Abstract
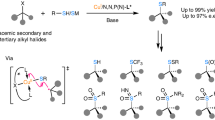
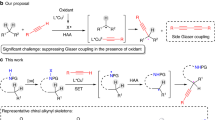
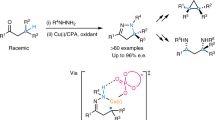
In contrast with the well-established enantioconvergent radical C(sp3)–C cross-coupling of racemic secondary alkyl electrophiles, the corresponding coupling of tertiary electrophiles to forge all-carbon quaternary stereocentres remains underexplored. The major challenge arises from the steric hindrance and the difficult enantio-differentiation of three distinct carbon substituents of prochiral tertiary radicals. Here we demonstrate a general copper-catalysed enantioconvergent C(sp3)–C(sp) cross-coupling of diverse racemic tertiary alkyl halides with terminal alkynes (87 examples). Key to the success is the rational design of chiral anionic N,N,N-ligands tailor-made for the computationally predicted outer-sphere radical group transfer pathway. This protocol provides a practical platform for the construction of chiral C(sp3)–C(sp/sp2/sp3) bonds, allowing for expedient access to an array of synthetically challenging quaternary carbon building blocks of interest in organic synthesis and related areas.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Data relating to the materials and methods, optimization studies, experimental procedures, mechanistic studies, DFT calculations, HPLC spectra, NMR spectra and mass spectrometry are available in the Supplementary Information. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition nos. CCDC 2074298 (44), 2074300 (55), 2074301 (79) and 2074302 (C1). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Johansson Seechurn, C. C. C., Kitching, M. O., Colacot, T. J. & Snieckus, V. Palladium-catalyzed cross-coupling: a historical contextual perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed. 51, 5062–5085 (2012).
de Meijere, A., Bräse, S. & Oestreich, M. (eds) Metal-Catalyzed Cross-Coupling Reactions and More (Wiley, 2014).
Biffis, A., Centomo, P., Del Zotto, A. & Zecca, M. Pd metal catalysts for cross-couplings and related reactions in the 21st century: a critical review. Chem. Rev. 118, 2249–2295 (2018).
Choi, J. & Fu, G. C. Transition metal-catalyzed alkyl-alkyl bond formation: another dimension in cross-coupling chemistry. Science 356, eaaf7230 (2017).
Fu, G. C. Transition-metal catalysis of nucleophilic substitution reactions: a radical alternative to SN1 and SN2 processes. ACS Cent. Sci. 3, 692–700 (2017).
Cherney, A. H., Kadunce, N. T. & Reisman, S. E. Enantioselective and enantiospecific transition-metal-catalyzed cross-coupling reactions of organometallic reagents to construct C-C bonds. Chem. Rev. 115, 9587–9652 (2015).
Bullock, R. M. et al. Using nature’s blueprint to expand catalysis with Earth-abundant metals. Science 369, eabc3183 (2020).
Wang, Z., Yin, H. & Fu, G. C. Catalytic enantioconvergent coupling of secondary and tertiary electrophiles with olefins. Nature 563, 379–383 (2018).
Wang, Z., Yang, Z.-P. & Fu, G. C. Quaternary stereocentres via catalytic enantioconvergent nucleophilic substitution reactions of tertiary alkyl halides. Nat. Chem. 13, 236–242 (2021).
Quasdorf, K. W. & Overman, L. E. Catalytic enantioselective synthesis of quaternary carbon stereocentres. Nature 516, 181–191 (2014).
Liu, Y., Han, S.-J., Liu, W.-B. & Stoltz, B. M. Catalytic enantioselective construction of quaternary stereocenters: assembly of key building blocks for the synthesis of biologically active molecules. Acc. Chem. Res. 48, 740–751 (2015).
Wu, L., Yang, G. & Zhang, W. Ni-catalyzed enantioconvergent coupling of epoxides with alkenylboronic acids: construction of oxindoles bearing quaternary carbons. CCS Chem. 2, 623–631 (2020).
Trost, B. M. & Jiang, C. Atom economic asymmetric creation of quaternary carbon: regio- and enantioselective reactions of a vinylepoxide with a carbon nucleophile. J. Am. Chem. Soc. 123, 12907–12908 (2001).
Zhang, P., Le, H., Kyne, R. E. & Morken, J. P. Enantioselective construction of all-carbon quaternary centers by branch-selective Pd-catalyzed allyl–allyl cross-coupling. J. Am. Chem. Soc. 133, 9716–9719 (2011).
Ma, S., Han, X., Krishnan, S., Virgil, S. C. & Stoltz, B. M. Catalytic enantioselective stereoablative alkylation of 3-halooxindoles: facile access to oxindoles with C3 all-carbon quaternary stereocenters. Angew. Chem. Int. Ed. 48, 8037–8041 (2009).
Tsuchida, K., Senda, Y., Nakajima, K. & Nishibayashi, Y. Construction of chiral tri- and tetra-arylmethanes bearing quaternary carbon centers: copper-catalyzed enantioselective propargylation of indoles with propargylic esters. Angew. Chem. Int. Ed. 55, 9728–9732 (2016).
Zhao, W., Wang, Z., Chu, B. & Sun, J. Enantioselective formation of all-carbon quaternary stereocenters from indoles and tertiary alcohols bearing a directing group. Angew. Chem. Int. Ed. 54, 1910–1913 (2015).
Li, X. et al. Catalytic enantioselective synthesis of chiral tetraarylmethanes. Nat. Catal. 3, 1010–1019 (2020).
Wendlandt, A. E., Vangal, P. & Jacobsen, E. N. Quaternary stereocentres via an enantioconvergent catalytic SN1 reaction. Nature 556, 447–451 (2018).
Zhang, X. et al. An enantioconvergent halogenophilic nucleophilic substitution (SN2X) reaction. Science 363, 400–404 (2019).
Li, J. et al. Formal enantioconvergent substitution of alkyl halides via catalytic asymmetric photoredox radical coupling. Nat. Commun. 9, 2445 (2018).
Murakata, M., Jono, T., Mizuno, Y. & Hoshino, O. Construction of chiral quaternary carbon centers by catalytic enantioselective radical-mediated allylation of α-iodolactones using allyltributyltin in the presence of a chiral Lewis acid. J. Am. Chem. Soc. 119, 11713–11714 (1997).
Evano, G. & Blanchard, N. (eds) Copper-Mediated Cross-Coupling Reactions (Wiley, 2014).
Chemler, S. R. Copper’s contribution to amination catalysis. Science 341, 624–626 (2013).
Gu, Q.-S., Li, Z.-L. & Liu, X.-Y. Copper(I)-catalyzed asymmetric reactions involving radicals. Acc. Chem. Res. 53, 170–181 (2020).
Dong, X.-Y. et al. A general asymmetric copper-catalysed Sonogashira C(sp3)–C(sp) coupling. Nat. Chem. 11, 1158–1166 (2019).
Jiang, S.-P. et al. Copper-catalyzed enantioconvergent radical Suzuki-Miyaura C(sp3)-C(sp2) cross-coupling. J. Am. Chem. Soc. 142, 19652–19659 (2020).
Su, X.-L. et al. Copper-catalyzed enantioconvergent cross-coupling of racemic alkyl bromides with azole C(sp2)–H bonds. Angew. Chem. Int. Ed. 60, 380–384 (2021).
Dong, X.-Y. et al. Copper-catalyzed asymmetric radical 1,2-carboalkynylation of alkenes with alkyl halides and terminal alkynes. J. Am. Chem. Soc. 142, 9501–9509 (2020).
Proctor, R. S. J., Colgan, A. C. & Phipps, R. J. Exploiting attractive non-covalent interactions for the enantioselective catalysis of reactions involving radical intermediates. Nat. Chem. 12, 990–1004 (2020).
Sladojevich, F., Trabocchi, A., Guarna, A. & Dixon, D. J. A new family of cinchona-derived amino phosphine precatalysts: application to the highly enantio- and diastereoselective silver-catalyzed isocyanoacetate aldol reaction. J. Am. Chem. Soc. 133, 1710–1713 (2011).
Hayashi, M., Shiomi, N., Funahashi, Y. & Nakamura, S. Cinchona alkaloid amides/dialkylzinc catalyzed enantioselective desymmetrization of aziridines with phosphites. J. Am. Chem. Soc. 134, 19366–19369 (2012).
Zhang, F.-H., Zhang, F.-J., Li, M.-L., Xie, J.-H. & Zhou, Q.-L. Enantioselective hydrogenation of dialkyl ketones. Nat. Catal. 3, 621–627 (2020).
Banik, B. K. (ed.) β-Lactams: Unique Structures of Distinction for Novel Molecules (Springer, 2013).
Decuyper, L. et al. Antibacterial and β-lactamase inhibitory activity of monocyclic β-lactams. Med. Res. Rev. 38, 426–503 (2018).
Galletti, P. & Giacomini, D. Monocyclic β-lactams: new structures for new biological activities. Curr. Med. Chem. 18, 4265–4283 (2011).
Pitts, C. R. & Lectka, T. Chemical synthesis of β-lactams: asymmetric catalysis and other recent advances. Chem. Rev. 114, 7930–7953 (2014).
Ojima, I., Zuniga, E. S. & Seitz, J. D. in β-Lactams: Unique Structures of Distinction for Novel Molecules (ed. Banik, B. K.) 1–64 (Springer, 2013).
Wu, L., Wang, F., Chen, P. & Liu, G. Enantioselective construction of quaternary all-carbon centers via copper-catalyzed arylation of tertiary carbon-centered radicals. J. Am. Chem. Soc. 141, 1887–1892 (2019).
Kainz, Q. M. et al. Asymmetric copper-catalyzed C-N cross-couplings induced by visible light. Science 351, 681–684 (2016).
Lin, C. Y., Coote, M. L., Gennaro, A. & Matyjaszewski, K. Ab initio evaluation of the thermodynamic and electrochemical properties of alkyl halides and radicals and their mechanistic implications for atom transfer radical polymerization. J. Am. Chem. Soc. 130, 12762–12774 (2008).
Isse, A. A., Bortolamei, N., De Paoli, P. & Gennaro, A. On the mechanism of activation of copper-catalyzed atom transfer radical polymerization. Electrochim. Acta 110, 655–662 (2013).
Fang, C. et al. Mechanistically guided predictive models for ligand and initiator effects in copper-catalyzed atom transfer radical polymerization (Cu-ATRP). J. Am. Chem. Soc. 141, 7486–7497 (2019).
Acknowledgements
We thank the National Natural Science Foundation of China (grants nos. 21831002 and 22025103 to X.-Y.L., 21901106 to F.-L.W., 22001109 to Q.-S.G. and 21702182, 21873081 and 22122109 to X.H.), Guangdong Innovative Program (no. 2019BT02Y335 to X.-Y.L.), Guangdong Provincial Key Laboratory of Catalysis (no. 2020B121201002 to X.-Y.L.), Shenzhen Special Funds (no. JCYJ20200109141001789 to X.-Y.L.), SUSTech Special Fund for the Construction of High-Level Universities (no. G02216303 to X.-Y.L.), Fundamental Research Funds for the Central Universities (no. 2020XZZX002-02 to X.H.), the Starry Night Science Fund of Zhejiang University Shanghai Institute for Advanced Study (no. SN-ZJU-SIAS-006 to X.H.), the State Key Laboratory of Clean Energy Utilization (no. ZJUCEU2020007 to X.H.) and Key Laboratory of Precise Synthesis of Functional Molecules of Zhejiang Province (no. PSFM2021-01 to X.H.) for financial support. We appreciate the assistance of SUSTech Core Research Facilities with compound characterization. We also appreciate the support of the Center of Chemistry for Frontier Technologies, Department of Chemistry, Zhejiang University. Calculations were performed on the high-performance computing system at the Department of Chemistry, Zhejiang University.
Author information
Authors and Affiliations
Contributions
X.-Y.L. and Q.-S.G. conceived and supervised the project. F.-L.W., C.-J.Y., N.-Y.Y., X.-Y.D., R.-Q.J., X.-Y.C., Z.-L.L., D.-L.Y. and Y.-S.Z. designed and performed the experiments and analysed the data. X.H. designed the DFT calculations. J.-R.L. and G.-X.X. performed the DFT calculations. X.-Y.L., X.H. and Q.-S.G. wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–31 and Tables 1–7, experimental procedures, synthetic procedures, characterization data, density functional theory (DFT) calculations and mechanistic discussion.
Supplementary Data 1
Crystallographic data for compound 44; CCDC reference 2074298.
Supplementary Data 2
Crystallographic data for compound 55; CCDC reference 2074300.
Supplementary Data 3
Crystallographic data for compound 79; CCDC reference 2074301.
Supplementary Data 4
Crystallographic data for compound C1; CCDC reference 2074302.
Supplementary Data 5
Tables of energies and coordinates in XYZ format.
Rights and permissions
About this article
Cite this article
Wang, FL., Yang, CJ., Liu, JR. et al. Mechanism-based ligand design for copper-catalysed enantioconvergent C(sp3)–C(sp) cross-coupling of tertiary electrophiles with alkynes. Nat. Chem. 14, 949–957 (2022). https://doi.org/10.1038/s41557-022-00954-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-022-00954-9