Abstract
Purpose
The present study aimed to develop, statistically optimize, and characterize hydroquinone-loaded transfersomes (HQ-TFs) for effective topical delivery by mitigating the problems associated with HQ.
Methods
HQ-TFs were prepared by the thin-film hydration method and characterized for particle size, zeta potential (ZP), entrapment efficiency (EE), in vitro drug release, and skin penetration potential. The optimized hydroquinone-loaded transfersome (OPT-HQ-TF) was incorporated in a gel and evaluated for ex vivo skin permeation and deposition profile, in vitro antioxidant activity, in vitro cytotoxicity study, in vitro tyrosinase inhibition assay, and dermal skin irritation study.
Results
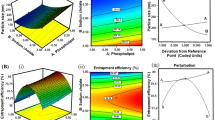
The OPT-HQ-TF showed a particle size of 210 nm, ZP of − 15.10 mV, and EE% of 67.61. The cumulative drug release % from transfersomal formulations ranged from 54.39 ± 1.92 to 76.05 ± 1.18%. The fluorescence microscopy investigation revealed the penetration of transfersomes into deeper skin layers. The skin permeation and deposition studies indicated that the OPT-HQ-TF gel improved permeation and drug retention in the skin compared to the HQ plain gel. The antioxidant assay revealed that HQ retained its antioxidant activity after encapsulation. The cytotoxicity study demonstrated that the OPT-HQ-TF gel significantly decreased the cytotoxicity towards L-929 mouse fibroblast. The tyrosinase inhibition assay specified that the OPT-HQ-TF gel has the potential to treat hyperpigmentation. The dermal skin irritation study indicated that the OPT-HQ-TF gel is safe and non-irritant.
Conclusion
The present study findings suggested the potential application of deformable nanovesicles as an innovative topical drug delivery system of HQ in the treatment of hyperpigmentation.










Similar content being viewed by others
References
Rathee P, Kumar S, Kumar D, Kumari B, Yadav SS. Skin hyperpigmentation and its treatment with herbs: an alternative method. Future J Pharm Sci. 2021;132(7):1–14.
Cichorek M, Wachulska M, Stasiewicz A, Tyminska A. Skin melanocytes: biology and development. Postepy Dermatol Alergol. 2013;30(1):30–41.
Cook-Bolden FE, Hamilton SF. An open-label study of the efficacy and tolerability of microencapsulated hydroquinone 4% and retinol 0.15% with antioxidants for the treatment of hyperpigmentation. Cutis. 2008;81:365–371.
Rendona M, Horwitzb S. Topical treatment of hyperpigmentation disorders. Ann Dermatol Venereol. 2012;139:153–8.
Taylor A, Pawaskar M, Taylor SL, Balkrishnan R, Feldman SR. Prevalence of pigmentary disorders and their impact on quality of life: a prospective cohort study. J Cosmet Dermatol. 2008;7:164–8.
Draelos ZD. Skin lightening preparations and the hydroquinone controversy. Dermatol Ther. 2007;20:308–13.
Rendon MI, Gaviria JI. Review of skin-lightening agents. Dermatol Surg. 2005;31(7):886–9.
Rossi AM, Perez MI. Treatment of hyperpigmentation. Facial Plast Surg Clin N Am. 2011;19(2):313–24.
Ghanbarzadeh S, Haririb R, Kouhsoltanic M, Shokrid J, Javadzadehe Y, Hamishehkarf H. Enhanced stability and dermal delivery of hydroquinone using solid lipid nanoparticles. Colloids Surf B. 2015;136:1004–10.
Obagi ZE. Taking the pulse of hydroquinone therapy: a plea for caution. Pract. Dermatol. 2013;39–42.
Serrano DR, Gordo MJ, Matji A, Gonzalez S, Lalatsa A, Torrado JJ. Tuning the transdermal delivery of hydroquinone upon formulation with novel permeation enhancers. Pharmaceutics. 2019;11(4):167.
Pandey P, Satija S, Wadhwa R, Mehta M, Purohit D, Gupta G, Prasher P, Chellappan DK, Awasthi R, Dureja H, Dua K. Emerging trends in nanomedicine for topical delivery in skin disorders: current and translational approaches. Dermatol Ther. 2020;33(3):1–12.
Kumar A, Pathak K, Bali V. Ultra-adaptable nanovesicular systems: a carrier for systemic delivery of therapeutic agents. Drug Discov Today. 2012;17(21–22):1233–41.
Madni A, Kousar R, Naeema N, Wahid F. Recent advancements in applications of chitosan-based biomaterials for skin tissue engineering. J Bioresour Bioprod. 2021;6(1):11–25.
Fatima A, Yasir S, Khan MS, Manan S, Ullah MW, Ul-Islam M. Plant extract-loaded bacterial cellulose composite membrane for potential biomedical applications. J Bioresour Bioprod. 2021;6(1):26–32.
Monteiro RC, Kishore BN, Bhat RM, Sukumar D, Martis J, Ganesh HK. A comparative study of the efficacy of 4% hydroquinone vs 0.75% kojic acid cream in the treatment of facial melasma. Indian J Dermatol. 2013;58(2):157.
Grimes PE. A microsponge formulation of hydroquinone 4% and retinol 0.15% in the treatment of melasma and postinflammatory hyperpigmentation. Cutis. 2004;74(6):362–368.
Taheri A, Mohammadi M. The use of cellulose nanocrystals for potential application in topical delivery of hydroquinone. Chem Biol Drug Des. 2015;86(1):102–6.
Okur NU, Caglar ES, Pekcan AN, Okur ME, Ayla S. Preparation, optimization and in vivo anti-inflammatory evaluation of hydroquinone loaded microemulsion formulations for melasma treatment. J Pharm Res. 2019;23(4):662–70.
Salimi A, Hajiani MK. Enhanced stability and dermal delivery of hydroquinone using microemulsion-based system. Asian J Pharm. 2017;11(4):773–81.
Wu PS, Lin CH, Kuo YC, Lin CC. Formulation and characterization of hydroquinone nanostructured lipid carriers by homogenization emulsification method. J Nanomater. 2017;1–7.
Khoshneviszadeh R, Bazzaz BSF, Housaindokht MR, Habibi AE, Rajabi A. A comparison of explanation methods of encapsulation efficacy of hydroquinone in a liposomal system. J Paramed Sci. 2016;7(2):23–8.
Azarbayjani AF, Talebi N, Diba K. Development and characterization of hydroquinone-loaded nanofiber for topical delivery: effect of chitosan. Int J Polym Anal Charact. 2019;1–9.
Abdellatif AAH, Tawfeek HM. Transfersomal nanoparticles for enhanced transdermal delivery of clindamycin. AAPS J. 2016;17(5):1067–74.
Arora D, Khurana B, Nanda S. DoE directed optimization, development and evaluation of resveratrol loaded ultradeformable vesicular cream for topical antioxidant benefits. Drug Dev Ind Pharm. 2020;46(2):227–35.
Tawfeek HM, Abdellatif AAH, Aleema JAA, Hassand YA, Fathallaf D. Transfersomal gel nanocarriers for enhancement the permeation of lornoxicam. J Drug Deliv Sci Technol. 2020;56:1–10.
Almehmady AM, Elsisi AM. Development, optimization, and evaluation of tamsulosin nanotransfersomes to enhance its permeation and bioavailability. J Drug Deliv Sci Technol. 2020;57.
Dudhipala N, Mohammed RP, Youssef AAA, Banala N. Effect of lipid and edge activator concentration on development of aceclofenac-loaded transfersomes gel for transdermal application: in vitro and ex vivo skin permeation. Drug Dev Ind Pharm. 2020;46(8):1334–44.
Chen M, Shamim MA, Shahid A, Yeung S, Andresen BT, Wang J, Nekkanti V, Meyskens FL Jr, Kelly KM, Huang Y. Topical delivery of carvedilol loaded nano-transfersomes for skin cancer chemoprevention. Pharmaceutics. 2020;12(12):1–17.
Peram MR, Jalalpure S, Kumbar V, Patil S, Joshi S, Bhat K, Diwan P. Factorial design-based curcumin ethosomal nanocarriers for the skin cancer delivery: in vitro evaluation. J Liposome Res. 2019;29(3):291–311.
Avadhani KS, Manikkath J, Tiwari M, Chandrasekhar M, Godavarthi A, Vidya SM, Hariharapura RC, Kalthur G, Udupa N, Mutalik S. Skin delivery of epigallocatechin-3-gallate (EGCG) and hyaluronic acid loaded nano-transfersomes for antioxidant and anti-aging effects in UV radiation induced skin damage. Drug Deliv. 2017;24(1):61–74.
Jain S, Kale1 DP, Swami R, Katiyar SS. Codelivery of benzoyl peroxide & adapalene using modified liposomal gel for improved acne therapy. Nanomedicine. 2018;13(12):1481–1493.
Gupta A, Bargode RS, Jain S, Shukla K. Formulation and evaluation of econazole transferosomal gel. IAR J Med Cse Rep. 2021;2(5):5–15.
Vasanth S, Dubey A, Ravi GS, Lewis SA, Ghate VM, El-Zahaby SA, Hebbar S. Development and investigation of vitamin C-enriched adapalene-loaded transfersome gel: a collegial approach for the treatment of acne vulgaris. AAPS Journal. 2020;21(61):1–17.
Ahad A, Al-Saleh AA, Al-Mohizea AM, Al-Jenoobi FI, Raish M, Yassin AEB, Alam MA. Pharmacodynamic study of eprosartan mesylate-loaded transfersomes Carbopol® gel under Dermaroller® on rats with methyl prednisolone acetate-induced hypertension. Biomed Pharmacother. 2017;89:177–84.
Shrotriya S, Ranpise N, Satpute P, Vidhate B. Skin targeting of curcumin solid lipid nanoparticles-engrossed topical gel for the treatment of pigmentation and irritant contact dermatitis. Artif Cells Nanomed Biotechnol. 2018;46(7):1471–82.
OECD. Test No. 404: Acute Dermal Irritation/Corrosion, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris. 2015. https://doi.org/10.1787/9789264242678-en.
Wanga J, Li Z, Sun F, Tang S, Zhang S, Lv P, Li J, Cao X. Evaluation of dermal irritation and skin sensitization due to vitacoxib. Toxicol Rep. 2017;4:287–90.
Banerjee S, Chattopadhyay P, Ghosh A, Pathak MP, Singh S, Veer V. Acute dermal irritation, sensitization, and acute toxicity studies of a transdermal patch for prophylaxis against (±) anatoxin-A poisoning. Int J Toxicol. 2013;32(4):308–13.
Aggarwal N, Goindi S. Preparation and evaluation of antifungal efficacy of griseofulvin loaded deformable membrane vesicles in optimized guinea pig model of Microsporum canis-Dermatophytosis. Int J Pharm. 2012;437(1–2):277–87.
Zhai Y, Xu R, Wang Y, Liu J, Wang Z, Zhai G. Ethosomes for skin delivery of ropivacaine: preparation, characterization and ex vivo penetration properties. J Liposome Res. 2015;25(4):316–24.
Opatha SAT, Titapiwatanakun V, Chutoprapat R. Transfersomes: a promising nanoencapsulation technique for transdermal drug delivery. Pharmaceutics. 2020;12(9):1–23.
Abdelmonem R, Hamed RR, Abdelhalim SA, ElMiligi MF, El-Nabarawi MA. Formulation and characterization of cinnarizine targeted aural transfersomal gel for vertigo treatment: a pharmacokinetic study on rabbits. Int J Nanomed. 2020;15:6211–23.
Carreras JJ, Tapia-Ramirez WE, Salal A, Guillot AJ, Garrigues TM, Melero A. Ultraflexible lipid vesicles allow topical absorption of cyclosporin A. Drug Deliv Transl Res. 2019;10(2):486–97.
Jangdey MS, Gupta A, Saraf S, Saraf S. Development and optimization of apigenin-loaded transfersomal system for skin cancer delivery: in vitro evaluation. Artif Cells Nanomed Biotechnol. 2017;45(7):1452–62.
Qushawy M, Nasr A, Abd-Alhaseeb M, Swidan S. Design, optimization and characterization of a transfersomal gel using miconazole nitrate for the treatment of candida skin infections. Pharmaceutics. 2018;10(1):26.
Pena-Rodriguez E, Moreno MC, Blanco-Fernandez B, Gonzalez J, Fernández-Campos F. Epidermal delivery of retinyl palmitate loaded transfersomes: Penetration and biodistribution studies. Pharmaceutics. 2020;12(2):1–14.
Chen J, Lu W, Gu W, Lu S, Chen Z, Cai B. Skin permeation behavior of elastic liposomes: role of formulation ingredients. Expert Opin Drug Deliv. 2013;10(6):845–56.
Arora D, Khurana B, Nanda S. Statistical development and in vivo evaluation of resveratrol loaded topical gel containing deformable vesicles for a significant reduction in photoinduced skin aging and oxidative stress. Drug Dev Ind Pharm. 2020;46(11):1898–910.
Doppalapudi S, Shaheen M, Khan W. Development and in vitro assessment of psoralen and resveratrol co-loaded ultradeformable liposomes for the treatment of vitiligo. J Photochem Photobiol B: Biol. 2017;174:44–57.
Elsayed I, El-Dahmy RM, El-Emam SZ, Elshafeey AH, El-Gawad NAA, El-Gazayerly ON. Response surface optimization of biocompatible elastic nanovesicles loaded with rosuvastatin calcium: enhanced bioavailability and anticancer efficacy. Drug Deliv Transl Res. 2020;10(5):1459–75.
Strzepek-Gomolka M, Gawel-Beben K, Angelis A, Antosiewicz B, Sakipova Z, Kozhanova K, Glowniak K, Kukula-Koch W. Identification of mushroom and murine tyrosinase inhibitors from Achillea biebersteinii Afan. extract. Molecules. 2021;26(4):964.
Zolghadri S, Bahrami A, Hassan Khan MT, Munoz-Munoz J, Garcia-Molina F, Garcia-Canovas F, Saboury AA. A comprehensive review on tyrosinase inhibitors. J Enzyme Inhib Med Chem. 2019;34(1):279–309.
Chaudhary H, Kohli K, Kumar V. A novel nano-carrier transdermal gel against inflammation. Int J Pharm. 2014;465(1–2):175–86.
Acknowledgements
The authors acknowledge the kind support of Lipoid GmBH, Ludwigshafen, Germany, for providing the gift sample Phospholipon® 90 G.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jamadar, A.T., Peram, M.R., Chandrasekhar, N. et al. Formulation, Optimization, and Evaluation of Ultradeformable Nanovesicles for Effective Topical Delivery of Hydroquinone. J Pharm Innov 18, 506–524 (2023). https://doi.org/10.1007/s12247-022-09657-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-022-09657-7