Abstract
Through dynamic activities of conserved master transcription factors (mTFs), the unfolded protein response (UPR) relieves proteostasis imbalance of the endoplasmic reticulum (ER), a condition known as ER stress1,2. Because dysregulated UPR is lethal, the competence for fate changes of the UPR mTFs must be tightly controlled3,4. However, the molecular mechanisms underlying regulatory dynamics of mTFs remain largely elusive. Here, we identified the abscisic acid-related regulator G-class bZIP TF2 (GBF2) and the cis-regulatory element G-box as regulatory components of the plant UPR led by the mTFs, bZIP28 and bZIP60. We demonstrate that, by competing with the mTFs at G-box, GBF2 represses UPR gene expression. Conversely, a gbf2 null mutation enhances UPR gene expression and suppresses the lethality of a bzip28 bzip60 mutant in unresolved ER stress. By demonstrating that GBF2 functions as a transcriptional repressor of the UPR, we address the long-standing challenge of identifying shared signalling components for a better understanding of the dynamic nature and complexity of stress biology. Furthermore, our results identify a new layer of UPR gene regulation hinged upon an antagonistic mTFs-GFB2 competition for proteostasis and cell fate determination.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within this paper and Supplementary Information. The ChIP–seq data supporting the finding of this study have been deposited in the NCBI Sequence Read Archive and are accessible through the BioProject accession code PRJNA810750. The full results of the eY1H screen, including gene accession numbers, are available in Supplementary Data 4. Source data are provided with this paper.
Code availability
The scripts used in this study are available in GitHub (https://github.com/DaeKwan-Ko/UPR-TFs.git).
Change history
30 September 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41477-022-01265-0
References
Howell, S. H. Endoplasmic reticulum stress responses in plants. Annu. Rev. Plant Biol. 64, 477–499 (2013).
Pastor-Cantizano, N., Ko, D. K., Angelos, E., Pu, Y. & Brandizzi, F. Functional diversification of ER stress responses in Arabidopsis. Trends Biochem. Sci. 45, 123–136 (2020).
Ko, D. K. & Brandizzi, F. A temporal hierarchy underpins the transcription factor–DNA interactome of the maize UPR. Plant J. 105, 254–270 (2020).
Ko, D. K. & Brandizzi, F. Advanced genomics identifies growth effectors for proteotoxic ER stress recovery in Arabidopsis thaliana. Commun. Biol. 5, 16 (2022).
Vihervaara, A., Duarte, F. M. & Lis, J. T. Molecular mechanisms driving transcriptional stress responses. Nat. Rev. Genet. 19, 385–397 (2018).
Lemon, B. & Tjian, R. Orchestrated response: a symphony of transcription factors for gene control. Genes Dev. 14, 2551–2569 (2000).
Ezer, D. et al. The G-box transcriptional regulatory code in Arabidopsis. Plant Physiol. 175, 628–640 (2017).
Komili, S. & Silver, P. A. Coupling and coordination in gene expression processes: a systems biology view. Nat. Rev. Genet. 9, 38–48 (2008).
Ko, D. K. & Brandizzi, F. Network‐based approaches for understanding gene regulation and function in plants. Plant J. 104, 302–317 (2020).
Martínez, I. M. & Chrispeels, M. J. Genomic analysis of the unfolded protein response in Arabidopsis shows its connection to important cellular processes. Plant Cell 15, 561–576 (2003).
Liu, J. X. & Howell, S. H. bZIP28 and NF-Y transcription factors are activated by ER stress and assemble into a transcriptional complex to regulate stress response genes in Arabidopsis. Plant Cell 22, 782–796 (2010).
Ruberti, C., Lai, Y. & Brandizzi, F. Recovery from temporary endoplasmic reticulum stress in plants relies on the tissue-specific and largely independent roles of bZIP28 and bZIP60, as well as an antagonizing function of BAX-Inhibitor 1 upon the pro-adaptive signaling mediated by bZIP28. Plant J. 93, 155–165 (2018).
Song, L. et al. A transcription factor hierarchy defines an environmental stress response network. Science 354, aag1550 (2016).
Lai, Y. S. et al. Salicylic acid-independent role of NPR1 is required for protection from proteotoxic stress in the plant endoplasmic reticulum. Proc. Natl Acad. Sci. USA 115, E5203–E5212 (2018).
Nawkar, G. M. et al. HY5, a positive regulator of light signaling, negatively controls the unfolded protein response in Arabidopsis. Proc. Natl Acad. Sci. USA 114, 2084–2089 (2017).
Zhang, S. S. et al. Tissue-specific transcriptomics reveals an important role of the unfolded protein response in maintaining fertility upon heat stress in Arabidopsis. Plant Cell 29, 1007–1023 (2017).
Zou, C. et al. Cis-regulatory code of stress-responsive transcription in Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 108, 14992–14997 (2011).
Jakoby, M. et al. bZIP transcription factors in Arabidopsis. Trends Plant Sci. 7, 106–111 (2002).
Gaudinier, A. et al. Enhanced Y1H assays for Arabidopsis. Nat. Methods 8, 1053–1055 (2011).
Reece-Hoyes, J. S. et al. Enhanced yeast one-hybrid assays for high-throughput gene-centered regulatory network mapping. Nat. Methods 8, 1059–1064 (2011).
Deng, Y. et al. Heat induces the splicing by IRE1 of a mRNA encoding a transcription factor involved in the unfolded protein response in Arabidopsis. Proc. Natl Acad. Sci. USA 108, 7247–7252 (2011).
Song, Z. T. et al. Transcription factor interaction with COMPASS-like complex regulates histone H3K4 trimethylation for specific gene expression in plants. Proc. Natl Acad. Sci. USA 112, 2900–2905 (2015).
Otero, J. H., Lizák, B. & Hendershot, L. M. Life and death of a BiP substrate. Semin. Cell Dev. Biol. 21, 472–478 (2010).
Smit, M. E. et al. Specification and regulation of vascular tissue identity in the Arabidopsis embryo. Development 147, dev186130 (2020).
Schindler, U., Menkens, A. E., Beckmann, H., Ecker, J. R. & Cashmore, A. R. Heterodimerization between light‐regulated and ubiquitously expressed Arabidopsis GBF bZIP proteins. EMBO J. 11, 1261–1273 (1992).
Kurihara, Y., Makita, Y., Shimohira, H. & Matsui, M. Time-course transcriptome study reveals mode of bZIP transcription factors on light exposure in Arabidopsis. Int. J. Mol. Sci. 21, 1993 (2020).
Yu, C. P., Lin, J. J. & Li, W. H. Positional distribution of transcription factor binding sites in Arabidopsis thaliana. Sci. Rep. 6, 25164 (2016).
Zhang, T., Marand, A. P. & Jiang, J. PlantDHS: a database for DNase I hypersensitive sites in plants. Nucleic Acids Res. 44, D1148–D1153 (2016).
Sparkes, I. A., Runions, J., Kearns, A. & Hawes, C. Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat. Protoc. 1, 2019–2025 (2006).
Sherf, B. A., Navarro, S. L., Hannah, R. R. & Wood, K. V. Dual-luciferase reporter assay: an advanced co-reporter technology integrating firefly and Renilla luciferase assays. Promega notes 57, 2–8 (1996).
Deng, Y., Srivastava, R. & Howell, S. H. Protein kinase and ribonuclease domains of IRE1 confer stress tolerance, vegetative growth, and reproductive development in Arabidopsis. Proc. Natl Acad. Sci. USA 110, 19633–19638 (2013).
Lai, Y. S. et al. Systemic signaling contributes to the unfolded protein response of the plant endoplasmic reticulum. Nat. Commun. 9, 3918 (2018).
Pobre, K. F. R., Poet, G. J. & Hendershot, L. M. The endoplasmic reticulum (ER) chaperone BiP is a master regulator of ER functions: getting by with a little help from ERdj friends. J. Biol. Chem. 294, 2098–2108 (2019).
Deppmann, C. D. et al. Dimerization specificity of all 67 B-ZIP motifs in Arabidopsis thaliana: a comparison to Homo sapiens B-ZIP motifs. Nucleic Acids Res. 32, 3435–3445 (2004).
Gordân, R. et al. Genomic regions flanking E-box binding sites influence DNA binding specificity of bHLH transcription factors through DNA shape. Cell Rep. 3, 1093–1104 (2013).
Nuruzzaman, M., Sharoni, A. M. & Kikuchi, S. Roles of NAC transcription factors in the regulation of biotic and abiotic stress responses in plants. Front. Microbiol. 4, 248 (2013).
Altman, B. J. et al. MYC disrupts the circadian clock and metabolism in cancer cells. Cell Metab. 22, 1009–1019 (2015).
Li, Z., Tang, J., Srivastava, R., Bassham, D. C. & Howell, S. H. The transcription factor bZIP60 links the unfolded protein response to the heat stress response in maize. Plant Cell 32, 3559–3575 (2020).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 17, 10–12 (2011).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Kim, D. et al. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 14, R36 (2013).
Anders, S., Pyl, P. T. & Huber, W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Robinson, J. T. et al. Integrative genomics viewer. Nat. Biotechnol. 29, 24–26 (2011).
Wu, T. et al. clusterProfiler 4.0: a universal enrichment tool for interpreting omics data. Innovation 2, 100141 (2021).
Langmead, B., Trapnell, C., Pop, M. & Salzberg, S. L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 (2009).
Li, H. et al. The sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Zhang, Y. et al. Model-based analysis of ChIP–Seq (MACS). Genome Biol. 9, R137 (2008).
Yu, G., Wang, L. G. & He, Q. Y. ChIPseeker: an R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics 31, 2382–2383 (2015).
Ramírez, F. et al. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 44, W160–W165 (2016).
Bailey, T. L. STREME: accurate and versatile sequence motif discovery. Bioinformatics 37, 2834–2840 (2021).
O’Malley, R. C. et al. Cistrome and epicistrome features shape the regulatory DNA landscape. Cell 165, 1280–1292 (2016).
Franco-Zorrilla, J. M. et al. DNA-binding specificities of plant transcription factors and their potential to define target genes. Proc. Natl Acad. Sci. USA 111, 2367–2372 (2014).
Gupta, S., Stamatoyannopoulos, J. A., Bailey, T. L. & Noble, W. S. Quantifying similarity between motifs. Genome Biol. 8, R24 (2007).
Deplancke, B., Dupuy, D., Vidal, M. & Walhout, A. J. A gateway-compatible yeast one-hybrid system. Genome Res. 14, 2093–2101 (2004).
Pruneda-Paz, J. L. et al. A genome-scale resource for the functional characterization of Arabidopsis transcription factors. Cell Rep. 8, 622–632 (2014).
Gaudinier, A. et al. Transcriptional regulation of nitrogen-associated metabolism and growth. Nature 563, 259–264 (2018).
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Tian, T. et al. agriGO v2.0: a GO analysis toolkit for the agricultural community, 2017 update. Nucleic Acids Res. 45, W122–W129 (2017).
Acknowledgements
This study was supported primarily by the National Institutes of Health (R35GM136637) with contributing support from by the Great Lakes Bioenergy Research Center, US Department of Energy, Office of Science, Office of Biological and Environmental Research (DE-SC0018409), Chemical Sciences, Geoscience and Biosciences Division, Office of Basic Energy Sciences, Office of Science, US Department of Energy (DE-FG02-91ER20021) and MSU AgBioResearch (MICL02598). This study was supported in part through Michigan State University’s Institute for Cyber-Enabled Research Cloud Computing Fellowship, with computational resources and services provided by Information Technology Services and the Office of Research and Innovation at Michigan State University. We thank the Genome Center and Proteomics Core, University of California, Davis for the eY1H screen.
Author information
Authors and Affiliations
Contributions
D.K.K. and F.B. conceived the project and designed the experiments and research plan. D.K.K. performed experiments and data analysis. F.B. supervised the project. D.K.K. and F.B. interpreted the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Yukio Kurihara, Xinhong Guo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Identification of DEGs under either ABA treatment or ER stress recovery in this study.
a, The number of DEGs at each time-point of either condition. The DEGs were identified relative to the corresponding mock controls in each set of treatments (ABA or ER stress recovery). ABA, ABA treated conditions. ERR, ER stress recovery conditions. A full list of UPR-ABA DEGs is provided in Supplementary Data 2. b, GO terms enriched for ERR-ABA DEGs (see Fig. 1a). The 30 GO terms with the largest gene ratios are plotted in order of gene ratio. The P values were calculated by a hypergeometric test with Benjamini–Hochberg correction. The size of the dots represents the number of genes annotated to the corresponding GO term and the colour of the dots represent the adjusted P-values. A full list of GO terms is provided in Supplementary Data 2.
Extended Data Fig. 2 Pairwise correlation analyses of relative expression levels of DEGs between between ABA (1, 4, 8, 12, 24 or 60 h) and ERR (0, 12 or 24 h) conditions.
a-f, The expression of each of the DEGs were subjected to Spearman correlation coefficient analysis between ABA treatment 1 h (a), 4 h (b), 8 h (c), 12 h (d), 24 h (e) or 48 h (f) and ER stress recovery conditions. The scatter plot (centre) shows the pairwise expression pattern of the genes between ABA treatment and each ER stress recovery time-point. The density plots at the top and right visualize the enrichment of Log2FC under ER stress recovery and ABA, respectively. The Spearman’s correlation coefficient (rho) for each comparison is shown along with the level of significance (P value) in the corresponding colour. Error bars (grey) denote 95% confidence intervals. The plot between ABA-36 h and each time-point of ERR is shown in Fig. 1b.
Extended Data Fig. 3 Enriched motifs on the promoters of the ERR-ABA DEGs.
The 1 kb promoter of each ERR-ABA DEG (n = 922; 8 of 928 ERR-ABA DEGs have no promoter sequences available) was split into 10 fragments (10 bp overlapping). The 1 kb promoter of each of the randomly selected 922 genes was also processed in the same way. De novo motif analyses were performed on each set of promoter fragments of DEGs relative to those of random genes. Below the scheme, the significantly enriched motifs are shown with the corresponding P values and TF families of which the binding site significantly matched the corresponding motif. CHC, CXC-hinge-CXC. DOF, DNA binding with one finger. MYB, myeloblastosis.
Extended Data Fig. 4 Location of G-box and ABRE on promoters of core UPR genes.
The 1 kb promoter and 5’UTR sequences of (a) BiP1, (b) BiP2, (c) BiP3, (d) ERdj3A, (e) ERdj3B, (f) bZIP17, (g) bZIP28, (h) bZIP60, (i) IRE1a and (j) IRE1b. G-box (5’-CACGT-3’) and its reverse-complement sequence (5’-ACGTG-3’) are marked by light blue. ABRE (5’-CACG-3’) and its reverse-complement sequence (5’-CGTG-3’). The transcriptional start site of each gene is indicated by bold letters.
Extended Data Fig. 5 DNA sequence-specific binding of TFs to the UPR gene promoters.
a, A heatmap showing frequency distribution for the binding of multiple TF family members to each of the promoter fragments used in our eY1H screen. TF families of which members bound to the promoter fragment ≥ 10 times were selected for this analysis. b, Schematic representation of TF binding motif locations for selected TF families. Black arrowheads indicate the locations of the core sequence of corresponding TF motifs.
Extended Data Fig. 6 GO analysis of TFs binding either exclusively to each bait gene or all bait genes.
Significantly enriched GO terms were identified with adjusted P < 0.05 using a Hypergeometric test with Bonferroni correction. 28, TFs binding exclusively to the bZIP28 promoters. 60, TFs binding exclusively to the bZIP60 promoters. B3, TFs binding exclusively to the BiP3 promoters. All, TFs binding exclusively to all of the promoters. A full list of GO terms with adjusted P values is provided in Supplementary Data 5. Red asterisks indicate phytohormone-related pathways.
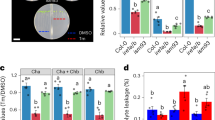
Extended Data Fig. 7 Hyposensitivity of gbf2 null mutants to ER stress.
Relative growth rate of primary root of Col-0, bzip28-2 bzip60-1, gbf2-1, gbf2-2 and gbf1 (-/-) gbf2-2 (-/+) gbf3 (-/-) after 7 days in ERR. Means ± SEM; n = 4 biological replicates (6 seedlings per replicate) for gbf2-1 and gbf2-2; n = 5 biological replicates (6 seedlings per replicate) for Col-0, bzip28-2 bzip60-1 and gbf1 (-/-) gbf2-2 (-/+) gbf3 (-/-). The significance (P value) measured by two-tailed Student’s t-test is shown. ns, not significant. The experiments were independently repeated at least two times with similar results.
Extended Data Fig. 8 Differential expression of ABA biomarker genes under ABA treatment in gbf2-3.
qRT–PCR assays of AT1G52680, AT4G35690, AT5G06090, and AT5G43770 expression (Log2[ABA/EtOH]) under ABA treatment (0 h, 12 h and 24 h). The genes were selected because they displayed high expression responsiveness to ABA and were bound by GBF213. Five-old-day plants were treated with 3 µM ABA or EtOH (solvent) only. Whole seedlings were harvested at the indicated time-points. The expression values were calculated relative to UBQ10. Means ± SEM; n = 3 biological replicates (12 seedlings per replicate). The significance (P value) measured by two-tailed Student’s t-test is shown. The experiments were independently repeated at least two times with similar results.
Extended Data Fig. 9 GBF2 temporally binds to the promoter of BiP3 during ERR.
ChIP–qPCR assays of temporal binding of GBF2 to the BiP3 promoters in ERR (0 h, 12 h and 24 h). The enrichment values are represented relative to the corresponding input values. Samples treated with DMSO served as the mock treatment. Mock samples (ChIP without antibodies) were used as negative controls for ChIP. The ACT7 gene promoter was used as a negative control for the enrichment. Means ± SEM; n = 3 biological replicates (≥ 100 seedlings per replicate). The significance (P value) measured by two-tailed Student’s t-test is shown. The experiments were independently repeated at least two times with similar results.
Supplementary information
Supplementary Tables
Supplementary Table 1. Creation of entry clones in our eY1H screen. For BP cloning, attB4 (GGGGACAACTTTGTATAGAAAAGTTG) and attB1R (GGGGACTGCTTTTTTGTACAAACTTG) sites are attached at 5′ of the forward and reverse primers, respectively. Supplementary Table 2. Primers used in this study. *G-box is labelled red. **The mutated G-box is labelled red. ***Restriction enzyme digestion sites are underlined.
Supplementary Data
Supplementary Data 1. Relative expression levels (log2(ABA/EtOH) or log2(Tm/DMSO)) of UPR-ABA DEGs. ABA, treatment with 10 µM ABA. ERR, recovery from ER stress (500 ng ml–1 Tm for 6 h). Supplementary Data 2. A full list of enriched GO terms for the UPR-ABA DEGs. Top-ranked GO terms are visualized in Extended Data Fig. 1b. Supplementary Data 3. TF motifs statistically matched with potential CREs found on the DEGs. CREs are described in Fig. 1c and Extended Data Fig. 3. Supplementary Data 4. PDIs obtained from our eY1H screen. Each row shows a PDI. The TF network built on PDIs are mapped in Fig. 1f. Supplementary Data 5. A full list of enriched GO terms for TF genes in the UPR-TF network shown in Fig. 1f. Top-ranked GO terms are visualized in Extended Data Fig. 6. P, biological process. F, molecular function. C, cellular component. Supplementary Data 6. UPR-specific binding peaks of GBF2 at 0 h of ERR. *1, sense. 2, antisense. Distal intergenic peaks were not included for gene annotation. Supplementary Data 7. A full list of enriched GO terms for genes cobound by GBF2 and bZIP28 (and/or bZIP60) at 0 h of ERR. Top-ranked GO terms are visualized in Fig. 2f.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3b,c,
Unprocessed EMSA blots.
Source Data Fig. 3e
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ko, D.K., Brandizzi, F. Transcriptional competition shapes proteotoxic ER stress resolution. Nat. Plants 8, 481–490 (2022). https://doi.org/10.1038/s41477-022-01150-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-022-01150-w
This article is cited by
-
Dynamics of ER stress-induced gene regulation in plants
Nature Reviews Genetics (2024)
-
An IRE1-proteasome system signalling cohort controls cell fate determination in unresolved proteotoxic stress of the plant endoplasmic reticulum
Nature Plants (2023)