Abstract
Purpose
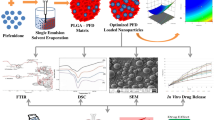
The success of the treatment of lung diseases with minimized side effects depends on the optimum design of microparticulate systems for pulmonary delivery that provide local delivery of drugs. This study aims to develop and evaluate optimized drug delivery systems against cystic fibrosis for pulmonary delivery.
Methods
Lysozyme, an antimicrobial peptide, was used as an alternative drug to conventional antibiotics. Since lysozyme is a water-soluble drug, lysozyme-loaded microparticles were prepared using the water-in-oil-in-water (w1/o/w2) double emulsion solvent evaporation method. Polycaprolactone (PCL) was chosen as a polymer due to its biocompatible and biodegradable properties. Designed microparticles were optimized utilizing 24 full factorial experimental design based on desired response factors including particle size and encapsulation efficiency (%EE).
Results and conclusion
Optimized formulation was found to display particle size of 8.75 ± 0.04 µm and %EE of 65.15 ± 0.00%. Results of SEM analysis have shown the spherical structure of microparticles with a smooth surface. Optimized microparticles were found to exhibit sustained release for up to 35 days after initial burst release. Mean median aerodynamic diameter (MMAD) and fine particle fraction (FPF) values were found to be 5.44 ± 0.19 μm and 50.99 ± 2.89%, respectively. These results demonstrated that optimized PCL microparticles can be used for pulmonary delivery of lysozyme.









Similar content being viewed by others
References
Garbuzenko OB, Kbah N, Kuzmov A, Pogrebnyak N, Pozharov V, Minko T. Inhalation treatment of cystic fibrosis with lumacaftor and ivacaftor co-delivered by nanostructured lipid carriers. J Control Release. 2019;296:225–31.
Zhu C, Chen J, Yu S, Que C, Taylor LS, Tan W, Wu C, Zhou QT. Inhalable nanocomposite microparticles with enhanced dissolution and superior aerosol performance. Mol Pharm. 2020;17:3270–80.
Porsio B, Craparo EF, Mauro N, Giammona G, Cavallaro G. Mucus and cell-penetrating nanoparticles embedded in nanointo-micro formulations for pulmonary delivery of ıvacaftor in patients with cystic fibrosis. ACS Appl Mater Interfaces. 2018;10:165–81.
Sardo C, Di Domenico EG, Porsio B, De Rocco D, Santucci R, Ascenzioni F, Giammona G, Cavallaro G. Nanometric ion pair complexes of tobramycin forming microparticles for the treatment of Pseudomonas aeruginosa infections in cystic fibrosis. Int J Pharm. 2019;563:347–57.
Chatterjee M, Anju CP, Biswas L, Anil Kumar V, Gopi Mohan C, Biswas R. Antibiotic resistance in Pseudomonas aeruginosa and alternative therapeutic options. Int J Med Microbiol. 2016;306:48–58.
Pang Z, Raudonis R, Glick BR, Lin TJ, Cheng Z. Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotechnol Adv. 2019;37:177–92.
Seo MD, Won HS, Kim JH, Mishig OT, Lee BJ. Antimicrobial peptides for therapeutic applications: a review. Molecules. 2012;17:12276–86.
Moghaddam MM, Barjini KA, Ramandi MF, Amani J. Investigation of the antibacterial activity of a short cationic peptide against multidrug-resistant Klebsiella pneumoniae and Salmonella typhimurium strains and its cytotoxicity on eukaryotic cells. World J Microbiol Biotechnol. 2014;30:1533–40.
Moghaddam MM, Aghamollaei H, Kooshki H, Barjini KA, Mirnejad R, Choopani A. The development of antimicrobial peptides as an approach to prevention of antibiotic resistance. Rev Med Microbiol. 2015;26:98–110.
Wang G, Mishra B, Lau K, Lushnikova T, Golla R, Wang X. Human antimicrobial peptides and proteins. Pharmaceuticals. 2014;7:545–94.
Grumezescu V, Holban AM, Sima LE, Chiritoiu MB, Chiritoiu GN, Grumezescu AM, Ivan L, Safciuc F, Antohe F, Florica C, Luculescu CR, Chifiriuc MC, Socol G. Laser deposition of poly(3-hydroxybutyric acid-co-3-hydroxyvaleric acid) - lysozyme microspheres based coatings with anti-microbial properties. Int J Pharm. 2017;521:184–95.
Wu T, Huang J, Jiang Y, Hu Y, Ye X, Liu D, Chen J. Formation of hydrogels based on chitosan/alginate for the delivery of lysozyme and their antibacterial activity. Food Chem. 2018;240:361–9.
Devrim B, Bozkır A, Canefe K. Preparation and evaluation of poly(lactic-co-glycolic acid) microparticles as a carrier for pulmonary delivery of recombinant human interleukin-2: II. In vitro studies on aerodynamic properties of dry powder inhaler formulations. Drug Dev Ind Pharm. 2011;37:1376–86.
Lee W-H, Loo C-Y, Traini D, Young PM. Inhalation of nanoparticle-based drug for lung cancer treatment: advantages and challenges. Asian J Pharm Sci. 2015;10:481–9.
Juntke J, Murgia X, Günday Türeli N, Türeli AE, Thorn CR, Schneider M, Schneider-Daum N, Carvalho-Wodarz CS, Lehr C-M. Testing of aerosolized ciprofloxacin nanocarriers on cystic fibrosis airway cells infected with P. aeruginosa biofilms. Drug Deliv Transl Res. 2021;11:1752–65.
Yang MY, Chan JG, Chan HK. Pulmonary drug delivery by powder aerosols. Journal of The Control Release Society. 2014;193:228–40.
Pulivendala G, Bale S, Godugu C. Inhalation of sustained release microparticles for the targeted treatment of respiratory diseases. Drug Deliv Transl Res. 2020;10:339–53.
Devrim B, Bozkır A, Canefe K. Preparation and evaluation of PLGA microparticles as carrier for the pulmonary delivery of rhIL-2: I. Effects of some formulation parameters on microparticle characteristics. J Microencapsulation. 2011;28:582–94.
Soomherun N, Kreua-Ongarjnukool N, Chumnanvej S, Thumsing S. Encapsulation of nicardipine hydrochloride and release from biodegradable poly(d,l-lactic-co-glycolic acid) microparticles by double emulsion process: effect of emulsion stability and different parameters on drug entrapment. Int J Biomater 2017;2017:1743765.
Salatin S, Jelvehgari M. expert design and optimization of ethyl cellulose-poly(ε-caprolactone) blend microparticles for gastro-retentive floating delivery of metformin hydrochloride. Curr Drug Deliv. 2021;18:1125–35.
Devrim B, Bozkır A. Preparation and evaluation of double-walled microparticles prepared with a modified water-in-oil-in-oil-in-water (w1/o/o/w3) method. J Microencapsulation. 2013;30:741–54.
Nikfarjam S, Jouravleva E, Anisimov M, Woehl T. Effects of protein unfolding on aggregation and gelation in lysozyme solutions. Biomolecules. 2020;10:1262.
Pharmacopoeia B. Preparations for inhalation. Aerodynamic assessment of fine particles–fine particle dose and particle size distribution (Ph. Eur. Method 2.9. 18). Br Pharmacop. 2005;4:A277–90.
Giménez VM, Sperandeo N, Faudone S, Noriega S, Manucha W, Kassuha D. Preparation and characterization of bosentan monohydrate/ε-polycaprolactone nanoparticles obtained by electrospraying. Biotechnol Prog. 2019;35(2):e2748.
Arunraj TR, Sanoj Rejinold N, Ashwin Kumar N, Jayakumar R. Doxorubicin-chitin-poly(caprolactone) composite nanogel for drug delivery. Int J Biol Macromol. 2013;62:35–43.
Kasten G, Silva LF, Lemos-Senna E. Development of low density azithromycin-loaded polycaprolactone microparticles for pulmonary delivery. Drug Dev Ind Pharm. 2016;42(5):776–87.
Bruinsmann FA, Buss JH, Souto GD, Schultze E, de Cristo Soares Alves A, Seixas FK, Collares TV, Pohlmann AR, Guterres SS. Guterres SS. Erlotinib-loaded poly(ε-caprolactone) nanocapsules improve in vitro cytotoxicity and anticlonogenic effects on human A549 lung cancer cells. AAPS PharmSciTech. 2020;21(6):229.
Su M, Hu H, Zhao X, Huang C, Yang B, Yin Z. Construction of mannose-modified polyethyleneimine-block-polycaprolactone cationic polymer micelles and its application in acute lung injury. Drug Deliv Transl Res. 2022;12(5):1080–95.
Oz UC, Devrim B, Bozkır A, Canefe K. Development of reconstitutable suspensions containing diclofenac sodium-loaded microspheres for pediatric delivery. J Microencapsul. 2015;32(4):317–28.
Mawazi SM, Doolaanea AA, Hadi HA, Chatterjee B. The impact of carbamazepine crystallinity on carbamazepine-loaded microparticle formulations. Int J Pharm. 2021;602:120638.
Sabbagh HAK, Hussein-Al-Ali SH, Hussein MZ, Abudayeh Z, Ayoub R, Abudoleh SM. A statistical study on the development of metronidazole-chitosan-alginate nanocomposite formulation using the full factorial design. Polymers. 2020;12:772.
Yang YY, Chung TS, Ngee NP. Morphology, drug distribution, and in vitro release profiles of biodegradable polymeric microspheres containing protein fabricated by double-emulsion solvent extraction/evaporation method. Biomaterials. 2001;22:231–41.
Jeffery H, Davis SS, O’hagan DT. The preparation and characterization of poly(lactide-co-glycolide) microparticles. II. The entrapment of a model protein using a (water-in-oil)-in-water emulsion solvent evaporation technique. Pharm Res. 1993;10:362–8.
Diez S, Conchita TDI. Versatility of biodegradable poly(d, l-lactic-co-glycolic acid) microspheres for plasmid DNA delivery. Eur J Pharm Biopharm. 2006;63:188–97.
Cui C, Stevens VC, Schwendeman SP. Injectable polymer microspheres enhance immunogenicity of a contraceptive peptide vaccine. Vaccine. 2007;25:500–9.
Feczkó T, Tóth J, Dósa G, Gyenis J. Optimization of protein encapsulation in PLGA nanoparticles. Chem Eng Process. 2011;50:757–65.
Sadoun O, Rezgui F, G’Sell C. Optimization of valsartan encapsulation in biodegradables polyesters using Box-Behnken design. Mater Sci Eng C Mater Biol Appl. 2018;90:189–97.
Van De Weert M, Van ’T Hof R, Van Der Weerd J, Heeren MAR, Posthuma G, Hennink WE, Crommelin DJA. Lysozyme distribution and conformation in a biodegradable polymer matrix as determined by FTIR techniques. J Control Release. 2000;68:31–40.
Liverani L, Lacina J, Roether JA, Boccardi E, Killian MS, Schmuki P, Schubert DW, Boccaccini AR. Incorporation of bioactive glass nanoparticles in electrospun PCL/chitosan fibers by using benign solvents. Bioact Mater. 2018;3:55–63.
Kerman I, Toppare L, Yilmaz F, Yagci Y. Thiophene ended ϵ-caprolactone conducting copolymers and their electrochromic properties. J Macromol Sci. 2005;42:509–20.
Jana S, Leung M, Chang J, Zhang M. Effect of nano- and micro-scale topological features on alignment of muscle cells and commitment of myogenic differentiation. Biofabrication. 2014;6:35012.
Bye JW, Falconer RJ. Thermal stability of lysozyme as a function of ion concentration: a reappraisal of the relationship between the Hofmeister series and protein stability. Protein Sci. 2013;22:1563–70.
Cao XM, Tian Y, Wang ZY, Liu YW, Wang CX. Effects of protein and phosphate buffer concentrations on thermal denaturation of lysozyme analyzed by isoconversional method. Bioengineered. 2016;7:235–40.
Trevino SR, Scholtz JM, Pace CN. Measuring and increasing protein solubility. J Pharm Sci. 2008;97(10):4155–66.
Farhangi M, Mahboubi A, Kobarfard F, Vatanara A, Mortazavi SA. Optimization of a dry powder inhaler of ciprofloxacin-loaded polymeric nanomicelles by spray drying process. Pharm Dev Technol. 2019;24:584–92.
Gao P, Nie X, Zou M, Shi Y, Cheng G. Recent advances in materials for extended-release antibiotic delivery system. J Antibiot (Tokyo). 2011;64(9):625–34.
Soares S, Fonte P, Costa A, Andrade J, Seabra V, Ferreira D, Reis S, Sarmento B. Effect of freeze-drying, cryoprotectants and storage conditions on the stability of secondary structure of insulin-loaded solid lipid nanoparticles. Int J Pharm. 2013;456:370–81.
Yang MY, Chan JGY, Chan H-K. Pulmonary drug delivery by powder aerosols. J Controlled Release. 2014;193:228–40.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Devrim Gökberk, B., Erdinç, N. Design, Optimization, and Characterization of Lysozyme-Loaded Poly(ɛ-Caprolactone) Microparticles for Pulmonary Delivery. J Pharm Innov 18, 325–338 (2023). https://doi.org/10.1007/s12247-022-09648-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-022-09648-8