Abstract
Background and Objective
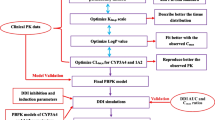
A physiologically based pharmacokinetic (PBPK) modeling approach for esketamine and its metabolite noresketamine after esketamine intranasal administration was developed to aid the prediction of drug–drug interactions (DDIs) during the clinical development of esketamine nasal spray (SPRAVATO®). This article describes the development of the PBPK model to predict esketamine and noresketamine kinetics after intranasal administration of esketamine and its verification and application in the prediction of prospective DDIs with esketamine using models of index perpetrator and victim drugs.
Methods
The intranasal PBPK (IN-PBPK) models for esketamine/noresketamine were constructed in Simcyp® v14.1 by combining the oral and intravenous esketamine PBPK models, with the dose divided in the ratio 57.7/42.3. Verification of the model was based on comparing the pharmacokinetics and DDI simulations with observed data in healthy volunteers.
Results
The simulated and observed (171 healthy volunteers) plasma pharmacokinetic profiles of intranasal esketamine/noresketamine showed a good match. The relative contributions of different cytochromes P450 (CYPs), mainly CYP3A4 and CYP2B6, involved in esketamine/noresketamine clearance was captured correctly in the IN-PBPK model using the DDI clinical studies of intranasal esketamine with clarithromycin and rifampicin and a published DDI study of oral esketamine with ticlopidine. The induction potential of esketamine toward CYP3A4 was also well captured. Inhibition of intranasal esketamine in the presence of ticlopidine was predicted to be not clinically relevant. Different scenarios tested with esketamine as a CYP3A4 perpetrator of midazolam also predicted the absence of clinically relevant CYP3A4 interactions.
Conclusion
This PBPK model of the intranasal route adequately described the pharmacokinetics and DDI of intranasal esketamine/noresketamine with potential perpetrator and victim drugs. This work was used to support regulatory submissions of SPRAVATO®.



Similar content being viewed by others
References
US Food and Drug Administration. FDA approves new nasal spray medication for treatment-resistant depression; availableonly at a certified doctor's office or clinic. 2019. https://www.fda.gov/news-events/press-announcements/fda-approves-new-nasal-spray-medication-treatment-resistant-depression-available-only-certified. Accessed 10 Jun 2020.
Mortensen MB, Nordestgaard BG. Elevated LDL cholesterol and increased risk of myocardial infarction and atherosclerotic cardiovascular disease in individuals aged 70–100 years: a contemporary primary prevention cohort. Lancet. 2020;396(10263):1644–52. https://doi.org/10.1016/S0140-6736(20)32233-9.
SPRAVATO Prescribing Information. Janssen Pharmaceutical Companies. 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211243lbl.pdf. Accessed 7 Aug 2020.
Zanos P, Moaddel R, Morris PJ, Riggs LM, Highland JN, Georgiou P, et al. Ketamine and ketamine metabolite pharmacology: insights into therapeutic mechanisms. Pharmacol Rev. 2018;70(3):621–60. https://doi.org/10.1124/pr.117.015198.
Portmann S, Kwan HY, Theurillat R, Schmitz A, Mevissen M, Thormann W. Enantioselective capillary electrophoresis for identification and characterization of human cytochrome P450 enzymes which metabolize ketamine and norketamine in vitro. J Chromatogr A. 2010;1217(51):7942–8. https://doi.org/10.1016/j.chroma.2010.06.028.
US Food and Drug Administration. Advisory Committee Briefing Document. Esketamine Nasal Spray for Patients with Treatment-resistant Depression. 2019. https://www.fda.gov/media/121377/download. Accessed 10 Jun 2020.
Hagelberg NM, Peltoniemi MA, Saari TI, Kurkinen KJ, Laine K, Neuvonen PJ, et al. Clarithromycin, a potent inhibitor of CYP3A, greatly increases exposure to oral S-ketamine. Eur J Pain. 2010;14(6):625–9. https://doi.org/10.1016/j.ejpain.2009.10.003.
Peltoniemi MA, Saari TI, Hagelberg NM, Laine K, Kurkinen KJ, Neuvonen PJ, et al. Rifampicin has a profound effect on the pharmacokinetics of oral S-ketamine and less on intravenous S-ketamine. Basic Clin Pharmacol Toxicol. 2012;111(5):325–32. https://doi.org/10.1111/j.1742-7843.2012.00908.x.
Peltoniemi MA, Saari TI, Hagelberg NM, Reponen P, Turpeinen M, Laine K, et al. Exposure to oral S-ketamine is unaffected by itraconazole but greatly increased by ticlopidine. Clin Pharmacol Ther. 2011;90(2):296–302. https://doi.org/10.1038/clpt.2011.140.
Jamei M. Recent advances in development and application of physiologically-based pharmacokinetic (PBPK) models: a transition from academic curiosity to regulatory acceptance. Curr Pharmacol Rep. 2016;2:161–9. https://doi.org/10.1007/s40495-016-0059-9.
Wagner C, Pan Y, Hsu V, Grillo JA, Zhang L, Reynolds KS, et al. Predicting the effect of cytochrome P450 inhibitors on substrate drugs: analysis of physiologically based pharmacokinetic modeling submissions to the US Food and Drug Administration. Clin Pharmacokinet. 2015;54(1):117–27. https://doi.org/10.1007/s40262-014-0188-4.
Kato M, Chiba K, Ito T, Koue T, Sugiyama Y. Prediction of interindividual variability in pharmacokinetics for CYP3A4 substrates in humans. Drug Metab Pharmacokinet. 2010;25(4):367–78. https://doi.org/10.2133/dmpk.dmpk-09-rg-038.
A Study to Evaluate the Absolute Bioavailability of Intranasal and Oral Esketamine and the Effects of Clarithromycin on the Pharmacokinetics of Intranasal Esketamine in Healthy Participants. ClinicalTrials.gov. 2015. https://clinicaltrials.gov/ct2/show/NCT02343289.
A Mass Balance Study With a Microtracer Dose of 14C-esketamine in Healthy Male Participants. ClinicalTrials.gov. 2016. https://clinicaltrials.gov/ct2/show/NCT02674295.
Jamei M, Dickinson GL, Rostami-Hodjegan A. A framework for assessing inter-individual variability in pharmacokinetics using virtual human populations and integrating general knowledge of physical chemistry, biology, anatomy, physiology and genetics: a tale of “bottom-up” vs “top-down” recognition of covariates. Drug Metab Pharmacokinet. 2009;24(1):53–75. https://doi.org/10.2133/dmpk.24.53.
Huang W, Lee SL, Yu LX. Mechanistic approaches to predicting oral drug absorption. AAPS J. 2009;11(2):217–24. https://doi.org/10.1208/s12248-009-9098-z.
Jamei M, Turner D, Yang J, Neuhoff S, Polak S, Rostami-Hodjegan A, et al. Population-based mechanistic prediction of oral drug absorption. AAPS J. 2009;11(2):225–37. https://doi.org/10.1208/s12248-009-9099-y.
Rodgers T, Rowland M. Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci. 2006;95(6):1238–57. https://doi.org/10.1002/jps.20502.
A Study to Assess the Pharmacokinetics, Safety, and Tolerability of Intranasally Administered Esketamine in Healthy Participants. ClinicalTrials.gov. 2013. https://clinicaltrials.gov/ct2/show/NCT01780259.
A Study to Assess the Pharmacokinetics of Intranasally Administered Esketamine in Healthy Japanese and Caucasian Volunteers. ClinicalTrials.gov. 2014. https://clinicaltrials.gov/ct2/show/NCT01980303.
A Study to Evaluate the Pharmacokinetics of Intranasal Esketamine Administered With and Without a Nasal Guide on the Intranasal Device. ClinicalTrials.gov. 2014. https://clinicaltrials.gov/ct2/show/NCT02060929.
Pharmacokinetic Study of Intranasal Esketamine and Its Effects on the Pharmacokinetics of Orally-Administered Midazolam and Bupropion in Healthy Participants. ClinicalTrials.gov. 2017. https://clinicaltrials.gov/ct2/show/NCT02568176.
Pharmacokinetic, Safety, and Tolerability Study of Intranasally Administered Esketamine in Elderly and and Healthy Younger Adult Participants. ClinicalTrials.gov. 2017. https://clinicaltrials.gov/ct2/show/NCT02345148.
Allen JA, Berger M, Querol L, Kuitwaard K, Hadden RD. Individualized immunoglobulin therapy in chronic immune-mediated peripheral neuropathies. J Peripher Nerv Syst. 2018;23(2):78–87. https://doi.org/10.1111/jns.12262.
Pharmacokinetic, Safety, and Tolerability Study of Intranasally Administered Esketamine in Healthy Han Chinese, Korean, Japanese, and Caucasian Participants and the Effects of Rifampin on the Pharmacokinetics of Intranasally Administered Esketamine. ClinicalTrials.gov. 2019. https://clinicaltrials.gov/ct2/show/NCT02846519.
A Study to Assess the Effect of Ticlopidine on the Pharmacokinetics, Safety, and Tolerability of Intranasally Administered Esketamine in Healthy Participants. ClinicalTrials.gov. 2017. https://clinicaltrials.gov/ct2/show/NCT03298906.
Hijazi Y, Boulieu R. Protein binding of ketamine and its active metabolites to human serum. Eur J Clin Pharmacol. 2002;58:37–40.
Edwards SR, Mather LE. Tissue uptake of ketamine and norketamine enantiomers in the rat. Indirect evidence for extrahepatic metabolic inversion. Life Sci. 2001;69:2051–66.
Acknowledgements
Priya Ganpathy, MPharm CMPP (SIRO Clinpharm Pvt. Ltd., India), provided writing assistance. Ellen Baum, PhD, and Harry Ma, PhD CMPP (both Janssen Global Services, LLC) provided additional editorial support. The authors thank the patients and investigators for their participation in the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Janssen Research & Development, LLC.
Conflicts of Interest
Marie-Emilie Willemin, Peter Zannikos, Geert Mannens, Loeckie de Zwart, and Jan Snoeys are employees of Janssen Research & Development, LLC, and may hold company stocks or stock options.
Ethics approval
Institutional review boards or independent ethics committees approved the study protocols of all primary pharmacokinetic and DDI studies. The studies were conducted in accordance with the ethical principles of the Declaration of Helsinki, good clinical practice, and applicable regulatory requirements.
Consent to participate
All participants provided written consent to participate
Consent for Publication
All authors read and approved the manuscript for publication, agreed on the journal to which the article was submitted, and agreed to be accountable for all aspects of the work.
Availability of Data and Material
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. Requests for access to the study data can be submitted through Yale Open Data Access Project site at http://yoda.yale.edu.
Code availability
Not applicable.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Willemin, ME., Zannikos, P., Mannens, G. et al. Prediction of Drug–Drug Interactions After Esketamine Intranasal Administration Using a Physiologically Based Pharmacokinetic Model. Clin Pharmacokinet 61, 1115–1128 (2022). https://doi.org/10.1007/s40262-022-01123-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-022-01123-4