Abstract
Ontogeny is expected to be a determinant factor affecting production of colour patches in lizards, while immune challenges or sudden weight loss may impair the maintenance of pigment-based coloration within a breeding season. We translocated males of the lizard Psammodromus algirus between two sampling plots that differed in distance to a road, vegetation structure, and predator abundance. We analysed variation in spectral reflectance of their colour patches the same and the following year. The change in the reflectance of the lizard colour patches within the first breeding season was explained by the interaction between plot and treatment, but not body condition. The maintenance of the breeding coloration was impaired only in those males translocated close to the road, probably reflecting that it is a poor-quality habitat for P. algirus. The following year, lizards that produced a more elaborate coloration were those that increased their body condition and controlled some parasitic infections, although suffered an increase of others. This study shows that colour patch production is plastic in P. algirus. Lizards increasing parasites or losing weight reduced pigmentation, although habitat quality can cushion these negative effects on pigmentation. However, not all parasites constrain the investment in coloration. In fact, some increased in those lizards that allocated more pigments to colour patches. In conclusion, longitudinal studies following experimental manipulation can contribute to understand pigment allocation rules in lizards.
Significance statement
Pigments involved in colour patches of animals are limiting resources that can be reallocated off the skin to other functions. However, longitudinal evidence of this phenomenon is scarce in reptiles. We designed a manipulative mark-recapture experiment to investigate effects of habitat and parasitic infections on colour patch maintenance (within-year variation) and production (between-year variation) in male free-ranging lizards that were reciprocally translocated between two patches of habitat that differed in quality. During the first year, lizards translocated to the habitat with more predators and worse vegetation impoverished their coloration, while lizards translocated to the more favourable habitat maintained it despite all translocated lizards loose body condition. The next year we detected different effects on the coloration of three different parasites investigated, suggesting that coloration can reflect the virulence of the infections.
Similar content being viewed by others
Introduction
Honesty of visual signals is a central concept in sexual selection theory. It stems from the dual properties of carotenoids, (i) contributing as pigments to animal coloration, and (ii) as antioxidants, which can be reallocated off colour patches to assist in immune response (Alonso-Álvarez et al., 2008; Johansen et al. 2019). Carotenoids cannot be synthetised endogenously, some may be scarce in nature, and can require an oxidisation process prior to their deposition as pigments (Cantarero and Alonso-Alvarez 2017). These characteristics explain why carotenoid-based colour patches have received massive attention as indicators of good individual quality (e.g., Møller et al. 2000; Hill and Hill 2002; Olsson et al. 2013). However, some animals, including many lizards, can express nuptial coloration derived from other pigment families that can be synthesised endogenously. This is the case for pterins and melanins (Cuervo et al. 2016; Megía-Palma et al. 2018a; Merkling et al. 2018). For these species, colour patch expression is expected to be cheaper compared to carotenoid-based patches (Johnson and Fuller 2015). Nonetheless, a combination of these three pigment families usually produces colour patches in lizards rather than separate pigments alone (Andrade et al. 2019; Megía-Palma et al. 2021).
Pterins are pigments present in yellow, orange, and red colour patches of lizards (Weiss et al. 2012; Cuervo et al. 2016; Andrade et al. 2019; Megía-Palma et al. 2021), while melanins participate in black, brown, and blue patches (Megía-Palma et al. 2018a). Recent investigations suggest that pterins and melanins are also reallocated off colour patches when lizards are immunologically challenged (Llanos-Garrido et al. 2017; Megía-Palma et al. 2018b). This trade-off supports the dual activity of these molecules as both pigments and antioxidants in living systems (McGraw 2005), and increases the suspicion that pterin and melanin synthesis are also costly processes that may be constrained by the energy budget of the bearer of the colour patch. In compliance with this trade-off hypothesis, environmental variables such as temperature, food availability, abundance of conspecifics, or sources of stress can influence pigment allocation rules in lizards (Fitze et al. 2009; Cote et al. 2010; Miñano et al. 2021; Masó et al. 2022). Complementarily, other cost–benefit models can also influence the evolution of colour patches of animals, namely photoprotection versus thermoregulation and crypsis versus conspicuousness (Møller 1989; Tibbetts and Dale 2004; Reguera et al. 2014; Díaz et al. 2017).
One sexual selection hypothesis relying on the honesty of visual signals connects pigment concentration in colour patches with parasite infection status of hosts (i.e., parasite-mediated selection hypothesis; Hamilton and Zuk 1982). Individuals in better nutritional state and with lower abundance, or lower proportion of certain parasites in relation to others, may afford greater allocation of pigments to colour patches (Mougeot et al. 2009; Biard et al. 2010). Therefore, this hypothesis proposes that host resistance to parasites can evolve through sexual selection operating over secondary sexual traits that (i) depend on the nutritional status of the individual and (ii) honestly signal resistance to parasites (Hamilton and Zuk 1982). It comes with no surprise that animals in the wild can be infected simultaneously by different parasite species that may differ in their virulence (Del Cerro et al. 2010; Johansen et al. 2019). Fighting off more virulent parasites may provoke trade-offs in pigment allocation to colour patches (Megía-Palma et al. 2018c). Nonetheless, assuming the primary importance of devoting resources to reproduction, pigment allocation to colour patches may be prioritised over assisting immune response against some parasites in individuals with good nutritional status (Folstad et al. 1994; Johansen et al. 2019). In addition, individuals may downregulate reproductive effort influenced by metabolic traumas (e.g., immune challenges, sudden loss of body mass, and/or adverse environmental conditions) that augur poor reproductive output during current reproductive season (Shine and Schwarzkopf 1992; Jørgensen et al. 2006). Under this circumstance, some parasites may benefit from host’s trade-offs in resource allocation and increase their intensity of infection in individuals allocating more resources to reproduction (Nordling et al. 1998; Knowles et al. 2009). Indeed, this latter hypothesis might explain why 58% of the published relationships between colour patch expression and parasite infection in lizards contradict the parasite-mediated selection hypothesis (see Megía-Palma et al. 2021).
We previously reported the negative effect on body condition of the translocation of males of Psammodromus algirus (Lacertidae: Gallotini) within a matrix of habitat (Barrientos and Megía-Palma et al. 2021). Here, we analyse the spectral variation of orange, yellow, and blue patches and the abundance of parasite infections of these same individuals at recapture both within the same and the following year. We will test the hypothesis of whether allocation of pigments to the nuptial coloration of P. algirus concords with trade-offs expected in lizards between body condition and defense against infections (Cote et al. 2008). We predict that (i) translocated males will exhibit a reduction of the pigmentation of orange and yellow patches during the first year influenced by the loose of body condition suffered after translocation (Barrientos and Megía-Palma et al. 2021), although (ii) those translocated to a habitat patch with better environmental quality might cushion these negative effects on pigmentation (Sumasgutner et al. 2018). (iii) We will also explore the effects of the translocation treatment on the coloration of the lizards recaptured one year later. According to the predictions of the Resource Allocation Theory (Beilharz et al. 1993), we expect that the lizards’ body condition and parasite load would influence the production of colour patches.
Material and methods
Study system
We studied the Algerian sand racer, P. algirus (adult snout-vent length range in the sample = 59–79 mm). This species is a precise thermoregulator and has generalist habitat occupancy in the Iberian Peninsula and North of Africa (Díaz 1997; Llanos-Garrido et al. 2021). The sampling area was Monte de El Pardo, in Madrid, Spain (40.5063°N, 3.7662°W; ~ 680 m above sea level). This is a seminatural and heterogeneous matrix of evergreen forest dominated by holm-oak trees (Quercus ilex) and gum rockrose bushes (Cistus ladanifer). It has a sandy substrate that resulted from the erosion of granite rock. This locality falls within the Mesomediterranean bioclimatic region, which is characterised by contrasting seasons (Rivas-Martínez et al. 2017). Thus, Monte de El Pardo has temperate and rainy springs (average 15.0 °C; 54 mm) with hot and dry summers (23.4 °C; 15 mm) (Díaz et al. 2006).
Sampling
We looked for lizards in sunny days between 9:30 a.m. and 7:30 p.m. by haphazardly walking throughout two sampling plots. One sampling plot was an area of 6.6 ha ranging between 0 and 150 m from a road (9,050 vehicles/day) (aka, low-quality plot), while the second plot was an area of 7.9 ha ranging between 350 and 550 m from the same road (aka, high-quality plot). This left a 200-m band between both sampling plots (Barrientos and Megía-Palma 2021). The size of the smallest of our sampling plots exceeded by approximately 75 times the maximum home range size described for the species, which is 0.09 ha (Díaz 1993).
Vegetation cover and structure
To investigate the vegetation structure of the two sampling plots, we performed 40 vegetation transects, each of 10-m long, in every sampling plot and in a single sampling event following Carrascal et al. (1989). Lizards are very abundant in this habitat system, and they can forage for long periods in the shadow (e.g., inside bushes) (Belliure et al. 1996). Thus, the vegetation transects performed are representative of microhabitats occupied by P. algirus. Each one of these 40 transects consisted of ten replicates of vegetation measurements that were performed every 1 m (N = 800 points). Every replicate had an approximate radius of 50 cm (Carrascal et al. 1989). In each one of these plots, we quantified the percentage of gramineous, bush, tree, and ground level vegetation covers, as well as the percentage of ground covered by leaf litter and sandy substrate (Carrascal et al. 1989). All vegetation cover percentages were visually estimated by a single researcher (RB). We carried out a principal component analysis (PCA) that included all the cover values. We performed varimax normalised rotation of the factors (Budaev 2010). In addition, we estimated the structural diversity of the different types of vegetation by calculating a Shannon–Weaver index. We analyzed the two principal factors extracted from the PCA (see results), and the vegetation structural diversity with three mixed models that included the plot as fixed effect and transect as random term.
Avian predator abundance
We estimated the abundance of birds that have been described as predators of P. algirus (Pica pica, Falco tinnunculus, Buteo buteo, and Hieraaetus pennatus) (Salvador 2015) by performing 13 censuses in each of the two sampling plots between the end of April and the beginning of July. Time expended per census and time of day when they were performed were randomised between the two plots. We summed the total number of aerial predators visually identified in every census in a belt of 100 m wide (50 m at every side of a progressing line). We calculated the kilometric abundance index (KAI) to standardise this metric because the length of the different sampling transects was variable (mean 340 m, range 112–840 m) (e.g., Paz et al. 2013). Differences in KAI were tested using Mann–Whitney U test based on the non-normal distribution of the data (visually evaluated with a histogram).
Experimental design
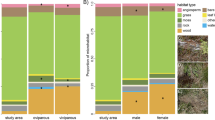
We captured 120 adult males, which were recognised by their coloration, proportionally bigger heads, and enlarged femoral pores. To minimise observer bias, blinded methods were use when data were recorded and/or analysed. Thus, males were randomly assigned to two treatments in each one of the two plots (30 males per treatment and plot): (i) control lizards, which were released in the same point where they had been captured (either in the low-quality plot –CLQ–, or in the high-quality plot –CHQ–), and (ii) translocated lizards, which were translocated to a random point in the opposite plot (i.e., release coordinate) and always at least 300 m from their capture point (translocated to high-quality plot –THQ–, and translocated to low-quality plot –TLQ–) (Fig. 1). This distance is above the average homing distance of mid-sized lacertids (Strijbosch et al. 1983). In fact, all but one lizard recaptured in 2018 moved between 3 and 131 m from their release point (mean ± SE = 27.98 ± 4.15 m). Therefore, our design ensured that the lizards did not move back to their area of origin (Megía-Palma et al. 2022). The sampling effort in 2018 was 23 days and took place between 24th of April and 2nd of July, which is a period of both maximum gonadal development and expression of the coloration in the males of this species (Díaz et al. 1994). Lizards were first recaptured in 2018, but we avoided recapturing lizards that had been captured less than 14 days before (range = 14–43 days) because this may be a necessary time to find changes in the coloration of lacertids (e.g., Megía-Palma et al. 2018b). We calculated the distance travelled by the lizards between the release coordinate and the recapture point as the Euclidean distance in metres between them, which were geo-referred with a GPS device (eTrex, Garmin, Olathe, KS, USA) (Barrientos and Megía-Palma, 2021). We always transported the lizards to the lab in individual cotton bags to collect all the data and were individually marked using a toe-clipping technique with a maximum of three clipped toes per lizard (maximum one per leg) (Langkilde and Shine 2006; Perry et al. 2011). A clean and sharp nail clipper was used. We checked that lizards stopped bleeding quickly. Photographs of the lizards were also made to assist in identification. During the next breeding season (2019), we resampled the area to recapture the individuals of the experiment. We performed a sampling effort in 2019 of 37 days in the same calendar days as the previous year.
Male lizards were sampled in two plots that differed in their habitat quality for lizards, due to the influence of distance to a road. Upper pictures show general overviews of the two sampling plots. During the first year, males were randomly assigned to treatments of soft translocation. Lizards were resampled within the same and the following breeding season. Four different experimental groups were created: control lizards in low-quality plot (CLQ), translocated lizards to high-quality plot (THQ), control lizards in high-quality plot (CHQ), and translocated lizards to low-quality plot (TLQ)
Quantification of ectoparasite and endoparasite abundances
We quantified the abundance of parasites from every lizard (sensu Rózsa et al. 2000) and at every capture. Mites of the genus Ophionyssus (Acari: Macronyssidae) were counted using a magnifying glass (× 10) in the field immediately after capture. This was done by checking the base of the tail, the interscale spaces in the belly of the lizards, as well as the tympani, and the mite pockets (Salvador et al. 1999; Barrientos and Megía-Palma 2021; Megía-Palma et al. 2022). In addition, we collected blood samples from the tail using sterile needles (Megía-Palma et al. 2016a). We smeared the blood samples (~ 5 µl) in microscope slides that were air-dried, fixed with methanol, and stained with Giemsa stain (azur-eosin methylene solution) and buffer at pH 7.2 (1:10, v/v) for 40 min. A single person (RM-P) used a light microscope (BX41TF; Olympus, Tokyo, Japan) to count blood parasites in a total of 10,000 erythrocytes per smear. These belonged to the genera Schellackia (Apicomplexa: Eimeriida) and Karyolysus (Apicomplexa: Adeleida). Both counts of ectoparasites and blood parasites were included in the models as raw count data.
Body condition
We used a transparent ruler to measure body length from the tip of the snout to the cloaca and tail length from the cloaca to the tip of the tail to the closest 1 mm. We weighed the lizards with a digital scale to the closest 0.01 g. We calculated body condition index as the residuals of the regression between the log10-transformed values of both body mass and length (Barrientos and Megía-Palma 2021). We included the length of the tail as covariate because 61% of the lizards in the population had regenerated tails and tail length was significantly correlated with body mass even when the analysis was controlled by body length (F1, 118 = 20.48, P < 0.001). Positive values of the body condition index calculated here corresponded to lizards being fatter than the sample mean for a given body length. This residual variable of body mass is statistically independent of body length.
Quantification of colour reflectance
We analysed colour patches of the lizards in two ways: (i) we scored the coloration of the males in six discrete groups attending to the extension of the colour patches following Díaz (1993); (ii) we used spectrophotometry to quantify the spectral reflectance of the orange patch on the right commissure of the mouth, the yellow patch on the throat, and the blue patch on the first and largest eyespot in the right flank of the lizards. To maintain the mouth commissure visible during the measurements, we offered one soft black rubber cap to the lizards that they grabbed with the mouth. We used a glass-fibre probe that was connected to a pulsed xenon lamp source (spectrophotometer Jaz DPU® Module, Ocean Optics Inc., Dunedin, FL, USA) and inserted in a black holder that reduced noise from environmental light. This facilitated measurements at a constant distance of 3 mm and 90° angle. Three consecutive measurements were taken per colour patch using a continuous strobe frequency of 10 ms, and a boxcar width of 4 (Megía-Palma et al. 2018b). We standardised the spectral measurements by comparing them to a 100% reflecting white-matte Teflon tape. We calculated the repeatability of the measurements by comparing the between- and within-subjects mean of squares of the three spectral measurements performed in each colour patch (Lessells and Boag 1987). We averaged the three spectra of each of the colour patches in wavelength bins of five nanometres using the CLR v1.1 software (Montgomerie 2009). We calculated luminance, chroma, and hue of the three colour patches (see Online Resource, Annex I) and calculated two values of spectral colour distance (SCD) (i.e., Endler 1990) as the Euclidean distance between the spectral variables of (i) the orange and yellow patches (hereafter ‘SCD_pigment’), and (ii) the three colour patches including the structural-based blue patch in the lateral eyespot (hereafter ‘SCD_all’) as follows:
Statistical analyses
Model averaging and z-standardisation of estimate coefficients
We ran all subsequent statistical models in R version 3.4.3 (R Core Team 2017). We tested normality, homoscedasticity, skewness, and kurtosis of the models on their residual errors. We applied a multimodel inference approach (package ‘MuMIn’; Barton 2018), which is a method of variable reduction recommended in ecology (Hegyi and Garamszegi 2011). In addition, we used an information criterion corrected for small sample sizes (Bedrick and Tsai 1994). We considered sufficiently informative all models with ΔAICc ≤ 4 in relation to the best model (i.e., the one with the lowest AICc) (Burnham and Anderson 2004). We checked if a null model was included within the list of most likely models under this criterium (it was never the case). Then, we summed the AICc weights of all the models where the predictor appeared (i.e., conditional average) to calculate the relative importance of each variable in the averaged model. Using this procedure, we also calculated the significance (α < 0.05) of the effects and their z-standardised ß coefficient ± adjusted standard error. The latter z-standardisation enables comparing the magnitude of the effects. For the non-mixed linear models (see the next subsection), we provide the adjusted R2 score. For the mixed models, which test predictors on the within-individual change, we provide the R2m (marginal) score of the final model extracted from model averaging. The R2 m depicts the proportion of the variance explained by the fixed predictors.
Spectral colour distance as predictor of individual quality
We applied a generalised model with ordinal multinomial distribution linked to a logit function to analyse the categorical variable of colour extension on the initial sample of 120 lizards. We analysed whether SVL, body condition, and SCD are significant predictors of the discrete groups of colour patch extension. We analysed if body length, body condition, and SCD are important traits that can explain the distance travelled by the lizards between captures. We controlled this analysis by adding the translocation treatment, the plot, and the number of elapsed days between captures (see Barrientos and Megía-Palma et al. 2021).
Within-year change in coloration
To investigate changes in the coloration within 2018, we analysed separately SCD_all and SCD_pigment. We used two-way within-subject mixed models of fixed slopes that fit Gaussian distribution (LMM). After this, we analysed the three spectral variables (luminance, chroma, and hue) for each of the three colour patches separately to finely identify predictors that significantly explain colour patch change during the breeding season (Online Resource, Annex II, Table S1). All models included the individual and the individual nested within treatment and within zone as random terms. As fixed terms, we included treatment and zone and their interaction, date of capture and recapture, SVL and body condition, abundances of mites and blood parasites, the two-way interactions between mites and Schellackia, and mites and Karyolysus, and the two-way interactions between body condition and (i) mites, (ii) Schellackia, and (iii) Karyolysus. We z-standardised all the variables of parasite counts by subtracting their mean to the parasite scores and dividing this by their standard deviation to increase the stability of the models (R Core Team 2017).
Between-year change in coloration
To analyse the changes in coloration between consecutive breeding seasons, we analysed in two ways the coloration of the lizards that were recaptured in 2019 (i.e., only CLQ and TLQ groups because only 5 lizards were recaptured in the plot far from the road). We analysed the change in discrete colour category with an ordinal multinomial mixed model linked to a logit function using the function ‘clmm’ of the R package ordinal (Christensen 2019) (Annex II, Table S2). Additionally, we analysed the between-years variation in SCD between colour patches with two separate mixed models for SCD_pigment and SCD_all and that fitted Gaussian distributions (LMM). We included in all models the individual and the individual nested within the treatment as random factors to control within-subject effects (Cnaan et al. 1997). As fixed factor, we included the treatment. We also included the body condition and the abundances of mites, Schellackia, and Karyolysus, and the two-way interactions between them. We z-standardised the predictors to improve the stability of the model (R Core Team 2017).
Between-years change in parasite abundances
To analyse the change in the abundance of the different parasites between years in the CLQ and TLQ groups, we performed generalised mixed models (GLMM) for Karyolysus and mites, which were best fit with binomial negative distribution of residual errors and log link function (Pennycuick 1971). However, the model performed to analyse the change between years of Schellackia abundance best fit a model with Gamma distribution of errors that was linked to a log function, after the 2 + log10-transformation of the infection scores. The three models included the body condition and the z-standardised values of date, treatment, and the abundances of the other two parasites. They included the individual nested within treatment as random effect (Annex II, Table S3).
Results
Vegetation structure and predator abundance
The PCA reduced our original variables of vegetation cover to two principal components that retained 72.56% of the variance. PC1 (eigenvalue = 2.82; variance retained = 46.94%) was positively related to bush and leaf litter covers, and negatively related to sand cover (Table S4). PC2 (eigenvalue = 1.54; variance retained = 25.62%) was positively related to herbaceous and ground level vegetation (Table S4). PC1(χ21, 78 = 14.10, P < 0.001) and PC2 (χ21, 78 = 54.39, P < 0.001) differed significantly between sampling plots. Close to the road, vegetation development was scarcer, as the sand cover was greater while bush cover was smaller, while far from the road both herb and ground vegetation covers were greater (Table S5). In addition, the vegetation structural diversity (Shannon–Weaver) was lower close to the road (χ21, 78 = 49.41, P < 0.0001, Mean low-quality plot ± Standard Error = 1.04 ± 0.02, Mean high-quality plot = 1.30 ± 0.01). Aerial predators were more abundant close to the road (IKA, mean ± SE = 2.30 ± 0.56) than far from it (IKA = 0.83 ± 0.65) (Z1, 24 = 2.25, P = 0.02).
Parasitic infections at first capture
Mites infested 95% of the lizards in the initial sample of 120 males (abundance range = 0–64 mites). We found two genera of blood parasites: Karyolysus that infected 80.0% of the lizards (abundance range = 0–133 blood parasites) and Schellackia that infected 31.7% of the lizards (abundance range = 0–157 blood parasites) (χ21, 118 = 58.32, P < 0.001; Annex III, Fig. S1).
Initial coloration and spectral colour distance as measures of individual quality
The within-individual repeatability of the spectral measurements was above 94% in the three colour patches. The coloration in the initial sample of 120 males was similar in low-quality and high-quality sampling plots (SCD_all: F1, 118 = 1.14, P = 0.28; SCD_pigment: F1, 118 = 2.30, P = 0.13). Both SCD_all and SCD_pigment, as well as body size and condition, were significant predictors of the category of colour patch extension in the initial sample of 120 adult males (R2 = 0.24; Table 1). Namely, lizards with the smallest colour patch extension (colour groups 1 and 2) also had significantly higher SCD between colour patches than the lizards ranked in groups 3 to 6 (Annex III, Fig. S2). Lizards in groups 1–3 were smaller, while those in groups 1 and 2 had body condition below average (Fig. 2). Both SCD_all (β = 0.36, F1, 32 = 5.37, P = 0.027; R2 = 0.33) and SCD_pigment (β = 0.39, F1, 32 = 6.89, P = 0.013; R2 = 0.34) significantly explained the distance travelled by the lizards independently of the treatment applied, as lizards with more elaborated coloration (i.e., lower SCD) tended to stay closer to their release point.
a Discrete groups according to Díaz (1993) based on the colour patch extension of the initial sample of 120 males. The spectral profiles reveal that the variation in male coloration is continuous, and not discrete. b Body length and c body condition significantly varied among colour groups as indicated by an ordinal multinomial model. The grey dotted line in c indicates the mean residual body condition of the 120 sampled males. All values represented show mean ± standard error
Within-year change in coloration
Even though lizards were randomly assigned to the translocation treatments, control lizards close to the road had significantly lower SCD initial scores (i.e., more coloration) than the other three groups (SCD_all: F3,116 = 4.31, P = 0.040; SCD_pigment: F3, 116 = 4.79, P = 0.030). Within the breeding season of 2018, we were able to recapture 40 males (11 CHQ + 9 THQ; and 9 CLQ + 11 TLQ). The two-way within-subject mixed model for both SCD_all and SCD_pigment consistently showed a significant effect of the interaction of the treatment with the plot (treatment*plot: SCD_all: z1, 36 = 2.42, P = 0.016, R2m = 0.12; SCD_pigment: z1, 36 = 2.14, P = 0.032, R2m = 0.11), with no significant effect of other predictors (Annex II, Table S1). That is, no effect of the treatment was observed on the coloration between CLQ and THQ lizards. However, we found a differential effect of the translocation treatment on the coloration between CHQ and TLQ lizards. Here, lizards from the TLQ group increased SCD — i.e., their coloration became duller at recapture — while lizards from the CHQ group intensified their coloration. This effect on the coloration was observed only in the lizards from the TLQ group (Fig. 3).
Within-year changes in nuptial coloration (z-standardised). For simplicity, the graph only shows the interaction effect between sampling plot and translocation treatment on SCD_pigment, but the treatment had similar significant effect also on SCD_all (see Table S1). Note that the lizards translocated to the low-quality plot (TLQ group) exhibited reduced coloration within the breeding season of 2018 (i.e., tended to increase SCD) as compared to CHQ lizards. The grey dotted line indicates null change. SCD spectral colour distance, CLQ control lizards in low-quality plot, THQ translocated lizards to high-quality plot, CHQ control lizards in high-quality plot, TLQ translocated lizards to low-quality plot
Between-years change in body condition
The change in body condition between years was not explained by the effect of the translocation (z1,39 = 0.44, P = 0.66). Only the change in mite abundance had a marginally non-significant negative effect (importance = 0.59, estimate = − 0.009, z = 1.81, P = 0.070; Table S6 in Annex II, Online Resource).
Between-year change in coloration
We were able to recapture 17 lizards in the low-quality plot close to the road the second breeding season: eight CLQ and nine TLQ lizards. We removed from the analyses the five lizards we captured in the high-quality plot far from the road due to low sample size. Both SCD_all (R2m = 0.60) and SCD_pigment (R2m = 0.67) models consistently showed that the interaction effect between the changes in the abundances of Karyolysus and Schellackia on the coloration was significant (Table 2). The interaction plots representing the change in the abundance of infection by the two blood parasites in relation to the change in SCD showed that the capability of the lizards to produce a complex coloration was putatively constrained by the between-years increase in the intensity of infection by Schellackia because those lizards that reduced the number of this parasite produced more complex coloration the following spring (dark red area in Fig. 4a, b). Similar results were obtained for the interaction between mites and Karyolysus (Table 2; Fig. 4c, d); that is, lizards reducing the number of mites between years produced more complex coloration in 2019. In opposition, lizards with both increased number of mites and Schellackia produced a duller coloration, although the hematic parasite showed a stronger effect (Fig. 4e). Interestingly, the interaction plots showed a positive relationship between the change in coloration and the abundance of Karyolysus. These models also showed a consistent effect of the change in body condition on the nuptial coloration of the male lizards in 2019 (Table 2; Fig. 4f). This relationship was also supported by the significant increase in both (i) the pigmentary component of the coloration (decreasing SCD_pigment) (Table 2; Fig. 5a) and (ii) and the abundance of infection by Karyolysus (z1,11 = 2.27, P = 0.023) in 2019 in lizards from the TLQ group as compared to those from the CLQ group (Fig. 5b; Annex II, Table S3).
a–e Double interactions showing the relationship of the change in the different parasite abundances (number of parasites) and the within-individual change in the nuptial coloration (NC) of male P. algirus between years as indicated by model averaging. SCD spectral Euclidean distance between colour patches. f Relationship between the changes in body condition and SCD. Warmer colours indicate more developed coloration
Mean ± SE changes between years in coloration and parasitisation of P. algirus as analysed by GLMM. a Male coloration significantly increased (mean ± SE SCD decreased) in 2019 in lizards that had been translocated to the low-quality plot in 2018 (TLQ group) as compared with controls in that plot (CLQ group). b Parasites of the genus Karyolysus significantly increased in 2019 in the same lizards. However, no effect of the treatment (including here only CLQ and TLQ groups due to sample size) was detected for the parasitaemia of Schellackia (z = 0.013, P = 0.99), or mites (z = 0.008, P = 0.99). The grey dotted line in b indicates null change
Discussion
Only TLQ lizards reduced their coloration during the first season, despite the negative effect of translocation on the body condition of both TLQ and THQ lizards (Barrientos and Megía-Palma 2021). The lack of significant effect on the colour patches of THQ lizards suggests that the more favourable environmental conditions far from the road may have cushioned the negative effects of translocation on the coloration of the lizards. Colour patch pigmentation in P. algirus can rapidly reflect metabolic traumas (e.g., immune challenge; Llanos-Garrido et al. 2017). Our results suggest that translocation of lizards to poor-quality habitats may also negatively impact the pigmentation of their colour patches. This is consistent with the idea that although some good-quality habitat patches can remain within the low-quality plot, our experiment did capture the overall effect of the worse environmental conditions for P. algirus close to the road. These results are not however reflecting direct effects of the road on the lizards but likely road-zone effects instead. Roads may increase human activity in nearby habitats, which can increase the negative impact on resident populations (Van der Ree et al. 2015; Whittington et al. 2019). In this sense, previous investigations demonstrated that small lizards avoid crossing roads (Tellería et al. 2011; Hibbitts et al. 2017), suggesting that they perceive roads as sources of danger. Moreover, the lizards’ perception of a greater predation risk in the presence of sparse vegetation or close to roads may alter their behaviour and have negative physiological impacts on them (Pérez-Tris et al. 2004; Amo et al. 2007; Kechnebbou et al. 2019). In further support of the negative impact of this road on the studied population of lizards, a previous survey of faecal glucocorticoid metabolites indicated a lower metabolic activity and possibly worse physiological capacity to respond to stress stimuli in the lizards captured close to the road (Megía-Palma et al. 2022; see also Owen et al. 2014).
The initial pigmentation (mean ± SE SCD) of the colour patches of the lizards in the CLQ group was greater than in the remaining groups. However, the change in coloration during the breeding season did not differ from that observed in the THQ group, which came from the same plot, suggesting that the initial differences do not undermine our findings. Indeed, the significant differences in coloration change within the first year of the experiment were found between the CHQ and the TLQ. Furthermore, the significant effects of the changes in the abundances of the different parasites studied on the production of the colour patches in 2019 in the lizards recaptured close to the road were statistically independent from the effects of the experimental group assigned during 2018, reinforcing the idea that initial differences in coloration did not affect our results.
Analysis of the luminance, chroma, and hue of the three colour patches within 2018 season revealed that the detected changes were mainly explained by the effect of the experiment on the chroma of both yellow and blue patches, as well as on the hue of the latter patch. This suggests that the allocation of carotenoids and melanin to yellow and blue patches, respectively, may plastically vary for needs other than signalling during the breeding season (e.g., Brawner et al. 2000). Furthermore, the observed changes in the reflectance properties of the blue coloration support previous findings suggesting that this patch can reflect stress levels in this species (Mészáros et al. 2019). Contrarily to the yellow and blue patches, no significant effect of the treatment on the orange patch was detected. However, the within-year change of its spectral variables (luminance, chroma, and hue) was explained by date. This suggests that the putative allocation of pterins to the orange patch decreased during the breeding season because the luminance of this colour patch increased (estimate = + 162.81), its chroma decreased (estimate = − 4.52), and its hue decreased (estimate = − 4.51) with date (Annex II, Table S7) (Megía-Palma et al. 2021). This further supports the concept that pigment allocation to colour patches of P. algirus is seasonal (Díaz et al. 1994; Carretero 2002) and likely influenced by hormones (Salvador et al. 1996).
Our analyses indicated that those males with higher development of colour patches were recaptured closer to the release point even if they had been translocated. This suggested that SCD correlated with reproductive investment and/or male competition ability (territoriality) in this population. Interestingly, during the second breeding season, lizards from TLQ group significantly increased their pigmentation and the infection by Karyolysus as compared to lizards from the CLQ group (Fig. 5), suggesting that the higher allocation of pigment to colour patches was associated with increased susceptibility to some parasites in line with a handicapping mechanism (Salvador et al. 1996; Megía-Palma et al. 2016b, 2021). Nonetheless, future experimental manipulation of the reproductive effort and/or the parasite load of the lizards is needed to fully discriminate their effects on coloration (e.g., Megía-Palma et al. 2018b). The abundances of mites and Schellackia did not change significantly between consecutive breeding seasons either in CLQ or TLQ lizards, suggesting that mites and Schellackia represent virulent infections in this population that need to remain under immunological control between years to maximise the reproductive effort in males of P. algirus in terms of colour patch production.
The variance explained by the model that analysed the between-year changes in the spectral properties of the nuptial coloration more than doubled the one explained by the model that analysed within-year changes. This supports the idea that the production of the nuptial coloration in P. algirus is strongly influenced by life trajectories in previous seasons. The significant interactions of the different parasites on the production of male coloration in P. algirus (CLQ and TLQ; Fig. 4) indicated a significant effect of the change in parasite abundances on the expression of colour patches. That is, lizards suffering a simultaneous increase of the infections by mites and Schellackia produced duller coloration (i.e., increased SCD = light blue in Fig. 4e), supporting the idea that pigment allocation to coloration of male P. algirus is offset by other functions in the face of mite–Schellackia coinfections. Similarly, lizards that increased their body condition between years produced a more elaborated coloration. This was congruent with the initial data of 120 males where lizards with higher development of the nuptial coloration had higher body condition than the average of the sample (Fig. 2) and it evidences that those male lizards that stored more reserves during past years can increase allocation of pigments to production of colour patches. Interestingly, the latter relationship only occurred in lizards where intensities of infection by one of the parasites were controlled (dark red in Fig. 4); lizards that simultaneously reduced the intensities of two of the parasites suffered a reduction of their pigmentation (light blue in Fig. 4a–c). This outcome suggested that colour production was only constrained when fighting off more than one infection. However, the production of nuptial coloration was not constrained in lizards with better body condition that fought off only one of the infections.
Our results support that habitat quality can compensate costs on coloration of lizards suffering from stress situations, such as translocation to a new environment and sudden loss of body condition. Male coloration reflected trade-offs between body condition and parasite infection. However, some parasites may escape immune defences and increase their number when hosts allocate pigments to colour patches (Megía-Palma et al. 2021). The rather complex relationships found between different parasites, body condition, and coloration suggested that parasitic infections can pose differential effects on the production of the colour patches of P. algirus. These differential effects suggested that the parasites differ in virulence in this host population.
Further implications and future directions
In a wider view, the taxon P. algirus is integrated by two mitochondrial lineages with a geographically well-structured distribution in the Iberian Peninsula (Carranza et al. 2006, 2010). One key phenotypic difference between males of these two lineages is the extension of the sexual coloration, which is smaller (and putatively with a lower investment of pigments) in the eastern clade (Carretero 2002; Moreno-Rueda et al. 2021). The reasons for this difference are unclear, but in line with ours and previous results, a combination of environmental and intrinsic factors can influence the expression of male sexual coloration of P. algirus (e.g., Salvador et al. 1996; Llanos-Garrido et al. 2017; Moreno-Rueda et al. 2021). In this sense, a recent correlational analysis of loci under selection across a climatic gradient supported that environmental factors can accurately predict the distributional range of this species (Llanos-Garrido et al. 2021). Parasites can also exert selective pressure in host populations as well as mediate sexual selection (Clayton 1991; Zuk 1992; Maan and Seehausen 2011). However, the susceptibility of the two lineages of P. algirus to environmental stress and parasitic infections, and the effect that parasites have on them, remains poorly explored (e.g., Megía-Palma et al. 2022). Environmental factors exert genetic selection on lizards and likely on their phenotypic expression. For example, our study shows a negative effect of a road on the nearby habitat and its influence on the expression of the sexual coloration in lizards that suffered an additional stress stimulus (translocation). Moreover, recent research showed that epistatic interactions between pteridine and carotenoid regulatory genes influence colour patch expression in lacertids (Aguilar et al. 2022). Therefore, as phenotypic traits result from the interaction between genotype and environment, whether the differential expression of male sexual coloration between the two lineages of P. algirus is influenced by the environment guarantees further investigation. In this sense, the eastern and western geologic facets of the Iberian Peninsula offer an interesting ecological scenario to investigate how differences in primary productivity, carotenoid availability, and parasitic pressure can influence the expression of colour patches in this and other species. For example, variation in pteridine/carotenoid balance in the colour patches of agamid lizards from Australia was explained by the environmental availability of carotenoids across a climatic gradient (Stuart-Fox et al. 2021). Although this resulted in an effective production of the colour badge in the lizards, the differences in pigment balance across sites did not necessarily result in the expression of colour patches with the same hue (Stuart-Fox et al. 2021). Similarly, endogenous synthesis of pteridine pigments can compensate the otherwise reduced expression of carotenoid-based colour patches in fish due to an environmental shortage in the latter pigments (Grether et al. 2005). Phenotypic expression of sexual coloration in lizards may be constrained by food availability and immune challenges (Cote et al. 2010). We argue that parasitic infections represent immune challenges for lizards and some of which may increase under climate warming (e.g., Jones et al. 2016; Megía-Palma et al. 2020). Therefore, we posit pigments deposited in colour patches of lizards as candidate biomarkers of environmental quality.
Data availability
See supplementary files.
References
Aguilar P, Andrade P, Pérez i de Lanuza G (2022) Epistatic interactions between pterin and carotenoid genes modulate intra-morph color variation in a lizard. Integr Zool 17:44–53
Alonso-Álvarez C, Pérez-Rodríguez L, Mateo R, Chastel O, Vinuela J (2008) The oxidation handicap hypothesis and the carotenoid allocation trade-off. J Evol Biol 21:1789–1797
Amo L, López P, Martín J (2007) Habitat deterioration affects antipredatory behavior, body condition, and parasite load of female Psammodromus algirus lizards. Can J Zool 85:743–751
Andrade P, Pinho C, Pérez i de Lanuza G et al (2019) Regulatory changes in pterin and carotenoid genes underlie balanced color polymorphisms in the wall lizard. P Natl Acad Sci U S A 116:5633–5642
Barrientos R, Megía-Palma R (2021) Associated costs of mitigation-driven translocation in small lizards. Amphibia-Reptilia 42:275–282
Barton K (2018) MuMIn: Multi-Model Inference. R package version 1.40.4. https://CRAN.R-project.org/package=MuMIn
Bedrick EJ, Tsai CL (1994) Model selection for multivariate regression in small samples. Biometrics 50:226–231
Beilharz RG, Luxford BG, Wilkinson JL (1993) Quantitative genetics and evolution: is our understanding of genetics sufficient to explain evolution? J Anim Breed Genet 110:161–170
Belliure J, Carrascal LM, Diaz JA (1996) Covariation of thermal biology and foraging mode in two Mediterranean lacertid lizards. Ecology 77:1163–1173
Biard C, Saulnier N, Gaillard M, Moreau J (2010) Carotenoid-based bill colour is an integrative signal of multiple parasite infection in blackbird. Naturwissenschaften 97:987–995
Brawner WR III, Hill G, Sundermann CA (2000) Effects of coccidial and mycoplasmal infections on carotenoid-based plumage pigmentation in male house finches. Auk 117:952–963
Budaev SV (2010) Using principal components and factor analysis in animal behaviour research: caveats and guidelines. Ethology 116:472–480
Burnham KP, Anderson DR (2004) Multimodel inference, understanding AIC and BIC in model selection. Sociol Method Res 33:261–304
Cantarero A, Alonso-Alvarez C (2017) Mitochondria-targeted molecules determine the redness of the zebra finch bill. Biol Lett 13:20170455
Carranza S, Harris DJ, Arnold EN, Batista V, González De La Vega JP (2006) Phylogeography of the lacertid lizard, Psammodromus algirus, in Iberia and across the Strait of Gibraltar. J Biogeogr 33:1279–1288
Carranza S, Salvador A, Verdú-Ricoy J, Díaz J, Busack S (2010) Phylogeography of Psammodromus algirus (Lacertidae) revisited: systematic implications. Amphibia-Reptilia 31:576–582
Carrascal LM, Diaz JA, Cano C (1989) Habitat selection in Iberian Psammodromus species along a Mediterranean successional gradient. Amphibia-Reptilia 10:231–242
Carretero MA (2002) Sources of colour pattern variation in Mediterranean Psammodromus algirus. Neth J Zool 52:43–60
Christensen RHB (2019) Ordinal—regression models for ordinal data. R package version 2019.12–10. https://CRAN.R-project.org/package=ordinal
Clayton DH (1991) The influence of parasites on host sexual selection. Parasitol Today 7:329–334
Cnaan A, Laird NM, Slasor P (1997) Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat Med 16:2349–2380
Cote J, Le Galliard JF, Rossi JM, Fitze PS (2008) Environmentally induced changes in carotenoid-based coloration of female lizards: a comment on Vercken et al. J Evol Biol 21:1165–1172
Cote J, Meylan S, Clobert J, Voituron Y (2010) Carotenoid-based coloration, oxidative stress and corticosterone in common lizards. J Exp Biol 213:2116–2124
Cuervo JJ, Belliure J, Negro JJ (2016) Coloration reflects skin pterin concentration in a red-tailed lizard. Comp Biochem Phys B 193:17–24
Del Cerro S, Merino S, Martínez-de la Puente J, Lobato E, Ruiz-de-Castañeda R, Rivero-de Aguilar J, Martínez J, Morales J, Tomás G, Moreno J (2010) Carotenoid-based plumage colouration is associated with blood parasite richness and stress protein levels in blue tits (Cyanistes caeruleus). Oecologia 162:825–835
Díaz JA (1993) Breeding coloration, mating opportunities, activity, and survival in the lacertid lizard Psammodromus algirus. Can J Zool 71:1104–1110
Díaz JA (1997) Ecological correlates of the thermal quality of an ectotherm’s habitat: a comparison between two temperate lizard populations. Funct Ecol 11:79–89
Díaz JA, Alonso-Gómez AL, Delgado MJ (1994) Seasonal variation of gonadal development, sexual steroids, and lipid reserves in a population of the lizard Psammodromus algirus. J Herpetol 28:199–205
Díaz JA, Iraeta P, Monasterio C (2006) Seasonality provokes a shift of thermal preferences in a temperate lizard, but altitude does not. J Therm Biol 31:237–242
Díaz JA, Verdú-Ricoy J, Iraeta P, Llanos-Garrido A, Pérez-Rodríguez A, Salvador A (2017) There is more to the picture than meets the eye: adaptation for crypsis blurs phylogeographical structure in a lizard. J Biogeogr 44:397–408
Endler JA (1990) On the measurement and classification of colour in studies of animal colour patterns. Biol J Linn Soc 41:315–352
Fitze PS, Cote J, San-Jose LM, Meylan S, Isaksson C, Andersson S, Rossi JM, Clobert J (2009) Carotenoid-based colours reflect the stress response in the common lizard. PLoS ONE 4:e5111
Folstad I, Hope AM, Karter A, Skorping A (1994) Sexually selected color in male sticklebacks: a signal of both parasite exposure and parasite resistance? Oikos 69:511–515
Grether GF, Cummings ME, Hudon J (2005) Countergradient variation in the sexual coloration of guppies (Poecilia reticulata): drosopterin synthesis balances carotenoid availability. Evolution 59:175–188
Hamilton WD, Zuk M (1982) Heritable true fitness and bright birds: a role for parasites? Science 218:384–387
Hegyi G, Garamszegi LZ (2011) Using information theory as a substitute for stepwise regression in ecology and behavior. Behav Ecol Sociobiol 65:69–76
Hibbitts TJ, Fitzgerald LA, Walkup DK, Ryberg WA (2017) Why didn’t the lizard cross the road? Dunes sagebrush lizards exhibit road-avoidance behaviour. Wildlife Res 44:194–199
Hill GE, Hill GE (2002) A red bird in a brown bag: the function and evolution of colorful plumage in the house finch. Oxford University Press, Oxford
Johansen IB, Henriksen EH, Shaw JC, Mayer I, Amundsen PA, Øverli Ø (2019) Contrasting associations between breeding coloration and parasitism of male Arctic charr relate to parasite species and life cycle stage. Sci Rep 9:10679
Johnson AM, Fuller RC (2015) The meaning of melanin, carotenoid, and pterin pigments in the bluefin killifish, Lucania goodei. Behav Ecol 26:158–167
Jones AR, Bull CM, Brook BW, Wells K, Pollock KH, Fordham DA (2016) Tick exposure and extreme climate events impact survival and threaten the persistence of a long-lived lizard. J Anim Ecol 85:598–610
Jørgensen C, Ernande B, Fiksen Ø, Dieckmann U (2006) The logic of skipped spawning in fish. Can J Fish Aquat Sci 63:200–211
Kechnebbou M, Martin J, Chammem M, Arregui L, Nouira S (2019) Roads and urban areas as physiological stressors of spiny-tailed lizards Uromastyx Acanthinura. J Arid Environ 170:103997
Knowles SC, Nakagawa S, Sheldon BC (2009) Elevated reproductive effort increases blood parasitaemia and decreases immune function in birds: a meta-regression approach. Funct Ecol 23:405–415
Langkilde T, Shine R (2006) How much stress do researchers inflict on their study animals? A case study using a scincid lizard, Eulamprus heatwolei. J Exp Biol 209:1035–1043
Lessells CM, Boag PT (1987) Unrepeatable Repeatabilities: a Common Mistake. Auk 104:116–121
Llanos-Garrido A, Briega-Álvarez A, Pérez-Tris J, Díaz JA (2021) Environmental association modelling with loci under divergent selection predicts the distribution range of a lizard. Mol Ecol 30:3856–3868
Llanos-Garrido A, Díaz JA, Pérez-Rodríguez A, Arriero E (2017) Variation in male ornaments in two lizard populations with contrasting parasite loads. J Zool 303:218–225
Maan ME, Seehausen O (2011) Ecology, sexual selection and speciation. Ecol Lett 14:591–602
Masó G, Vicente-Sastre D, Fitze PS (2022) Intrinsic climatic predictability affects ornamental coloration of adult males: evidence for compensation among carotenoid-and melanin-based coloration. Funct Ecol 36:1087–1097
McGraw KJ (2005) The antioxidant function of many animal pigments: are there consistent health benefits of sexually selected colourants? Anim Behav 69:757–764
Megía-Palma R, Arregui L, Pozo I, Žagar A, Serén N, Carretero MA, Merino S (2020) Geographic patterns of stress in insular lizards reveal anthropogenic and climatic signatures. Sci Total Environ 749:141655
Megía-Palma R, Barja I, Barrientos R (2022) Fecal glucocorticoid metabolites and ectoparasites as biomarkers of heat stress close to roads in a Mediterranean lizard. Sci Total Environ 802:149919
Megía-Palma R, Barrientos R, Gallardo M, Martínez J, Merino S (2021) Brighter is darker: the Hamilton-Zuk hypothesis revisited in lizards. Biol J Linn Soc 134:461–473
Megía-Palma R, Jorge A, Reguera S (2018a) Raman spectroscopy reveals the presence of both eumelanin and pheomelanin in the skin of lacertids. J Herpetol 52:67–73
Megía-Palma R, Martínez J, Merino S (2016a) Structural- and carotenoid-based throat colour patches in males of Lacerta schreiberi reflect different parasitic diseases. Behav Ecol Sociobiol 70:2017–2025
Megía-Palma R, Martínez J, Merino S (2016b) A structural colour ornament correlates positively with parasite load and body condition in an insular lizard species. Sci Nat 103:52
Megía-Palma R, Martínez J, Merino S (2018b) Manipulation of parasite load induces significant changes in the structural-based throat color of male Iberian green lizards. Curr Zool 64:293–303
Megía-Palma R, Paranjpe D, Reguera S, Martínez J, Cooper RD, Blaimont P, Merino S, Sinervo B (2018c) Multiple color patches and parasites in Sceloporus occidentalis: Differential relationships by sex and infection. Curr Zool 64:703–711
Merkling T, Chandrasoma D, Rankin KJ, Whiting MJ (2018) Seeing red: pteridine-based colour and male quality in a dragon lizard. Biol J Linn Soc 124:677–689
Mészáros B, Jordán L, Bajer K, Martín J, Török J, Molnár O (2019) Relationship between oxidative stress and sexual coloration of lizards depends on thermal habitat. Sci Nat 106:55
Miñano MR, While GM, Yang W, Burridge CP, Sacchi R, Zuffi M, Scali S, Salvi D, Uller T (2021) Climate shapes the geographic distribution and introgressive spread of color ornamentation in common wall lizards. Am Nat 198:379–393
Møller AP (1989) Natural and sexual selection on a plumage signal of status and on morphology in house sparrows, Passer domesticus. J Evol Biol 2:125–140
Montgomerie R (2009) CLR, version 1.1. Queen’s University, Kingston, Canada. http://post.queensu.ca/~mont/color/analyze.html
Moreno-Rueda G, Reguera S, Zamora-Camacho FJ, Comas M (2021) Inter-individual differences in ornamental colouration in a Mediterranean lizard in relation to altitude, season, sex, age, and body traits. Diversity 13:158
Mougeot F, Pérez-Rodríguez L, Sumozas N, Terraube J (2009) Parasites, condition, immune responsiveness and carotenoid-based ornamentation in male red-legged partridge Alectoris rufa. J Avian Biol 40:67–74
Nordling D, Andersson M, Zohari S, Lars G (1998) Reproductive effort reduces specific immune response and parasite resistance. Proc R Soc Lond B 265:1291–1298
Olsson M, Stuart-Fox D, Ballen C (2013) Genetics and evolution of colour patterns in reptiles. Sem Cell Dev Biol 24:529–541
Owen DA, Carter ET, Holding ML, Islam K, Moore IT (2014) Roads are associated with a blunted stress response in a North American pit viper. Gen Comp Endocr 202:87–92
Paz A, Jareño D, Arroyo L, Viñuela J, Arroyo B, Mougeot F, Luque-Larena JJ, Fargallo JA (2013) Avian predators as a biological control system of common vole (Microtus arvalis) populations in north-western Spain: experimental set-up and preliminary results. Pest Manag Sci 69:444–450
Pennycuick L (1971) Frequency distributions of parasites in a population of three-spined sticklebacks, Gasterosteus aculeatus L., with particular reference to the negative binomial distribution. Parasitology 63:389–406
Pérez-Tris J, Díaz JA, Tellería JL (2004) Loss of body mass under predation risk: cost of antipredatory behaviour or adaptive fit-for-escape? Anim Behav 67:511–521
Perry G, Wallace MC, Perry D, Curzer H, Muhlberger P (2011) Toe clipping of amphibians and reptiles: science, ethics, and the law. J Herpetol 45:547–555
R Core Team (2017) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Reguera S, Zamora-Camacho FJ, Moreno-Rueda G (2014) The lizard Psammodromus algirus (Squamata: Lacertidae) is darker at high altitudes. Biol J Linn Soc 112:132–141
Rivas-Martínez S, Penas Á, del Río S, González TED, Rivas-Sáenz S (2017) Bioclimatology of the Iberian Peninsula and the Balearic Islands. In: Loidi J (ed) The vegetation of the Iberian Peninsula. Springer, Cham, pp 29–80
Rózsa L, Reiczigel J, Majoros G (2000) Quantifying parasites in samples of hosts. J Parasitol 86:228–232
Salvador A (2015) Lagartija colilarga - Psammodromus algirus. In: Salvador A, Marco A (eds) Enciclopedia Virtual de los Vertebrados Españoles. Museo Nacional de Ciencias Naturales, Madrid. http://www.vertebradosibericos.org/
Salvador A, Veiga JP, Civantos E (1999) Do skin pockets of lizards reduce the deleterious effects of ectoparasites? An experimental study with Psammodromus algirus. Herpetologica 55:1–7
Salvador A, Veiga JP, Martin J, Lopez P, Abelenda M, Puerta M (1996) The cost of producing a sexual signal: testosterone increases the susceptibility of male lizards to ectoparasitic infestation. Behav Ecol 7:145–150
Shine R, Schwarzkopf L (1992) The evolution of reproductive effort in lizards and snakes. Evolution 46:62–75
Strijbosch H, van Rooy PTJ, Voesenek LACJ (1983) Homing behaviour of Lacerta agilis and Lacerta vivipara (Sauria, Lacertidae). Amphibia-Reptilia 4:43–47
Stuart-Fox D, Rankin KJ, Lutz A, Elliott A, Hugall AF, McLean CA, Medina I (2021) Environmental gradients predict the ratio of environmentally acquired carotenoids to self-synthesised pteridine pigments. Ecol Lett 24:2207–2218
Sumasgutner P, Adrion M, Gamauf A (2018) Carotenoid coloration and health status of urban Eurasian kestrels (Falco tinnunculus). PLoS ONE 13:e0191956
Tellería JL, Díaz JA, Pérez-Tris J, De Juana E, De la Hera I, Iraeta P, Salvador A, Santos T (2011) Barrier effects on vertebrate distribution caused by a motorway crossing through fragmented forest landscape. Anim Biodiv Conserv 34:331–340
Tibbetts EA, Dale J (2004) A socially enforced signal of quality in a paper wasp. Nature 432:218–222
Van Der Ree R, Smith DJ, Grilo C (2015) Handbook of road ecology. John Wiley & Sons, Hoboken
Weiss SL, Foerster K, Hudon J (2012) Pteridine, not carotenoid, pigments underlie the female-specific orange ornament of striped plateau lizards (Sceloporus virgatus). Comp Biochem Phys B 161:117–123
Whittington J, Low P, Hunt B (2019) Temporal road closures improve habitat quality for wildlife. Sci Rep 9:3772
Zuk M (1992) The role of parasites in sexual selection: current evidence and future directions. Adv Stud Behav 21:39–68
Acknowledgements
C. Ponce, I. Pozo, S. Reguera, M. Fernández, Z. Rohrer, C. Luque, and P. Quiles helped with logistics. Two reviewers improved the manuscript.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. RMP received a contract (CEECIND/04084/2017) by BIOPOLIS – Program in Genomics, Biodiversity and Land Planning and Fundação para a Ciência e a Tecnologia and RB a postdoctoral grant from Comunidad de Madrid (2018T1/AMB10374).
Author information
Authors and Affiliations
Contributions
RMP and RB designed methodology; RB and RMP collected the data; RMP and SM analyzed the data. All authors contributed critically to the draft and gave final approval for publication.
Corresponding author
Ethics declarations
Ethical approval
All applicable international, national, and/or institutional guidelines for the use of animals were followed. Handling protocols and general ethics of our research were approved by the ethic committee of University Complutense de Madrid (Ref. 5005), and by Comunidad de Madrid (Ref. 10/165944.9/18, PROEX 271/19) in accordance with current Spanish laws.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by S. Joy Downes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Megía-Palma, R., Merino, S. & Barrientos, R. Longitudinal effects of habitat quality, body condition, and parasites on colour patches of a multiornamented lizard. Behav Ecol Sociobiol 76, 73 (2022). https://doi.org/10.1007/s00265-022-03182-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-022-03182-w