Abstract
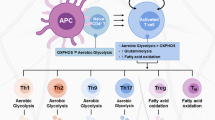
Regulatory T (Treg) cells are critical for maintaining immune homeostasis and preventing autoimmunity. Here, we show that the non-oxidative pentose phosphate pathway (PPP) regulates Treg function to prevent autoimmunity. Deletion of transketolase (TKT), an indispensable enzyme of non-oxidative PPP, in Treg cells causes a fatal autoimmune disease in mice, with impaired Treg suppressive capability despite regular Treg numbers and normal Foxp3 expression levels. Mechanistically, reduced glycolysis and enhanced oxidative stress induced by TKT deficiency triggers excessive fatty acid and amino acid catabolism, resulting in uncontrolled oxidative phosphorylation and impaired mitochondrial fitness. Reduced α-KG levels as a result of reductive TCA cycle activity leads to DNA hypermethylation, thereby limiting functional gene expression and suppressive activity of TKT-deficient Treg cells. We also find that TKT levels are frequently downregulated in Treg cells of people with autoimmune disorders. Our study identifies the non-oxidative PPP as an integrator of metabolic and epigenetic processes that control Treg function.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The GEO accession numbers for our RNA-seq and ATAC-seq data are GSE172048 and GSE198995. The link of mm10_gencodewe used for the ATAC-seq analysis is: https://hgdownload.cse.ucsc.edu/goldenpath/mm10/chromosomes/. The data used to generate the results in this paper are available as source data. All data and mouse lines included in this study are available from the corresponding authors upon reasonable request. Source data are provided with this paper.
References
Chen, Y., Kuchroo, V. K., Inobe, J., Hafler, D. A. & Weiner, H. L. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science 265, 1237–1240 (1994).
Hori, S., Nomura, T. & Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 299, 1057–1061 (2003).
Fontenot, J. D., Gavin, M. A. & Rudensky, A. Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4, 330–336 (2003).
Li, M. O. & Rudensky, A. Y. T cell receptor signalling in the control of regulatory T cell differentiation and function. Nat. Rev. Immunol. 16, 220–233 (2016).
Savage, P. A., Klawon, D. E. J. & Miller, C. H. Regulatory T cell development. Annu Rev. Immunol. 38, 421–453 (2020).
Su, W. et al. Protein prenylation drives discrete signaling programs for the differentiation and maintenance of effector Treg cells. Cell Metab. 32, 996–1011.e1017 (2020).
Xu, M. et al. c-MAF-dependent regulatory T cells mediate immunological tolerance to a gut pathobiont. Nature 554, 373–377 (2018).
Vasanthakumar, A. et al. The TNF receptor superfamily–NF-κB axis is critical to maintain effector regulatory T cells in lymphoid and non-lymphoid tissues. Cell Rep. 20, 2906–2920 (2017).
Vasanthakumar, A. et al. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat. Immunol. 16, 276–285 (2015).
Koizumi, S. I. & Ishikawa, H. Transcriptional regulation of differentiation and functions of effector T regulatory cells. Cells 8, 939 (2019).
Cretney, E. et al. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat. Immunol. 12, 304–311 (2011).
Dominguez-Villar, M. & Hafler, D. A. Regulatory T cells in autoimmune disease. Nat. Immunol. 19, 665–673 (2018).
Alissafi, T. et al. Mitochondrial oxidative damage underlies regulatory T cell defects in autoimmunity. Cell Metab. 32, 591–604.e597 (2020).
Campbell, C. et al. Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature 581, 475–479 (2020).
Zeng, H. et al. mTORC1 couples immune signals and metabolic programming to establish Treg-cell function. Nature 499, 485–490 (2013).
Pacella, I. et al. Fatty acid metabolism complements glycolysis in the selective regulatory T cell expansion during tumor growth. Proc. Natl Acad. Sci. USA 115, E6546–e6555 (2018).
Zeng, H. & Chi, H. Metabolic control of regulatory T cell development and function. Trends Immunol. 36, 3–12 (2015).
MacIver, N. J., Michalek, R. D. & Rathmell, J. C. Metabolic regulation of T lymphocytes. Annu Rev. Immunol. 31, 259–283 (2013).
Michalek, R. D. et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 186, 3299–3303 (2011).
Gerriets, V. A. et al. Foxp3 and Toll-like receptor signaling balance Treg cell anabolic metabolism for suppression. Nat. Immunol. 17, 1459–1466 (2016).
Field, C. S. et al. Mitochondrial integrity regulated by lipid metabolism is a cell-intrinsic checkpoint for Treg suppressive function. Cell Metab. 31, 422–437.e425 (2020).
Tian, N. et al. Transketolase deficiency in adipose tissues protects mice from diet-induced obesity by promoting lipolysis. Diabetes 69, 1355–1367 (2020).
Li, M. et al. Transketolase deficiency protects the liver from DNA damage by increasing levels of ribose 5-phosphate and nucleotides. Cancer Res. 79, 3689–3701 (2019).
Wang, T. et al. Inosine is an alternative carbon source for CD8+-T-cell function under glucose restriction. Nat. Metab. 2, 635–647 (2020).
Ge, T. et al. The role of the pentose phosphate pathway in diabetes and cancer. Front. Endocrinol. 11, 365 (2020).
Rubtsov, Y. P. et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity 28, 546–558 (2008).
Crotty, S. T follicular helper cell biology: a decade of discovery and diseases. Immunity 50, 1132–1148 (2019).
Sakaguchi, S., Yamaguchi, T., Nomura, T. & Ono, M. Regulatory T cells and immune tolerance. Cell 133, 775–787 (2008).
Zhu, J. & Shevach, E. M. TCR signaling fuels Treg cell suppressor function. Nat. Immunol. 15, 1002–1003 (2014).
Ren, J. et al. Foxp1 is critical for the maintenance of regulatory T-cell homeostasis and suppressive function. PLoS Biol. 17, e3000270 (2019).
Xu, I. M. et al. Transketolase counteracts oxidative stress to drive cancer development. Proc. Natl Acad. Sci. USA 113, E725–E734 (2016).
Patra, K. C. & Hay, N. The pentose phosphate pathway and cancer. Trends Biochem. Sci. 39, 347–354 (2014).
Chen, L. et al. NADPH production by the oxidative pentose-phosphate pathway supports folate metabolism. Nat. Metab. 1, 404–415 (2019).
Martínez-Reyes, I. & Chandel, N. S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 11, 102 (2020).
Steinert, E. M., Vasan, K. & Chandel, N. S. Mitochondrial metabolism regulation of T cell-mediated immunity. Annu Rev. Immunol. 39, 395–416 (2021).
Jiang, L. et al. Reductive carboxylation supports redox homeostasis during anchorage-independent growth. Nature 532, 255–258 (2016).
Mullen, A. R. et al. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature 481, 385–388 (2011).
Mullen, A. R. et al. Oxidation of alpha-ketoglutarate is required for reductive carboxylation in cancer cells with mitochondrial defects. Cell Rep. 7, 1679–1690 (2014).
Zhang, G. F. et al. Reductive TCA cycle metabolism fuels glutamine- and glucose-stimulated insulin secretion. Cell Metab. 33, 804–817.e805 (2021).
Xiao, M. et al. Inhibition of α-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes Dev. 26, 1326–1338 (2012).
Arts, R. J. et al. Glutaminolysis and fumarate accumulation integrate immunometabolic and epigenetic programs in trained immunity. Cell Metab. 24, 807–819 (2016).
Carey, B. W., Finley, L. W., Cross, J. R., Allis, C. D. & Thompson, C. B. Intracellular α-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature 518, 413–416 (2015).
Kaelin, W. G. Jr. Cancer and altered metabolism: potential importance of hypoxia-inducible factor and 2-oxoglutarate-dependent dioxygenases. Cold Spring Harb. Symp. Quant. Biol. 76, 335–345 (2011).
Weinberg, S. E. et al. Mitochondrial complex III is essential for suppressive function of regulatory T cells. Nature 565, 495–499 (2019).
Ohkura, N., Kitagawa, Y. & Sakaguchi, S. Development and maintenance of regulatory T cells. Immunity 38, 414–423 (2013).
Ohkura, N. et al. T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity 37, 785–799 (2012).
Xu, W. et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell 19, 17–30 (2011).
Yue, X., Lio, C. J., Samaniego-Castruita, D., Li, X. & Rao, A. Loss of TET2 and TET3 in regulatory T cells unleashes effector function. Nat. Commun. 10, 2011 (2019).
Asadi Shahmirzadi, A. et al. Alpha-ketoglutarate, an endogenous metabolite, extends lifespan and compresses morbidity in aging mice. Cell Metab. 32, 447–456.e446 (2020).
Pearce, E. L. & Pearce, E. J. Metabolic pathways in immune cell activation and quiescence. Immunity 38, 633–643 (2013).
Dias, S. et al. Effector regulatory T cell differentiation and immune homeostasis depend on the transcription factor Myb. Immunity 46, 78–91 (2017).
Oh, H. et al. An NF-κB transcription-factor-dependent lineage-specific transcriptional program promotes regulatory T cell identity and function. Immunity 47, 450–465.e455 (2017).
De Rosa, V. et al. Glycolysis controls the induction of human regulatory T cells by modulating the expression of FOXP3 exon 2 splicing variants. Nat. Immunol. 16, 1174–1184 (2015).
Procaccini, C. et al. The proteomic landscape of human ex vivo regulatory and conventional T cells reveals specific metabolic requirements. Immunity 44, 406–421 (2016).
Franco, F., Jaccard, A., Romero, P., Yu, Y. R. & Ho, P. C. Metabolic and epigenetic regulation of T-cell exhaustion. Nat. Metab. 2, 1001–1012 (2020).
Roy, D. G. et al. Methionine metabolism shapes T helper cell responses through regulation of epigenetic reprogramming. Cell Metab. 31, 250–266.e259 (2020).
He, H. et al. Histone methylation mediates plasticity of human FOXP3+ regulatory T cells by modulating signature gene expressions. Immunology 141, 362–376 (2014).
Liu, Y. et al. Epigenome-wide association data implicate DNA methylation as an intermediary of genetic risk in rheumatoid arthritis. Nat. Biotechnol. 31, 142–147 (2013).
Dor, Y. & Cedar, H. Principles of DNA methylation and their implications for biology and medicine. Lancet 392, 777–786 (2018).
Acknowledgements
We thank members of Xuemei Tong’s and Bin Li’s laboratory for their suggestions. We thank Nan Shen and Pingjing Zhang for technical supports and advice. We acknowledge Tianle Xu for Tktfl/fl mice. We are grateful for the support from Core Facility of Basic Medical Sciences, Shanghai Jiao Tong University School of Medicine. This work was supported by grants from National Key Research and Development Program of China (2021YFF0702100 to X.T.; 2019YFA0906100 to B.L. and X.T.), the National Natural Science Foundation of China (92057117 and 81972210 to X.T.; 32130041, 81830051 and 31961133011 to B.L.) and the Shanghai Municipal Science and Technology Major Project (19JC1410200 to X.T.).
Author information
Authors and Affiliations
Contributions
Q.L., B.L. and X.T. designed the research. Q.L., H.Y., F.Z. and X.L. performed experiments. Q.L. analysed and interpreted data. Y.L., N.T., L.T., Y.H. and T.X. contributed to mouse and cellular experiments. K.Y. and Z.H. contributed to metabolic analysis. H.H. and D.F. contributed to CRISPR–Cas9-mediated gene knockout experiments. X.G., X.W. and H.X. contributed to human samples. Y.L., Y.Z., L.H., Y.J., N.X., D.L., R.L., G.G., L.W., P.Z. and D.Y. contributed to scientific discussion. Q.L. and X.T. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
B. L. is a co-founder of Biotheus and chair of its scientific advisory board. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Navdeep Chandel, Margarita Dominguez-Villar and Wayne Hancock for their contribution to the peer review of this work. Primary handling editors: Christoph Schmitt and George Caputa, in collaboration with the Nature Cardiovascular Research team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
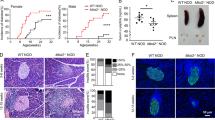
Extended Data Fig. 1 Loss of TKT in Tregs results in a fatal autoimmune disease.
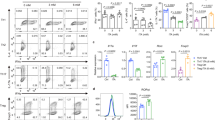
a, Schematic diagram of two reactions catalyzed by TKT in non-oxidative PPP. G6P: Glucose-6-phosphate; 6PGL: 6-phosphogluconolactone; 6PG: 6-phosphogluconate; Ru5P: Bibulose-5-phosphate; Xu5P: Xylulose-5-phosphate; R5P: Ribose-5-phosphate S7P: Sedoheptulose-7-phosphate; G3P: Glyceraldehyde-3-phosphate; E4P: Erythrose-4-phosphate; F6P: Fructose-6-phosphate; F1,6BP: Fructose 1,6-bisphosphate; DHAP: Dihydroxyacetone phosphate. b, Targeting strategy to generate Treg-specific TKT knockout mice. c, Protein levels of TKT, Foxp3 and β-actin in Treg cells (Tregs) and CD4+CD25-YFP- conventional T cells (Tconvs) sorted from lymphoid tissues of 5-week-old WT or cKO mice. d, Total cell numbers of thymus, spleen and pLN of 5-week-old WT or cKO mice. n=5 mice. e, Flow cytometric analysis and quantification of CXCR5hiPD-1CXCR5hi germinal center follicular helper T (GC-Tfh) cells (gated from TCRβ+CD4+ T cells) in the pLN of 5-week-old WT and cKO mice. n=6 mice. Data are representative of at least two independent experiments. Data are shown as mean ± s.d.. P value are determined by two-way ANOVA followed by Sidak’s multiple-comparisons test (d) and unpaired two-tailed Student’s t-test (e). Numbers in gates indicate percentage of cells.
Extended Data Fig. 2 Loss of TKT in Tregs disturbs immune homeostasis in multiple organs.
a,b, Flow cytometric analysis of the percentage (a) and number (b) of CD4–CD8– (DN), CD4+CD8+ (DP), CD4+CD8– (SP-CD4) and CD4–CD8+ (SP-CD8) in the thymus as well as CD4+ and CD8+ T cells in the spleen and pLN of 2-week-old WT and cKO mice. n=5 mice. c-e, Expression of Ki-67 (c), CD62L and CD44 (d,e) in CD4+ T cells and CD8+ T cells in the spleen and pLN of 2-week-old WT and cKO mice. n=5 mice. f,g, Percentage (f) and cell number (g) of CD4+ and CD8+ T cells in the pLN from 5-week-old WT and cKO mice. n=6 mice. h,i, Expression of Ki67 in CD4+ T cells and CD8+ T cells in the spleen (h) and pLN (i) of 5-week-old WT and cKO mice. n=5 mice. j-l, Number or percentage of effector (CD44hiCD62Llo) cells in CD4+ T cells and CD8+ T cells in the spleen (j) and pLN (k,l) of 5-week-old WT and cKO mice. n=6 mice. m,n, IFN-γ, IL-17A and IL-13 productions in CD4+ T cells (m) and IFN-γ production in CD8+ T cells (n) in the pLN, liver, lung and colon from 5-week-old WT or cKO mice. n=5 mice. Data are representative of at least two independent experiments. Data are shown as mean ± s.d.. P value are determined by two-way ANOVA followed by Sidak’s multiple-comparisons test. ns, not significant.
Extended Data Fig. 3 TKT deficiency does not alter Treg cellularity.
a, Flow cytometric analysis (left) and quantification (right) of Tregs from the thymus, spleen and pLN of 2-week-old WT and cKO mice. n=5 mice. b, Schematic diagram of chimeric Tregs in female Foxp3Cre/+Tktfl/fl mice. c,d, Total cell number (c) and absolute CD4+ and CD8+ T cell number in the spleen and pLN (d) of 7-week-old chimeric WT or chimeric cKO mice. n=7 mice. e, Percentage of effector (CD44hiCD62Llo) cells in CD4+ T cells and CD8+ T cells in the spleen and pLN of 7-week-old chimeric WT or chimeric cKO mice. n=7 mice. f, Schematic representation of the bone marrow chimeric (BMC) experiment. g, Flow cytometric analysis of Tregs from the spleen and pLN of BMC WT and cKO mice. n=6 mice. h, Protein levels of TKT, Foxp3 and β-actin in CD4+CD8+ T cells and Tregs sorted from thymus and spleen of Foxp3YFP-cre mice respectively (top). n=3 independent samples, Tregs is a positive control. mRNA expression of Tkt and Foxp3 during T cell development (bottom, data from the ImmGen RNA-seq database). T.DP.Th: double positive T cells in thymus; T.4.Th: CD4 single positive T cells in thymus; Treg.4.25.Sp: CD4+CD25+ T cells in spleen. i, Flow cytometric analysis (left) and quantification (right) of Tregs from 7-week-old Tktfl/fl and Cd4CreTktfl/fl mice. n=5 mice. Data are representative of one (g) and at least two (a,c-e,h,i) independent experiments. Data are shown as mean ± s.d.. P value are determined by unpaired two-tailed Student’s t-test (c), and two-way ANOVA followed by Sidak’s multiple-comparisons test (a,d,e,g,i). ns, not significant. Numbers in gates indicate percentage of cells.
Extended Data Fig. 4 TKT depletion impairs Treg function by abolishing aTreg signature gene expression and transaldolase deficiency also dampens Treg function.
a, Schematic representation of the transfer colitis model for assessing Treg suppressive function in vivo by transferring WT or cKO Tregs into Rag1–/– recipients. b-e, In vivo Treg suppressive assay by a T cell-dependent adoptive transfer colitis experiment. b, n=5 mice; d, n=4 mice. (b), Representative ratio curve of post-transfer body weight relative to initial body weight of Rag1–/– recipients. (c), Representative H&E staining of distal colons of Rag1-/- recipients at 6 weeks after the transfer. Scale bars, 100 μm. (d), Representative flow cytometric plots of IFN-γ- or IL-17A-producing T cells in the spleen and colon (left). Right, cell number of IFN-γ- or IL-17A-producing T cells (gated from CD45.1+ TCRβ+CD4+ T cells). (e), Representative image of spleens from Rag1–/– recipients in (a). f, Schematic representation of the transfer colitis model for assessing Treg suppressive function in vivo by transferring BMC-WT and BMC-cKO Tregs into Rag1–/– recipients. g, RT-PCR analysis of mRNA levels of indicated genes in WT and Taldo1-deficient iTregs. n=3 cultures. h, Heatmap of the differentially regulated genes in chimeric WT Tregs relative to chimeric cKO Tregs isolated from 7-week-old female mice. Padj <0.05. i, Expression of CD44 (left, n=6 mice) and KLRG1 (right, n=5 mice) in chimeric WT and cKO Tregs from 7-week-old female mice (gated from CD4+CD25+YFP+ Tregs). j, Percentages of active Tregs (CD62LloCD44hi) in the spleen and pLN of 7-week-old chimeric WT or chimeric cKO mice (gated from CD4+CD25+YFP+ Tregs). n=6 mice. k, Genome browser views of indicated gene loci in chimeric WT and chimeric cKO Tregs. Data are representative of at least two independent experiments (b,d,g,i,j). ns, not significant. Data are shown as mean ± s.d. (b,g,i,j); box plots (d) include minima, maxima, center line, median; box limits, upper and lower quartiles; whiskers, 1.5x interquartile range; points, outliers. P value are determined by unpaired two-tailed Student’s t-test (g), log-rank test (b) and two-way ANOVA followed by Sidak’s multiple-comparisons test (b,i,j). Numbers in gates indicate percentage of cells.
Extended Data Fig. 5 Loss of TKT leads to metabolic reprogramming, defective mitochondrial function and impaired energy production.
a, Analysis of ECAR of WT and cKO Tconvs isolated from Tktfl/fl and Cd4CreTktfl/fl mice. n=4 cultures. b, Analysis of ECAR of WT and cKO iTregs. n=5 cultures. c, Analysis of OCR of WT and cKO iTregs. WT iTregs: n=6 cultures; cKO iTregs: n=5 cultures. d, Relative isotopomer abandance of M+2 citrate, α-KG, succinate, fumarate and malate in WT and cKO Tregs following incubation with [13C6]glucose for 12 hours. n=3 independent samples. e, LC-MS/MS analysis of amino acids in the serum of 3-week-old WT and cKO mice. FC means fold change. n=8 mice. f, Representative quantifications of MitoSOX, MitoTrackerTM Red CMXRos and Mitotracker Green in WT and TKT-deficient Tregs by flow cytometry. n=5 mice. Tregs for MitoSOX and MitoTrackerTM Red staining were isolated from 3-week-old WT and cKO mice. Tregs for Mitotracker Green staining were isolated from 7-week-old Tktfl/fl and Cd4CreTktfl/fl mice. g, Level of ATP in WT and cKO Tregs isolated from 2-3-week-old mice. n=6 independent samples. h, Representative electron micrographs of mitochondria and quantification of mitochondrial area and cristae width of WT and cKO iTregs induced in vitro. Scale bars, 1 μm and 200 nm, respectively. n=10 independent samples. i, Western blotting analysis of levels of indicated proteins in WT and TKT-deficient Tregs. j, Representative ratio curve of post-transfer body weight relative to initial body weight of Rag1–/– recipients in the adoptive transfer colitis experiment supplied with or without NAC in drinking water. Water: WT Tregs: n=6 mice, cKO Tregs: n=7 mice, No Tregs: n=5 mice; NAC: WT Tregs: n=6 mice, cKO Tregs: n=7 mice, No Tregs: n=8 mice. P value were analyzed by using the body weight changes at 7 week. k, Representative IFN-γ production in CD45.1+CD4+ T cells of Rag1–/– recipients in the adoptive transfer colitis experiment supplied with or without NAC in drinking water. n=5 mice. Data are representative of three (a,b,c,h,i) and one (d,e,f,g) independent experiments. Data are shown as mean ± s.d. (a-d,f-h,k); box plots (e) include minima, maxima, center line, median; box limits, upper and lower quartiles; whiskers, 1.5x interquartile range; points, outliers. P value are determined by unpaired two-tailed Student’s t-test (g,h,j), and two-way ANOVA followed by Sidak’s multiple-comparisons test (a-d,f,k).
Extended Data Fig. 6 α-KG supplementation restores Treg function and aTreg-associated functional gene expression in TKT deficient Tregs.
a, Flow cytometric analysis (left) and quantification (right) of in vitro suppressive assay of Tregs isolated from 7-week-old WT mice. Tregs were pretreated with DMαKG or vehicle for 4 h and co-cultured with CTV-labelled Tconvs for 3 days. n=4 cultures. b, RT-PCR analysis of indicated genes in WT Tregs, cKO Tregs and DMαKG-treated WT and cKO Tregs. n=3 cultures. c, Global 5hmC/dG levels in Tregs treated with or without DMαKG in vitro. n=3 cultures. d, Representative flow cytometric analysis of CD62L and CD44 expression in CD8+ T cells in the spleen of 6-week-old WT and cKO mice supplemented with or without DMαKG in drinking water. n=7 mice. e, Global 5hmC/dG levels in Tregs isolated from WT and cKO mice supplemented with or without DMαKG in drinking water. n=3 independent samples. f, Heatmap of RNA sequencing of selected genes differentially expressed between WT and cKO Tregs from mice supplied with or without DMαKG in drinking water. g, RT-PCR analysis of indicated genes in Tregs isolated from WT and cKO mice supplemented with or without DMαKG in drinking water. n=3 independent samples. Data are representative of two (a,b,c,g) independent experiments. Data are shown as mean ± s.d.. P value are determined by two-way ANOVA followed by Sidak’s multiple-comparisons test. ns, not significant.
Extended Data Fig. 7 AS and SLE patients feature downregulated expression of TKT, ICOS and KLRG1 in Tregs.
a, Flow cytometric gating strategy of CD3+CD4+FOXP3+ Tregs in human PBMC. b,c, Representative flow cytometric analysis of IFN-γ and IL-17A production in CD4+ and CD8+ cells of in PBMC of AS patients (b) and SLE patients (c) relative to healthy controls. b, n=11 independent samples; c, CTRL: n=10 independent samples, SLE: n=7 independent samples. d,e, Representative flow cytometric analysis of TKT in CD4+FOXP3+ Tregs from PBMC of AS (d) and SLE (e) patients relative to healthy controls. d, n=11 independent samples; e, CTRL: n=10 independent samples, SLE: n=7 independent samples. f,g, Representative flow cytometric analysis of ICOS and KLRG1 expression in AS (f) and SLE patients (g) relative to controls. f, CTRL: n=9 independent samples, AS: n=7 independent samples; g, CTRL: n=8 independent samples, SLE: n=7 independent samples. Box plots (b-g) include minima, maxima, center line, median; box limits, upper and lower quartiles; whiskers, 1.5x interquartile range; points, outliers. P value are determined by two-way ANOVA followed by Sidak’s multiple-comparisons test (b,c) and unpaired two-tailed Student’s t-test (d-g). The number above the histogram indicated percentage of cells (a-c) and MFI (d,e).
Extended Data Fig. 8 Non-oxidative pentose phosphate pathway controls regulatory T cell function by integrating metabolism and epigenetics.
By balancing glucose flux between glycolysis and PPP as well as carbons feeding into the TCA cycle in Tregs, TKT in non-oxidative PPP controls Treg function by maintaining metabolite-dependent functional gene expression. In the absence of TKT, reduced glucose catabolism triggers excessive amino acids and fatty acids to compensatorily feed into mitochondria, which further impairs mitochondrial function and energy production. Meanwhile, enhanced oxidative stress triggers reductive TCA cycle and reduces α-ketoglutarate/succinate and α-ketoglutarate/fumarate ratios, leading to increased DNA methylation, further limiting aTreg-associated functional gene expression and suppressive activity in Tregs.
Supplementary information
Supplementary Information
Supplementary Figure 1 and Supplementary Tables 1–4
Source data
Source Data Fig. 1
Statistical Source Data
Source Data Fig. 1
Unprocessed immunoblots and images
Source Data Fig. 2
Statistical Source Data
Source Data Fig. 3
Statistical Source Data
Source Data Fig. 3
Unprocessed images
Source Data Fig. 4
Statistical Source Data
Source Data Fig. 4
Unprocessed immunoblots
Source Data Fig. 5
Statistical Source Data
Source Data Fig. 6
Statistical Source Data
Source Data Fig. 7
Statistical Source Data
Source Data Fig. 7
Unprocessed immunoblots and images
Source Data Extended Data Fig. 1
Statistical Source Data
Source Data Extended Data Fig. 1
Unprocessed immunoblots
Source Data Extended Data Fig. 2
Statistical Source Data
Source Data Extended Data Fig. 3
Statistical Source Data
Source Data Extended Data Fig. 3
Unprocessed immunoblots
Source Data Extended Data Fig. 4
Statistical Source Data
Source Data Extended Data Fig. 4
Unprocessed images
Source Data Extended Data Fig. 5
Statistical Source Data
Source Data Extended Data Fig. 5
Unprocessed immunoblots and images
Source Data Extended Data Fig. 6
Statistical Source Data
Source Data Extended Data Fig. 7
Statistical Source Data
Rights and permissions
About this article
Cite this article
Liu, Q., Zhu, F., Liu, X. et al. Non-oxidative pentose phosphate pathway controls regulatory T cell function by integrating metabolism and epigenetics. Nat Metab 4, 559–574 (2022). https://doi.org/10.1038/s42255-022-00575-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-022-00575-z
This article is cited by
-
Lipid metabolism in tumor-infiltrating regulatory T cells: perspective to precision immunotherapy
Biomarker Research (2024)
-
Identification of co-expressed gene networks promoting CD8+ T cell infiltration and having prognostic value in uveal melanoma
BMC Ophthalmology (2023)
-
The pentose phosphate pathway in health and disease
Nature Metabolism (2023)
-
Spatiotemporal resolution of germinal center Tfh cell differentiation and divergence from central memory CD4+ T cell fate
Nature Communications (2023)
-
Histone demethylases in the regulation of immunity and inflammation
Cell Death Discovery (2023)