Abstract
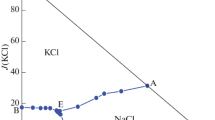
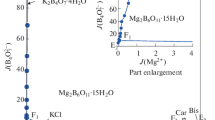
Solid-liquid phase equilibria of ternary system (LiBO2 + NaBO2 + H2O) and quaternary system (NaBO2 + NaCl + Na2SO4 + H2O) at 323.15 K and 0.1 MPa were determined by isothermal solution equilibrium method. The experimental results show that there are one invariant point, two univariant solubility curves and two crystallization regions in the ternary system (LiBO2 + NaBO2 + H2O). The phase region of LiBO2·2H2O is the largest, indicating that its solubility is the smallest, and no double salt and solid solution was formed in this system. In the quaternary system (NaBO2 + NaCl + Na2SO4 + H2O), there are two invariant points, five univariant solubility curves, and four crystallization regions. Among them, the crystallization region of Na2SO4 is the largest, and the double salt (NaCl·NaBO2·2H2O) is formed in the system. The physicochemical properties including refractive index, density and pH value of saturated solutions in the ternary system and quaternary system all changes regularly with the increasing of NaBO2/NaCl concentration.







Similar content being viewed by others
REFERENCES
Y. Liu, X. Liu, S. Liu, et al., Angew. Chem. Int. Edit. 59, 7793 (2020). https://doi.org/10.1002/anie.202001042
P. M. Wang, J. J. Kosinski, M. M. Lencka, et al., Pure Appl. Chem. 85, 2117 (2013). https://doi.org/10.1351/pac-con-12-07-09
P. S. Song, B. Sun, D. W. Zeng, Pure Appl. Chem. 85, 2097 (2013). https://doi.org/10.1351/PAC-CON-13-04-05
L. S. Pechen, E. V. Makhonina, A. E. Medvedeva, et al., Russ. J. Inorg. Chem. 66, 777 (2021). https://doi.org/10.1134/S0036023621050144
X. Y. Zheng, M. G. Zhang, Y. Xu, et al., Salt Lakes in China (Science Press, Beijing, 2002).
F. Yuan, J. Jiang, S. Q. Wang, et al., J. Mol. Liq. 337, 116334 (2021). https://doi.org/10.1016/j.molliq.2021.116334
H. W. Ge, M. Wang, Y. Yao, et al., J. Chem. Eng. Data 65, 26 (2019). https://doi.org/10.1021/acs.jced.9b00663
Y. Z. Jia, S. Y. Gao, S. P. Xia, et al., Spec. Acta A 56, 1291 (2000). https://doi.org/10.1016/S1386-1425(99)00227-9
W. J. Cui, H. F. Hou, J. Y. Hu, et al., J. Chem. 2019, 983051 (2019). https://doi.org/10.1155/2019/1983051
S. Q. Chen, M. X. Wang, J. Y. Hu, et al., J. Chem. Eng. Data 63, 4662 (2018). https://doi.org/10.1021/acs.jced.8b00715
S. Q. Chen, W. J. Cui, J. Y. Hu, et al., J. Chem. Eng. Data 64, 2809 (2019). https://doi.org/10.1021/acs.jced.9b00177
T. Vilarinho-Francoa, A. Teyssiera, R. Tenub, et al., Fluid Phase Equilib. 360, 212 (2013). https://doi.org/10.1016/j.fluid.2013.09.035
A. V. Churikov, K. V. Zapsis, V. V. Khramkov, et al., J. Chem. Eng. Data 56, 383 (2011). https://doi.org/10.1021/je1007422
S. Q. Wang, J. Yang, C. C. Shi, et al., J. Chem. Eng. Data 64, 3122 (2019). https://doi.org/10.1021/acs.jced.9b00218
S. Q. Wang, C. C. Shi, J. Yang, et al., J. Solution Chem. 49, 353 (2020). https://doi.org/10.1007/s10953-020-00962-8
Y. F. Guo, L. Li, L. N. Cao, et al., J. Chem. Eng. Data 62, 508 (2017). https://doi.org/10.1021/acs.jced.6b00777
L.N. Cao, L. Li, N. Zhang, et al., CIESC J. 67, 1117 (2016). https://doi.org/10.11949/j.issn.0438-1157.20151204
P. Gu, S.Q. Wang, Y. F. Guo, et al., Russ. J. Phys. Chem. A 95, 717 (2021). https://doi.org/10.1134/S0036024421040087
D. C. Li, T. Shi, H. X. Zhao, et al., J. Chem. Eng. Data 65, 5266 (2020). https://doi.org/10.1021/acs.jced.0c00435
D. C. Li, R. Fan, S. N. Yang, et al., Chem. Res. Chin. Univ. 34, 803 (2018). https://doi.org/10.1007/s40242-018-7395-8
L. Zhang, J. T. Wu, S. Q. Wang, et al., Russ. J. Inorg. Chem. 66, 924 (2021). https://doi.org/10.1134/S0036023621060231
P. S. Song, Chin. J. Salt Lake Res. 1, 15 (1991).
Funding
The work was supported by the Program of the Natural Science Foundation of Hebei Province (B2021202058), the Key Research and Development Program of Hebei Provence (19251804D), and the National Natural Science Foundation of China (22078247, 21406048 and U1707602).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Dong-chan Li, Li, C., Gu, Sq. et al. Phase Equilibria and Physicochemical Properties of the Ternary System (LiBO2 + NaBO2 + H2O) and Quaternary system (NaBO2 + NaCl + Na2SO4 + H2O) at 323.15 K. Russ. J. Inorg. Chem. 67, 1041–1050 (2022). https://doi.org/10.1134/S0036023622070142
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023622070142