Abstract
Background
The impact of underlying comorbidities on the clinical presentation, management and outcomes in patients with ARDS is poorly understood and deserves further investigation.
Objectives
We examined these issue in patients with ARDS enrolled in the Large observational study to UNderstand the Global impact of Severe Acute respiratory FailurE (LUNG SAFE) study.
Methods
In this secondary analysis of the patient cohort enrolled in the LUNG SAFE study, our primary objective was to determine the frequency, and impact of comorbidities on the management and ICU survival of patients with ARDS. Secondary outcomes relating to comorbidities included their impact on ventilatory management, the development of organ failures, and on end-of-life care.
Results
Of 2813 patients in the study population, 1692 (60%) had 1 or more comorbidities, of whom 631 (22.4%) had chronic respiratory impairment, 290 (10.3%) had congestive heart failure, 286 (10.2%) had chronic renal failure, 112 (4%) had chronic liver failure, 584 (20.8%) had immune incompetence, and 613 (21.8%) had diabetes. Multiple comorbidities were frequently present, with 423 (25%) having 2 and 182 (11%) having at least 3 or more comorbidities. The use of invasive ventilation (1379 versus 998, 82 versus 89%), neuromuscular blockade (301 versus 249, 18 versus 22%), prone positioning (97 versus 104, 6 versus 9%) and ECMO (32 versus 46, 2 versus 4%) were each significantly reduced in patients with comorbidities as compared to patients with no comorbidity (1692 versus 1121, 60 versus 40%). ICU mortality increased from 27% (n = 303) in patients with no comorbidity to 39% (n = 661) in patients with any comorbidity. Congestive heart failure, chronic liver failure and immune incompetence were each independently associated with increased ICU mortality. Chronic liver failure and immune incompetence were independently associated with more decisions to limitation of life supporting measures.
Conclusions
Most patients with ARDS have significant comorbidities, they receive less aggressive care, and have worse outcomes. Enhancing the care of these patients must be a priority for future clinical studies.
Trial registration LUNG-SAFE is registered with ClinicalTrials.gov, number NCT02010073.
Similar content being viewed by others
Background
The impact of underlying comorbidities on the clinical presentation, management and outcomes in patients with ARDS is poorly understood and deserves further investigation. This knowledge gap is exacerbated by the fact that the evidence base for management of ARDS comes from clinical trials that frequently exclude these patients [1]. These trials have led to important clinical advances, including the recognition of the protective effects of lowered tidal volume [2], the role of prone positioning [3] and of muscle relaxants in early moderate–severe ARDS [4] among others.
A concern that has arisen is the generalizability of the findings of studies carried out in patients with no comorbidities, to the patient population with comorbidities, and the validity of these trials if only performed in a minority of the entire ARDS patient cohort. Furthermore, these concerns may limit the degree to which these interventions are applied to patients with significant underlying comorbidities [5].
Given the potential for important impact of comorbidities on the management and outcomes from ARDS, we wished to address these issues in this secondary analysis of the LUNG SAFE patient cohort. Briefly, LUNG SAFE was an international, multicentre, prospective cohort study of patients undergoing invasive or non-invasive ventilation, conducted during 4 consecutive weeks in the winter of 2014 in a convenience sample of 459 ICUs from 50 countries across 5 continents, that recruited 3,022 patients that fulfilled ARDS criteria [6].
Our primary objective, in this secondary LUNG SAFE analysis, was to determine the frequency, and impact of comorbidities on the management and ICU survival of patients with ARDS. Secondary outcomes included determination of the impact of these comorbidities on ventilatory management, the development of organ failures, and on end-of-life care.
Materials and methods
This is a sub-study of the LUNG-SAFE study, an international, multicenter, prospective cohort study of patients receiving invasive or non-invasive ventilation, and the detailed methods and protocol have been published elsewhere [6]. The study, led by the European Society of Intensive Care Medicine (ESICM), was endorsed by multiple national societies/networks (Additional file 1: Appendix S1). All participating ICUs obtained ethics committee approval, and either patient consent or ethics committee waiver of consent. National coordinators and site investigators (Additional file 1: Appendix S1) were responsible for obtaining ethics committee approval and for ensuring data integrity and validity.
Patients, study design and data collection
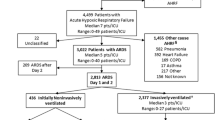
Inclusion criteria were admission to a study ICU (including ICU transfers) within the 4-week enrollment window and receipt of invasive or non-invasive ventilation. Exclusion criteria were age < 16 years or inability to obtain informed consent (where required). Patients were classified as having ARDS based on whether or not they fulfilled all of the Berlin criteria rather than by clinician determination, as previously described [6]. We restricted subsequent analyses to patients (93%, n = 2813) that fulfilled ARDS criteria within 48 h of the onset of acute hypoxemic respiratory failure (AHRF) (Fig. 1). All data were recorded for each patient at the same time each day within participating ICUs, normally as close as possible to 10am each day. Data on ventilatory settings were recorded simultaneously with arterial blood gas analysis.
Data definitions
Our data definitions have been previously reported [6,7,8]. Data collected on comorbidities were the following:
-
Chronic respiratory impairment, a patient has known or suspected chronic obstructive pulmonary disease or home ventilation therapy;
-
Congestive heart failure, a patient has chronic heart failure with marked limitation of physical activity or is unable to carry out any physical activity without chest discomfort (NYHA Classes III–IV);
-
Chronic renal failure, a patient has chronic renal failure with a creatinine clearance less than 60 ml per minute:
-
Chronic liver failure, a patient has chronic liver disease with a calculated Child Pugh score ≥ 10;
-
Immune incompetence: a patient has (a) a solid tumor which has not been resected or in remission, which is still requiring treatment or with metastasis; (b) viral immunosuppression, neoplastic disease, immunosuppressive drugs (including steroids), chemotherapy or congenital immunosuppression illness; or (c) an active hematologic neoplasm still requiring treatment;
-
Diabetes: a patient has known diabetes mellitus treated by drugs or diet.
For the purposes of this analysis, patients with more than one comorbidity appear in each relevant comorbidity category. Duration of invasive mechanical ventilation was calculated as the number of days between the date of intubation and the date of extubation in ICU (or death, if the patient died while receiving invasive mechanical ventilation). Survival was evaluated at ICU discharge or at hospital discharge up to a 90-day follow-up. Data about limitation of life-sustaining measures were reported. We defined new and/or worsening systemic acute organ dysfunction as an increase of ≥ 1 in SOFA score, in patients with an admission score of < 3 for that component of the SOFA score at 28-day follow-up.
Data management and statistical analyses
Descriptive statistics included proportions for categorical and mean (standard deviation) or median (interquartile range) for continuous variables. The study population was defined at patient cohort that developed ARDS within the first 2 days of developing hypoxic respiratory failure [6, 7, 9]. Comparisons between patients with any comorbidities or a specific type of comorbidities with patients with no comorbidities were performed using Chi-squared test (or Fisher exact test) for discrete variables, Student’s t-test (or Wilcoxon–Mann–Whitney test) for continuous variables. The Shapiro–Wilk test and the visual inspection of the data distribution was used to assess normality.
To evaluate factors associated with outcome from ARDS (i.e., ICU and hospital mortality), we applied multivariable logistic regression model and the independent predictors (demographic characteristics and clinical parameters measured at the first day of AHRF or ARDS) were identified through stepwise regression approach. The level of association was evaluated by OR with 95% confidence interval (90% CI). This approach combines forward and backward selection methods in an iterative procedure to select predictors in the final multivariable model. This approach was also applied to identify factors associated with decisions to limit life-sustaining measures.
In order to test the difference in mortality and limitation of life-sustaining measures among patients with increasing numbers of comorbidities, patients were stratified according to 4 categories (i.e., no comorbidities, a single comorbidity, 2 comorbidities, or 3 or more comorbidities). A Kaplan–Meier analysis was performed to detect differences across these 4 groups 90-day (i.e., ICU and hospital mortality, and limitation of life-sustaining measures) follow-up. Statistical difference between survival curves was assessed by log-rank test. Furthermore, we applied survival analysis with competing risk in order to investigate the relationship between increasing number of comorbidities and likelihood of limitation of life-sustaining treatment during 90-day follow-up, considering ICU death as competing risk (i.e., event that precludes the occurrence of limitation of life-sustaining measures). In this case, Fine and Gray competing risk regression model was used to assess the effect of comorbidities by the estimation of subhazard ratios with 95% confidence interval.
All p-values were two-sided, with p-values < 0.05 considered as statistically significant.
Statistical analyses were performed with STATA/16 MP (Texas, USA), GraphPad Prism 8.0.2 (La Jolla, California, USA), R software, version 3.3.3 (R Project for Statistical Computing, http://www.R-project.org) and SAS software, version 9.4 (SAS Institute, Cary, NC, USA).
Results
Of 4,499 patients that developed acute hypoxic respiratory failure (AHRF), 2,813 patients developed ARDS within the first 2 days of developing AHRF, of which 1692 (60.1%) had at least 1 major comorbidity (Fig. 1, Table 1). Of these, 1087 (64%) had one major comorbidity, 423 (25%) had two major comorbidities, while 182 (11%) had three or more major comorbidities. The order of frequency of comorbid conditions was chronic respiratory impairment (n = 631, 22.4%), diabetes (n = 613, 21.8%) immune incompetence (n = 584, 20.8%), chronic renal failure (n = 286, 10.2%), congestive heart failure (n = 290, 10.3%), and chronic liver failure (n = 112, 4%) (Fig. 1, Table 1).
Patient demographics
Patient with no comorbidities were younger than patients with comorbidities, except for those with chronic liver failure. Patients with chronic renal failure and those with diabetes had higher BMI than patients with no comorbidities (Table 1). There was a higher frequency of chronic renal failure, chronic liver failure, and diabetes in patients from high-income countries outside Europe, compared to European high-income country patients or those from middle-income countries (Additional file 1: Table S1). ARDS was less likely to be recognized in patients with chronic respiratory impairment and congestive heart failure or immune incompetence, compared to those with no comorbidities (Table 1). A medical reason for admission was more commonly present in patients with any type of comorbidities compared to those with no comorbidities (Table 1). Additional data regarding the patient population are reported in Additional file 1: Table S2.
Illness severity profiles
Patient with no comorbidities had a higher rate of severe ARDS compared to patients with chronic respiratory impairment, CHF and chronic renal failure. SOFA scores were lower in patients with chronic respiratory impairment, and higher in patients with renal or hepatic failure and immune incompetence, compared to those with no comorbidities. Non-pulmonary SOFA scores were lower in patients with chronic respiratory impairment, and higher in patients with chronic renal failure, chronic liver failure and immune incompetence.
Management of ARDS
The use of invasive ventilation (n = 1379 versus n = 998, 82 versus 89%), neuromuscular blockade (n = 301 versus n = 249, 18 versus 22%), prone positioning (n = 97 versus n = 104, 6 versus 9%) and ECMO (n = 32 versus n = 46, 2 versus 4%) were each significantly reduced in patients with comorbidities. More patients with chronic respiratory impairment, CHF, chronic renal failure, immune incompetence and diabetes received non-invasive ventilation, while more patients with chronic liver failure received invasive MV, compared to patients with no comorbidities (Additional file 1: Table S3). Overall, the use of adjunctive strategies was less frequent in patients with comorbidities. Fewer patients with chronic respiratory impairment and with CHF, renal and liver failure, and diabetes received continuous neuromuscular blockade. A similar pattern was seen for prone position ventilation (Additional file 1: Table S3). Factors independently associated with the use of any adjunctive measure within 28-day follow-up, included invasive ventilation, clinical recognition at baseline, hypoxemia and higher ventilatory pressures (i.e., PIP and PEEP). Among the comorbidities, immune incompetence and diabetes were independently correlated with the use of adjunctive measures within 28-day follow-up (Table 2).
Outcomes from ARDS
There were no major differences in the rates development of new or worsening systemic organ dysfunction in ARDS patients with comorbidities compared to those with no comorbidities (Additional file 1: Table S4). Similar proportions of patients with and without comorbidities no longer fulfilled ARDS criteria after 24 h. The number of MV-free days was higher in patients with no comorbidity compared to patients with any of the studied comorbidity except in patients with chronic respiratory impairment. Similar findings were observed regarding ICU and hospital length of stay (Table 3).
ICU mortality increased from 27% (n = 303) in patients with no comorbidity to 39% (n = 661) in patients with any comorbidity. ICU mortality was lowest in patients with no comorbidity (n = 303, 27%) and increased progressively in patients with chronic respiratory impairment (n = 209, 33%), diabetes (n = 210, 34%), chronic renal failure (n = 113, 40%), chronic heart failure (n = 120, 41%), immune incompetence (n = 266, 46%), and chronic liver failure (n = 75, 67%). A similar pattern was seen regarding hospital mortality, where mortality increased from 31% (n = 347) in patients with no comorbidity to 72% (n = 81) in patients with chronic liver failure (Table 3).
At the univariate analysis, all the studied comorbidities were significantly associated with a higher risk of ICU (Fig. 2 panel A) and hospital mortality at 90 days (Fig. 2 panel B). The risk of ICU and hospital mortality stratified by the type of immune incompetence is reported in Additional file 1: Fig. S1, panel A and B.
After adjusting these findings for multiple confounders, we observed that CHF, chronic liver failure and immune incompetence remained independent predictors of ICU mortality among all the comorbidities (Table 4). Chronic liver failure and immune incompetence, but not CHF, were predictive of hospital mortality at 90-day follow-up (Additional file 1: Table S5).
Furthermore, the presence of 1, 2, or > 3 comorbidities was significantly associated with a lower ICU (n = 675, n = 246, n = 110 versus no comorbidity—n = 818; 62%, 58%, 60% versus 73%, log-rank p < 0.001) (Fig. 3, panel A) and hospital survival (n = 609, n = 221, n = 91 versus no comorbidity—n = 774; 56%, 53%, 50% versus 69%, log-rank p < 0.001) (Fig. 3, panel B) at 90-day follow-up.
Decisions to limit life-sustaining measures were more frequent in patients with any comorbidities compared to those without comorbidities (n = 493 versus n = 209; 29% versus 19%, p < 0.001). Differences in limitation of care remained significant when patients were stratified by single groups of comorbidities (Table 3).
At univariate analysis, all the studied comorbidities were significantly associated with a higher proportion of limitation of life-sustaining measures (Fig. 2 panel C). The risk of a decision to limit life-sustaining measures by the type of immune incompetence is reported in Additional file 1: Fig. S1, panel C.
After adjusting for multiple confounders, both chronic liver failure and immune incompetence were predictive of limitation of life-sustaining measures at 90-day follow-up (Table 5). Furthermore, the presence of 1, 2, or > 3 comorbidities were each significantly associated with an increase in the proportion of limitation of life-sustaining measures (n = 315, n = 124, n = 54 versus no comorbidity—n = 208; 29%, 29%, 30% versus 19%, log-rank p < 0.001) (Fig. 3, panel C) at 90-day follow-up. Similar findings were obtained considering ICU mortality as a competing risk that precludes the occurrence of the event of interest (i.e., limitation of life-sustaining measures) (Additional file 1: Fig. S2).
With regard to the organ system failure considered by clinicians most responsible for death, cardiovascular failure was more frequent in patients with CHF and less frequent in patients with chronic liver failure—who died more of hepatic failure. Respiratory failure was more common in patients with immune incompetence, compared to patients with no comorbidities (Additional file 1: Table S6).
Discussion
The frequency and impact of underlying comorbidities on the clinical presentation, management and outcomes in patients with ARDS remain incompletely understood. We report that in the LUNG SAFE patient cohort, a large and geographically diverse cohort of ‘real world’ patient with ARDS, most patients had at least one significant comorbidity. We further found that patients with comorbidities were managed differently, being less likely to receive higher intensity modalities of organ support and adjunctive therapies. Patients with comorbidities experienced differences in end-of-life care and were more likely to die in the ICU and in hospital. These findings raise concerns regarding the management and outcomes of these patients and suggest that enhancing the care of these patients must be a priority for future clinical studies.
Critically ill ARDS patients with comorbidities were older and were more frequently admitted with acute medical condition. Geo-economic differences existed in comorbidity profiles, with a higher frequency of chronic renal failure, chronic liver failure, immune incompetence and diabetes in patients from high-income countries outside Europe. Of interest, ARDS was less likely to be recognized in patients with comorbidities, especially chronic respiratory impairment, congestive heart failure or immune incompetence. Overall, patients with comorbidities had less severe ARDS, and there were potentially important differences in the pattern of extrapulmonary organ injury.
The presence of comorbidities in our study population had a major impact on the management of ARDS patients. Patients with underlying comorbidities were managed more frequently with non-invasive ventilation, they received less adjunctive measures including NMBs, prone positioning and ECMO, regardless of their level of evidence [10], raising the concern of a potential bias towards less aggressive treatment of patients with comorbidities. It should be acknowledged that patients with comorbidities may have more contra-indications to the use of these management strategies, and/or the treating clinician may consider that these approaches may not be suitable for specific patients. Factors such as clinician recognition of ARDS at baseline, the use of invasive mechanical ventilation, greater ARDS severity (i.e., a lower PF ratio), a higher peak inspiratory and positive end-expiratory pressure were all independently associated with the use of adjunctive measures within 28-day follow-up. Furthermore, among all comorbidities immune incompetence and diabetes were independent predictors of using ARDS adjuncts during ICU stay.
The fact that ARDS patients with significant underling comorbidities are frequently excluded from interventional clinical trials, particularly trials of novel investigative medicinal products, has raised concerns regarding the generalizability of the findings of these studies. These concerns were first raised by Azoulay et al., in their study of the impact of comorbidities in a French cohort of patients with ARDS [11], where 51% of their cohort had significant comorbidities. Our findings confirm and extend their findings, demonstrating that the majority of patients in this global ARDS cohort had comorbidities that may have led to their being excluded from a clinical trial of an intervention for ARDS. Furthermore, clinician concerns regarding the generalizability of findings from ARDS trials of novel therapeutics to patients with underlying comorbidities may contribute to the differences seen in patient management.
The differences between cohorts of ARDS patients with and without comorbidities, in terms of their demographic profile, and the clinical pattern of ARDS, raises the possibility that patients with significant comorbidities may respond differently to these management strategies. These concerns are underlined by the findings that patients with different ARDS subphenotypes appear to respond differently to PEEP and to statin therapy [12, 13], while patients with different patterns of ARDS may respond differently to ventilation strategies [14]. These issues underline the need for additional studies focused on this patient population to develop the evidence base for their management.
ARDS patients with comorbidities had worse outcomes than those with no comorbidities. Specifically, these patients had less ventilator-free days, while ICU and hospital mortality was higher in this cohort. ICU survival in patients with no comorbidities at 73% compares favorably with outcomes from recent clinical trials [15, 16] and provides a ‘real world’ benchmark for outcomes in control populations in studies focused on this cohort. In contrast, ICU survival decreased to 61% in patients with at least 1 significant comorbidity.
The presence of even 1 major comorbidity increased the risk of death, there further increase in the risk of death with a greater number of comorbidities present. Specific comorbidities, including congestive heart failure, chronic liver failure and immune incompetence were each independent predictors of ICU mortality, while both chronic liver failure and immune incompetence were independently associated with hospital mortality. The presence of 1 or more comorbidities was associated with a higher proportion of limitation of life-sustaining measures compared to patients without comorbidities. Both chronic liver failure and immune incompetence were both independently associated with more decisions regarding end-of-life care. Of interest, we observed that a higher number of beds per physician (i.e., an index of resource ‘strain’) and location in a high-income European ICU were both positive predictors of a higher decision to limit treatment.
Limitations
This study has several limitations. Our patient cohort, while large and geographically diverse, may not be representative of actual clinical practice in ICUs across the globe. We did not have access to the source data for the patients in the enrolling ICUs, and it is possible that not all patients with ARDS in participating centers were enrolled. However, enrollment of patients with ARDS from participating ICUs met expectations based on their recorded 2013 admission rates, while data from lower recruiting ICUs were not different from that from higher enrolling ICUs, suggesting the absence of reporting biases. We instituted a robust data quality control program in which all centers were requested to verify data that appeared inconsistent or erroneous. While we have adjusted our analyses to account for known measured confounders, the possibility remains that some of our findings may arise from unmeasured or residual confounding. Moreover, we cannot make causal inferences for the associations seen, given the observational nature of our study. It should be acknowledged that the LUNG SAFE study unveiled that a significant proportion of patients did not receive protective mechanical ventilation, PEEP levels stratified by ARDS severity [6] and widely available adjuncts such as neuromuscular blockade and prone position [10] despite guidelines recommendations [17, 18]. This may imply that the relationship between comorbidities and outcome (i.e., mortality and limitation of life-sustaining measures) may differ as compared to cohort of ARDS patients treated according to the ARDS guidelines. The lack of information regarding the use of non-pulmonary organ supports should be considered as a limitation of the study.
Furthermore, as the patient inclusion period was before the pandemic era, the findings of the present study may not apply to patients with COVID-19 ARDS.
Conclusions
Our findings demonstrate that 60% of patients with ARDS have 1 or more significant comorbidities. These comorbidities profoundly influence patient management, with these patients less likely to receive invasive ventilation, neuromuscular blockade, prone positioning or ECMO. These findings raise concerns regarding both the use, and the applicability, of current management strategies in these patients. The impact on patient outcome is clear, with patients with comorbidities being more likely to receive treatment limitation decisions, while ICU mortality is over 40% higher than in patients without these comorbidities. Advancing the care of these patients must be a priority for future clinical studies.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AHRF:
-
Hypoxemic respiratory failure
- ARDS:
-
Acute respiratory distress syndrome
- CHF:
-
Congestive heart failure
- CRF:
-
Chronic renal failure
- ECMO:
-
Extracorporeal membrane oxygenation
- ICU:
-
Intensive Care Units
- MV:
-
Mechanical ventilation
- NMBA:
-
Neuromuscular blocking agent
- PEEP:
-
Positive end-expiratory pressure
- PF ratio:
-
PaO2/FiO2
- PEEP:
-
Positive end-expiratory pressure
- PP:
-
Prone positioning
- SOFA:
-
Sequential Organ Failure Assessment
References
Pais FM, Sinha P, Liu KD, Matthay MA. Influence of clinical factors and exclusion criteria on mortality in ARDS observational studies and randomized controlled trials. Respir Care. 2018. https://doi.org/10.4187/respcare.06034.
Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000. https://doi.org/10.1056/NEJM200005043421801.
Guerin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013. https://doi.org/10.1056/NEJMoa1214103.
Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010. https://doi.org/10.1056/NEJMoa1005372.
Juschten J, Tuinman PR, Guo T, Juffermans NP, Schultz MJ, Loer SA, et al. Between-trial heterogeneity in ARDS research. Intensive Care Med. 2021. https://doi.org/10.1007/s00134-021-06370-w.
Bellani G, Laffey JG, Pham T, Fan F, Brochard L, Esteban A, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016. https://doi.org/10.1001/jama.2016.0291.
Madotto F, Pham T, Bellani G, Bos LD, Simonis FD, Fan E, et al. Resolved versus confirmed ARDS after 24 h: insights from the LUNG SAFE study. Intensive Care Med. 2018. https://doi.org/10.1007/s00134-018-5152-6.
Madotto F, Rezoagli E, McNicholas BA, Pham T, Slutsky AS, Bellani G, et al. Patterns and impact of arterial CO2 management in patients with acute respiratory distress syndrome: insights from the LUNG SAFE study. Chest. 2020. https://doi.org/10.1016/j.chest.2020.05.605.
Laffey JG, Bellani G, Pham T, Fan E, Madotto F, Bajwa EK, et al. Potentially modifiable factors contributing to outcome from acute respiratory distress syndrome: the LUNG SAFE study. Intensive Care Med. 2016. https://doi.org/10.1007/s00134-016-4571-5.
Duggal A, Rezoagli E, Pham T, McNicholas BA, Fan E, Bellani G, et al. Patterns of use of adjunctive therapies in patients with early moderate to severe ARDS: insights from the LUNG SAFE study. Chest. 2020. https://doi.org/10.1016/j.chest.2020.01.041.
Azoulay E, Lemiale V, Mourvillier B, Garrouste-Orgeas M, Schwebel C, Ruckly S, et al. Management and outcomes of acute respiratory distress syndrome patients with and without comorbid conditions. Intensive Care Med. 2018. https://doi.org/10.1007/s00134-018-5209-6.
Calfee CS, Delucchi K, Parsons PE, Thompson BT, Ware LB, Matthay MA, et al. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014. https://doi.org/10.1016/S2213-2600(14)70097-9.
Calfee CS, Delucchi KL, Sinha P, Matthay MA, Hackett J, Shankar-Hari M, et al. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. Lancet Respir Med. 2018. https://doi.org/10.1016/S2213-2600(18)30177-2.
Constantin JM, Jabaudon M, Lefrant JY, Jaber S, Quenot JP, Langeron O, et al. Personalised mechanical ventilation tailored to lung morphology versus low positive end-expiratory pressure for patients with acute respiratory distress syndrome in France (the LIVE study): a multicentre, single-blind, randomised controlled trial. Lancet Respir Med. 2019. https://doi.org/10.1016/S2213-2600(19)30138-9.
Spragg RG, Bernard GR, Checkley W, Curtis JR, Gajic O, Guyatt G, et al. Beyond mortality: future clinical research in acute lung injury. Am J Respir Crit Care Med. 2010. https://doi.org/10.1164/rccm.201001-0024WS.
McNicholas BA, Rooney GM, Laffey JG. Lessons to learn from epidemiologic studies in ARDS. Curr Opin Crit Care. 2018. https://doi.org/10.1097/MCC.0000000000000473.
Rezoagli E, Bellani G. How I set up positive end-expiratory pressure: evidence- and physiology-based! Crit Care. 2019. https://doi.org/10.1186/s13054-019-2695-z.
Papazian L, Aubron C, Brochard L, Chiche JD, Combes A, Dreyfuss D, Forel JM, Guérin C, Jaber S, Mekontso-Dessap A, Mercat A, Richard JC, Roux D, Vieillard-Baron A, Faure H. Formal guidelines: management of acute respiratory distress syndrome. Ann Intensive Care. 2019. https://doi.org/10.1186/s13613-019-0540-9.
Acknowledgements
We wish to acknowledge the help of Guy Francois of the European Society of Intensive Care Medicine, Brussels, Belgium, for his assistance with the study.
Funding
This work was funded and supported by the European Society of Intensive Care Medicine (ESICM), Brussels, Belgium, by St Michael’s Hospital, Toronto, Canada, and by the University of Milan-Bicocca, Monza, Italy.
Author information
Authors and Affiliations
Consortia
Contributions
ER, GB, and JL conceived of and designed this study, interpreted the data, drafted the manuscript, and revised the manuscript for important intellectual content. FM, TP, BM, and ER contributed to the acquisition of data, conducted data cleaning, analyzed the data, interpreted the data, and revised the manuscript for important intellectual content. All authors interpreted the data and revised the manuscript for important intellectual content, and reviewed, discussed, and approved the final manuscript. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All participating ICUs obtained ethics committee approval and obtained either patient consent or ethics committee waiver of consent in LUNG-SAFE study.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Figure S1. A) ICU mortality, B) Hospital mortality and C) Limitation of care as a function of patients with different comorbidities that characterize the immune incompetence (i.e., solid neoplasm, hematological malignancy and immune-suppression). Unadjusted odds ratio calculated versus patients with no comorbidities. Figure S2. Survival analysis with competing risk to investigate the relationship between increasing number of comorbidities and the likelihood of limitation of life-sustaining measures during 90-day follow-up, considering ICU death as competing risk (i.e., event that precludes the occurrence of limitation of life-sustaining measures). Table S1. Comorbidities stratified by major geoeconomic areas of patients with ARDS. Table S2. ARDS risk factors, comorbidity profile and ICU related variables of patients with ARDS with and without comorbidities. Table S3. Management of patients with ARDS with and without comorbidities. Table S4. Development of new and/or worsening systemic acute organ dysfunction† in the study population. Table S5. Multivariate logistic regression model of factors associated with the hospital mortality in all patients. Table S6. Organ system failure considered as most important factors leading to death in ICU in non-surviving patients with ARDS at 90-day follow-up. Appendix S1.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rezoagli, E., McNicholas, B.A., Madotto, F. et al. Presence of comorbidities alters management and worsens outcome of patients with acute respiratory distress syndrome: insights from the LUNG SAFE study. Ann. Intensive Care 12, 42 (2022). https://doi.org/10.1186/s13613-022-01015-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13613-022-01015-7