Abstract
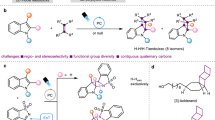
Many therapeutic agents are macrocyclic trisubstituted alkenes but preparation of these structures is typically inefficient and non-selective. A possible solution would entail catalytic macrocyclic ring-closing metathesis, but these transformations require high catalyst loading, conformationally rigid precursors and are often low yielding and/or non-stereoselective. Here we introduce a ring-closing metathesis strategy for synthesis of trisubstituted macrocyclic olefins in either stereoisomeric form, regardless of the level of entropic assistance. The goal was achieved by addressing several unexpected difficulties, including complications arising from pre-ring-closing metathesis alkene isomerization. The power of the method is highlighted by two examples. The first is the near-complete reversal of substrate-controlled selectivity in the formation of a macrolactam related to an antifungal natural product. The other is a late-stage stereoselective generation of an E-trisubstituted alkene in a 24-membered ring, en route to the cytotoxic natural product dolabelide C.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data in support of the findings of this study are available within the Article and its Supplementary Information.
References
Hoveyda, A. H. & Zhugralin, A. R. The remarkable metal-catalysed olefin metathesis. Nature 450, 243–251 (2007).
Hughes, D., Wheeler, P. & Ene, D. Olefin metathesis in drug discovery and development—examples from recent patent literature. Org. Process Res. Dev. 21, 1938–1962 (2017).
Yu, M., Lou, S. & Gonzalez-Bobes, F. Ring-closing metathesis in pharmaceutical development: fundamentals, applications, and future directions. Org. Process Res. Dev. 22, 918–946 (2018).
Nicolaou, K. C., Montagnon, T., Vassilikogiannakis, G. & Mathison, C. J. N. The total synthesis of coleophomones B, C, and D. J. Am. Chem. Soc. 127, 8872–8888 (2005).
Wang, Y. et al. Control of olefin geometry in macrocyclic ring-closing metathesis using a removable silyl group. J. Am. Chem. Soc. 133, 9196–9199 (2011).
Anketell, M. J., Sharrock, T. M. & Paterson, I. A unified total synthesis of the actinallolides, a family of anti-trypanosomal macrolides. Angew. Chem. Int. Ed. 59, 1572–1576 (2020).
Wasser, P. & Altmann, K.-H. An RCM-based total synthesis of the antibiotic disciformycin B. Angew. Chem. Int. Ed. 59, 17393–17397 (2020).
Smith, A. B. III, Mesaros, E. F. & Meyer, E. A. Total synthesis of (−)-kendomycin exploiting a Petasis–Ferrier rearrangement/ring-closing metathesis synthetic strategy. J. Am. Chem. Soc. 127, 6948–6949 (2005).
Toelle, N., Weinstabl, H., Gaich, T. & Mulzer, J. Light-mediated total synthesis of 17-deoxyprovidencin. Angew. Chem. Int. Ed. 53, 3859–3862 (2014).
Fuwa, H., Saito, A. & Sasaki, M. A concise total synthesis of (+)-neopeltolide. Angew. Chem. Int. Ed. 49, 3041–3044 (2010).
Terayama, N. et al. Total synthesis and structural revision of sekothrixide. Org. Lett. 16, 2794–2797 (2014).
Matsuzawa, A., Shiraiwa, J., Kasamatsu, A. & Sugita, K. Enantioselective, protecting-group-free total synthesis of boscartin F. Org. Lett. 20, 1031–1033 (2018).
Fürstner, A. Teaching metathesis ‘simple’ stereochemistry. Science 341, 1229713 (2013).
Hoveyda, A. H., Khan, R. K. M., Torker, S. & Malcolmson, S. J. in Handbook of Metathesis, Vol. 2 (eds Grubbs, R. H. & O’Leary, D. J.) 503–562 (Wiley-VCH, 2015).
Montgomery, T. P., Ahmed, T. S. & Grubbs, R. H. Stereoretentive olefin metathesis: an avenue to kinetic selectivity. Angew. Chem. Int. Ed. 56, 11024–11036 (2017).
Nguyen, T. T. et al. Synthesis of E- and Z-tri-substituted alkenes by catalytic cross-metathesis. Nature 552, 347–354 (2017).
Xu, C. et al. Synthesis of E- and Z-trisubstituted allylic alcohols and ethers by kinetically controlled cross-metathesis with a Ru catechothiolate complex. J. Am. Chem. Soc. 139, 15640–15643 (2017).
Mu, Y. et al. E- and Z-, di- and trisubstituted alkenyl nitriles through catalytic cross-metathesis. Nat. Chem. 11, 478–487 (2019).
Rummelt, S. M., Preindl, J., Sommer, H. & Fürstner, A. Selective formation of a trisubstituted alkene motif by trans-hydrostannation/Stille coupling: application to the total synthesis and late-stage modification of 5,6-dihydrocineromycin B. Angew. Chem. Int. Ed. 54, 6241–6245 (2015).
Houri, A. F., Xu, Z., Cogan, D. A. & Hoveyda, A. H. Cascade catalysis in synthesis. An enantioselective route to Sch 38516 (and fluvirucin B1) aglycon macrolactam. J. Am. Chem. Soc. 117, 2943–2944 (1995).
Llàcer, E., Urpì, F. & Vilarassa, J. Efficient approach to fluvirucins B2–B5, Sch 38518, and Sch 39185. First synthesis of their aglycon, via CM and RCM reactions. Org. Lett. 11, 3198–3201 (2009).
Guignard, G. et al. Enantioselective total synthesis of fluvirucinin B1. Org. Lett. 18, 1788–1791 (2016).
Wang, C., Haeffner, F., Schrock, R. R. & Hoveyda, A. H. Molybdenum-based complexes with two aryloxides and a pentafluoroimido ligand: catalysts for efficient Z-selective synthesis of a macrocyclic trisubstituted alkene by ring-closing metathesis. Angew. Chem. Int. Ed. 52, 1939–1943 (2013).
Meng, D. et al. Total syntheses of epothilones A and B. J. Am. Chem. Soc. 119, 10073–10092 (1997).
Wang, C. et al. Efficient and selective formation of macrocyclic disubstituted Z alkenes by ring-closing metathesis (RCM) reactions catalyzed by Mo- or W-based monoaryloxide pyrrolide (MAP) complexes: applications to total syntheses of epilachnene, Yuzu lactone, anbrettolide, epothiolone C, and Nakadomarin A. Chem. Eur. J. 19, 2726–2740 (2013).
Shen, X. et al. Kinetically E-selective macrocyclic ring-closing metathesis. Nature 541, 380–386 (2017).
Xu, C., Shen, X. & Hoveyda, A. H. In situ methylene capping: a general strategy for efficient stereoretentive catalytic olefin metathesis. The concept, methodological implications, and applications to synthesis of biologically active compounds. J. Am. Chem. Soc. 139, 10919–10928 (2017).
Ahmad, T. S. & Grubbs, R. H. A highly efficient synthesis of Z-macrocycles using stereoretentive, ruthenium-based metathesis catalysts. Angew. Chem. Int. Ed. 36, 11213–11216 (2017).
Xu, Z. et al. Applications of Zr-catalyzed carbomagnesation and Mo-catalyzed macrocyclic ring-closing metathesis in asymmetric synthesis. Enantioselective total synthesis of Sch 38516 (fluvirucin B1). J. Am. Chem. Soc. 119, 10302–10316 (1997).
Fürstner, A., Thiel, O. R. & Ackermann, L. Exploiting the reversibility of olefin metathesis. Syntheses of macrocyclic trisubstituted alkenes and (R,R)-(−)-pyrenophorin. Org. Lett. 3, 449–451 (2001).
Sytniczuk, A., Forcher, G., Grotjahn, D. B. & Grela, K. Sequential alkene isomerization and ring-closing metathesis in production of macrocyclic musks from biomass. Chem. Eur. J. 24, 10403–10408 (2018).
Sytniczuk, A., Milewski, M., Kajetanowicz, A. & Grela, K. Preparation of macrocyclic musks via olefin metathesis: comparison with classical syntheses and recent advances. Russ. Chem. Rev. 89, 469–490 (2020).
Peng, L. F. et al. Syntheses of aminoalcohol-derived macrocycles leading to a small-molecule binder to and inhibitor of Sonic Hedgehog. Bioorg. Med. Chem. Lett. 19, 6319–6325 (2009).
Yi, S., Varun, B. B., Choi, Y. & Park, S. B. A brief overview of two major strategies in diversity-oriented synthesis: build/couple/pair and ring-distortion. Front Chem. 6, 507 (2018).
Mallinson, J. & Collins, I. Macrocycles in new drug discovery. Future Med. Chem. 4, 1409–1438 (2012).
Taylor, R. D., MacCoss, M. & Lawson, A. D. G. Rings in drugs. J. Med. Chem. 57, 5845–5859 (2014).
Ojika, M., Nagoya, T. & Yamada, K. Dolabelides A and B, cytotoxic 22-membered macrolides isolated from the sea hare Dolabella auricularia. Tetrahedron Lett. 36, 7491–7494 (1995).
Suenaga, K. et al. Dolabelides C and D, cytotoxic macrolides isolated from the sea hare Dolabella auricularia. J. Nat. Prod. 60, 155–157 (1997).
Hanson, P. R. et al. Total synthesis of dolabelide C: a phosphate-mediated approach. J. Org. Chem. 76, 4358–4370 (2011).
Park, P. K., O’Malley, S. J., Schmidt, D. R. & Leighton, J. L. Total synthesis of dolabelide D. J. Am. Chem. Soc. 128, 2796–2797 (2006).
Grimaud, L., de Mesmay, R. & Prunet, J. Diastereoselective synthesis of protected syn 1,3-diols: prepration of the C16–C24 portion of dolabelides. Org. Lett. 4, 419–421 (2002).
Tiniakos, A. F., Wittman, S., Audic, A. & Prunet, J. Novel synthesis of trisubstituted olefins for the preparation of the C16–C30 fragment of dolabelide C. Org. Lett. 21, 589–592 (2019).
Nguyen, T. T. et al. Kinetically controlled E-selective catalytic olefin metathesis. Science 352, 569–575 (2016).
Hoveyda, A. H. et al. Impact of ethylene on efficiency and stereocontrol in olefin metathesis: when to add it, when to remove it, and when to avoid it. Angew. Chem. Int. Ed. 59, 22324–22348 (2020).
Meek, S. J. et al. The significance of degenerate processes to enantioselective olefin metathesis reactions promoted by stereogenic-at-Mo complexes. J. Am. Chem. Soc. 131, 16407–16409 (2009).
Schrock, R. R. & Hoveyda, A. H. Molybdenum and tungsten imido alkylidene complexes as efficient olefin-metathesis catalysts. Angew. Chem. Int. Ed. 42, 4592–4633 (2003).
Girvin, Z. C., Andrews, M. K., Liu, X. & Gellman, S. H. Foldamer-templated catalysis of macrocycle formation. Science 366, 1528–1531 (2019).
Ferreira, M. A. B. et al. Noncovalent interactions drive the efficiency of molybdenum imido alkylidene catalysts for olefin metathesis. J. Am. Chem. Soc. 141, 10788–10800 (2019).
Chou, T.-C. et al. Design and total synthesis of a superior family of epothilone analogues, which eliminate xenograft tumors to a nonrelapsable state. Angew. Chem. Int. Ed. 42, 4762–4767 (2003).
Paterson, I. et al. A practical synthesis of (+)-discodermolide and analogues: fragment union by complex aldol reactions. J. Am. Chem. Soc. 123, 9535–9544 (2001).
Roethle, P. A. & Trauner, D. Expedient synthesis of (±)-bipinnatin J. Org. Lett. 8, 345–347 (2006).
Yu, X. & Sun, D. Macrocyclic drugs and synthetic methodologies toward macrocycles. Molecules 18, 6230–6268 (2013).
Parenty, A., Moreau, X., Niel, G. & Campagne, J.-M. Update 1 of: macrolactonizations in the total synthesis of natural products. Chem. Rev. 113, PR1–PR40 (2013).
Acknowledgements
Financial support was provided by the National Institutes of Health (GM-130395). F.W.W.H. was supported as a Deutsche Forschungsgemeinschaft postdoctoral fellow. K.E.L. was supported by a Complex Systems Chemistry (CSC) graduate fellowship funded by the French National Research Agency (CSC-IGS ANR-17-EURE-0016). We thank T. Koengeter, C. Qin and S. Xu for helpful discussions and suggestions.
Author information
Authors and Affiliations
Contributions
Y.M., F.W.W.H., F.R. and E.C.Y. developed the method and performed the dolabelide C total synthesis. Y.M., F.R. and K.E.L. carried out the total synthesis of the E-alkene isomer related to fluvirucin B1. The molybdenum complexes used in this study were designed and developed as part of an ongoing collaboration between the research groups of A.H.H. and R.R.S. The approach was conceived by A.H.H. and F.R. The investigations were directed by A.H.H. and A.H.H. composed the manuscript with the other authors suggesting revisions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks N. Lemcoff, Damian Young and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
The file contains an extended bibliography regarding preparation of trisubstituted macrocyclic natural products. It also contains the experimental procedures for preparation of organometallic complexes, substrates, reagents and products and all the corresponding analytical/characterization data (tabulated and reproductions of 1H and 13C NMR spectra).
Rights and permissions
About this article
Cite this article
Mu, Y., Hartrampf, F.W.W., Yu, E.C. et al. E- and Z-trisubstituted macrocyclic alkenes for natural product synthesis and skeletal editing. Nat. Chem. 14, 640–649 (2022). https://doi.org/10.1038/s41557-022-00935-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-022-00935-y
This article is cited by
-
Trisubstituted triumph
Nature Chemistry (2022)