiol: effects of dose, preparation and timing on plasma estradiol levels. Climacteric 118, 121–134 (2014).
Raz, R. & Stamm, W. E. A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract Infections. N. Engl. J. Med. 329, 753–756 (1993).
Lethaby, A., Ayeleke, R. O. & Roberts, H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst. Rev. 8, CD001500 (2016).
Lyytinen, H., Pukkala, E. & Ylikorkala, O. Breast cancer risk in postmenopausal women using estrogen-only therapy. Obstet. Gynecol. 108, 1354–1360 (2006).
Crandall, C. J. et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women’s Health Initiative Observational Study. Menopause 25, 11–20 (2018).
O’Meara, E. S. et al. Hormone replacement therapy after a diagnosis of breast cancer in relation to recurrence and mortality. J. Natl Cancer Inst. 93, 754–762 (2001).
Santen, R. J. et al. Managing menopausal symptoms and associated clinical issues in breast cancer survivors. J. Clin. Endocrinol. Metab. 102, 3647–3661 (2017). A very useful guideline for clinicians caring for women with menopausal symptoms after breast cancer.
Labrie, F. et al. Intravaginal dehydroepiandrosterone (prasterone), a highly efficient treatment of dyspareunia. Climacteric 14, 282–288 (2011).
Bouchard, C. et al. Decreased efficacy of twice-weekly intravaginal dehydroepiandrosterone on vulvovaginal atrophy. Climacteric 18, 590–607 (2014).
Barton, D. L. et al. Systemic and local effects of vaginal dehydroepiandrosterone (DHEA): NCCTG N10C1 (Alliance). Support. Care Cancer 26, 1335–1343 (2018).
Kangas, L. & Unkila, M. Tissue selectivity of ospemifene: pharmacologic profile and clinical implications. Steroids 78, 1273–1280 (2013).
Goldstein, S. R. et al. Ospemifene 12-month safety and efficacy in postmenopausal women with vulvar and vaginal atrophy. Climacteric 17, 173–182 (2013).
Di Donato, V. et al. Ospemifene for the treatment of vulvar and vaginal atrophy: a meta-analysis of randomized trials. Part II: Evaluation of tolerability and safety. Maturitas 121, 93–100 (2019).
McLendon, A. N., Clinard, V. B. & Woodis, C. B. Ospemifene for the treatment of vulvovaginal atrophy and dyspareunia in postmenopausal women. Pharmacotherapy 34, 1050–1060 (2014).
Simon, J., Portman, D. & Mabey, R. G. Jr. Long-term safety of ospemifene (52-week extension) in the treatment of vulvar and vaginal atrophy in hysterectomized postmenopausal women. Maturita 77, 274–281 (2014).
Berman, J. R. et al. Correlation of androgen receptors, aromatase, and 5-α reductase in the human vagina with menopausal status. Fertil. Steril. 79, 925–931 (2003).
Baldassarre, M. et al. Androgen receptor expression in the human vagina under different physiological and treatment conditions. Int. J. Impot. Res. 25, 7–11 (2013).
Soderberg, M. W. et al. Pelvic floor sex steroid hormone receptors, distribution and expression in pre- and postmenopausal stress urinary incontinent women. Acta Obstet. Gynecol. Scand. 86, 1377–1384 (2007).
Fernandes, T., Costa-Paiva, L. H. & Pinto-Neto, A. M. Efficacy of vaginally applied estrogen, testosterone, or polyacrylic acid on sexual function in postmenopausal women: a randomized controlled trial. J. Sex. Med. 11, 1262–1270 (2014).
Witherby, S. et al. Topical testosterone for breast cancer patients with vaginal atrophy related to aromatase inhibitors: a phase I/II study. Oncologist 16, 424–431 (2011).
Raghunandan, C., Agrawal, S., Dubey, P., Choudhury, M. & Jain, A. A comparative study of the effects of local estrogen with or without local testosterone on vulvovaginal and sexual dysfunction in postmenopausal women. J. Sex. Med. 7, 1284–1290 (2010).
Melisko, M. E. et al. Vaginal testosterone cream vs estradiol vaginal ring for vaginal dryness or decreased libido in women receiving aromatase inhibitors for early-stage breast cancer: a randomized clinical trial. JAMA Oncol. 3, 313–319 (2017).
Dahir, M. & Travers-Gustafson, D. Breast cancer, aromatase inhibitor therapy, and sexual functioning: a pilot study of the effects of vaginal testosterone therapy. Sex. Med. 2, 8–15 (2014).
Davis, S. R. et al. Intravaginal testosterone improves sexual satisfaction and vaginal symptoms associated with aromatase inhibitors. J. Clin. Endocrinol. Metab. 103, 4146–4154 (2018).
Simon, J. A. et al. The role of androgens in the treatment of genitourinary syndrome of menopause (GSM): International Society for the Study of Women’s Sexual Health (ISSWSH) expert consensus panel review. Menopause 25, 837–847 (2018).
Mension, E. et al. Vaginal laser therapy for genitourinary syndrome of menopause–systematic review. Maturitas 156, 37–59 (2022).
Li, F. G. et al. Effect of fractional carbon dioxide laser vs sham treatment on symptom severity in women with postmenopausal vaginal symptoms: a randomized clinical trial. JAMA 326, 1381–1389 (2021).
Potter, N. & Panay, N. Vaginal lubricants and moisturizers: a review into use, efficacy, and safety. Climacteric 24, 19–24 (2021).
Mintziori, G. et al. EMAS position statement: non-hormonal management of menopausal vasomotor symptoms. Maturitas 81, 410–413 (2015).
McCormick, C. A., Brennan, A. & Hickey, M. Managing vasomotor symptoms effectively without hormones. Climacteric 23, 532–538 (2020).
Cantineau, R., Kremers, P., De Graeve, J., Gielen, J. E. & Lambotte, R. 15- and 16-hydroxylations of androgens and estrogens in the human fetal liver: a critical step in estetrol biosynthesis. J. Steroid Biochem. 22, 195–201 (1985).
Holinka, C. F., Diczfalusy, E. & Coelingh Bennink, H. J. Estetrol: a unique steroid in human pregnancy. J. Steroid Biochem. Mol. Biol. 110, 138–143 (2008).
Visser, M., Foidart, J. M. & Coelingh Bennink, H. J. In vitro effects of estetrol on receptor binding, drug targets and human liver cell metabolism. Climacteric 11, 64–68 (2008).
Benoit, T. et al. Estetrol, a fetal selective estrogen receptor modulator, acts on the vagina of mice through nuclear estrogen receptor α activation. Am. J. Pathol. 187, 2499–2507 (2017).
Montt-Guevara, M. M. et al. Estetrol modulates endothelial nitric oxide synthesis in human endothelial cells. Front. Endocrinol. 6, 111 (2015).
Arnal, J. F. et al. Membrane and nuclear estrogen receptor alpha actions: from tissue specificity to medical implications. Physiol. Rev. 97, 1045–1087 (2017).
Duijkers, I. et al. Effects of an oral contraceptive containing estetrol and drospirenone on ovarian function. Contraception 103, 386–393 (2021).
Hammond, G. L., Hogeveen, K. N., Visser, M. & Coelingh Bennink, H. J. Estetrol does not bind sex hormone binding globulin or increase its production by human HepG2 cells. Climacteric 11, 41–46 (2008).
Klipping, C. et al. Hemostatic effects of a novel estradiol-based oral contraceptive: an open-label, randomized, crossover study of estradiol valerate/dienogest versus ethinylestradiol/levonorgestrel. Drugs R. D. 11, 159–170 (2011).
Coelingh Bennink, H. J. T. et al. Pharmacodynamic effects of the fetal estrogen estetrol in postmenopausal women: results from a multiple-rising-dose study. Menopause 24, 677–685 (2017).
Gaspard, U. et al. A multicenter, randomized study to select the minimum effective dose of estetrol (E4) in postmenopausal women (E4Relief): part 1. Vasomotor symptoms and overall safety. Menopause 27, 848–857 (2020).
Schmidt, M. et al. Tumor suppression, dose-limiting toxicity and wellbeing with the fetal estrogen estetrol in patients with advanced breast cancer. J. Cancer Res. Clin. Oncol. 147, 1833–1842 (2021).
Skorupskaite, K., George, J. T. & Anderson, R. A. The kisspeptin-GnRH pathway in human reproductive health and disease. Hum. Reprod. Update 20, 485–500 (2014).
Morrison, A. E., Fleming, S. & Levy, M. J. A review of the pathophysiology of functional hypothalamic amenorrhoea in women subject to psychological stress, disordered eating, excessive exercise or a combination of these factors. Clin. Endocrinol. 95, 229–238 (2021).
Rometo, A. M. & Rance, N. E. Changes in prodynorphin gene expression and neuronal morphology in the hypothalamus of postmenopausal women. J. Neuroendocrinol. 20, 1376–1381 (2008).
Jayasena, C. N. et al. Neurokinin B administration induces hot flushes in women. Sci. Rep. 5, 8466 (2015).
Page, N. M. New challenges in the study of the mammalian tachykinins. Peptides 26, 1356–1368 (2005).
Rance, N. E., Dacks, P. A., Mittelman-Smith, M. A., Romanovsky, A. A. & Krajewski-Hall, S. J. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front. Neuroendocrinol. 34, 211–227 (2013).
Prague, J. K. et al. Neurokinin 3 receptor antagonism as a novel treatment for menopausal hot flushes: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 389, 1809–1820 (2017).
Fraser, G. L. et al. A phase 2b, randomized, placebo-controlled, double-blind, dose-ranging study of the neurokinin 3 receptor antagonist fezolinetant for vasomotor symptoms associated with menopause. Menopause 27, 382–392 (2020).
Astellas. Positive topline results from two phase 3 pivotal global trials of fezolinetant for the nonhormonal treatment of vasomotor symptoms in postmenopausal women. Astellas https://www.astellas.com (2021).
Schaffalitzky De Muckadell, O. B., Aggestrup, S. & Stentoft, P. Flushing and plasma substance P concentration during infusion of synthetic substance P in normal man. Scand. J. Gastroenterol. 21, 498–502 (1986).
Trower, M. et al. Effects of NT-814, a dual neurokinin 1 and 3 receptor antagonist, on vasomotor symptoms in postmenopausal women: a placebo-controlled, randomized trial. Menopause 27, 498–505 (2020).
The North American Menopause Society The 2017 hormone therapy position statement of The North American Menopause Society. Menopause 24, 728–753 (2017).
Stuenkel, C. A. et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 100, 3975–4011 (2015).
Brunner, R. L. et al. Menopausal symptom experience before and after stopping estrogen therapy in the Women’s Health Initiative randomized, placebo-controlled trial. Menopause 17, 946–954 (2010).
Mikkola, T. S. et al. Increased cardiovascular mortality risk in women discontinuing postmenopausal hormone therapy. J. Clin. Endocrinol. Metab. 100, 4588–4594 (2015).
Jane, F. M. & Davis, S. R. A practitioner’s toolkit for managing the menopause. Climacteric 17, 564–579 (2014).
Mann, E. et al. Cognitive behavioural treatment for women who have menopausal symptoms after breast cancer treatment (MENOS 1): a randomised controlled trial. Lancet Oncol. 13, 309–318 (2012).
Ee, C. et al. Acupuncture for menopausal hot flashes: a randomized trial. Ann. Intern. Med. 164, 146–154 (2016).
Loprinzi, C. L. et al. Newer antidepressants and gabapentin for hot flashes: an individual patient pooled analysis. J. Clin. Oncol. 27, 2831–2837 (2009).
Thacker, H. L. Assessing risks and benefits of nonhormonal treatments for vasomotor symptoms in perimenopausal and postmenopausal women. J. Womens Health 20, 1007–1016 (2011).
Shan, D. et al. Efficacy and safety of gabapentin and pregabalin in patients with vasomotor symptoms: a systematic review and meta-analysis. Am. J. Obstet. Gynecol. 222, 564–579 (2020).
Loprinzi, C. L. et al. Phase III, randomized, double-blind, placebo-controlled evaluation of pregabalin for alleviating hot flashes, N07C1. J. Clin. Oncol. 28, 641–647 (2010).
Cheema, D., Coomarasamy, A. & El-Toukhy, T. Non-hormonal therapy of post-menopausal vasomotor symptoms: a structured evidence-based review. Arch. Gynecol. Obstet. 276, 463–469 (2007).
Simon, J. A., Gaines, T. & LaGuardia, K. D. Extended-release oxybutynin therapy for vasomotor symptoms in women: a randomized clinical trial. Menopause 23, 1214–1221 (2016).
Sexton, T., Younus, J., Perera, F., Kligman, L. & Lock, M. Oxybutynin for refractory hot flashes in cancer patients. Menopause 14, 505–509 (2007).
Acknowledgements
S.R.D. is an NHMRC senior principal research fellow (grant no. 1135843).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
S.R.D. has been paid for developing and delivering educational presentations for Besins Healthcare, Abbott, Mayne Pharma and BioFemme, has been on advisory boards for Theramex, Abbott Laboratories, Mayne Pharma and Roche and a consultant to Lawley Pharmaceuticals, Southern Star Research and Que Oncology, and has received institutional grant funding from Que Oncology and Ovova Bio. R.J.B. has been paid for delivering educational presentations for Besins Healthcare, Abbott, Viatris and Pfizer Australia, and has served on medical advisory boards for Theramex, Mayne Pharma and Besins Healthcare.
Peer review
Peer review information
Nature Reviews Endocrinology thanks Haitham Hamoda and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Davis, S.R., Baber, R.J. Treating menopause — MHT and beyond. Nat Rev Endocrinol 18, 490–502 (2022). https://doi.org/10.1038/s41574-022-00685-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-022-00685-4
This article is cited by
-
Adipose-derived stem cells promote the repair of chemotherapy-induced premature ovarian failure by inhibiting granulosa cells apoptosis and senescence
Stem Cell Research & Therapy (2023)
-
Factors predicting age at menopause among Iranian women in the Bandare-Kong cohort study (a cross-sectional survey of PERSIAN cohort study)
Women's Midlife Health (2023)
-
Post-pubertal developmental trajectories of laryngeal shape and size in humans
Scientific Reports (2023)
-
Effects of alpha-adrenergic receptor blockade on coronary circulation in postmenopausal women
European Journal of Applied Physiology (2023)
-
Recognizing the importance of ovarian aging research
Nature Aging (2022)