Renal Artery Catheterization for Microcapsules’ Targeted Delivery to the Mouse Kidney
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microcapsules Labeled by Cyanine 7 and RITC: Preparation and Characterization
2.2.1. Preparation of Cy7-Conjugated BSA
2.2.2. Preparation of RITC-Conjugated BSA
2.2.3. Microcapsules’ Preparation
2.2.4. Confocal Laser Scanning Microscopy
2.3. Animal Studies
2.3.1. Blood Flow Response to Capsules’ Administration
2.3.2. Laser Speckle Contrast Imaging System
2.3.3. Photoacoustic Visualization of Kidney’s Blood Flow
2.3.4. Microcapsules’ Biodistribution
2.3.5. Microcapsules’ Localization in the Kidney Tissue
2.3.6. Histological Studies
2.3.7. Quantification of Fluorescent Dye Content in Kidneys
3. Results
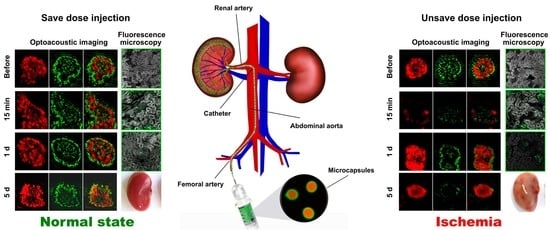
3.1. Minimally Invasive Mouse Renal Artery Catheterization Technique
3.2. Reversible/Irreversible Changes of Kidney Blood Flow Associated with Various Sizes and Concentrations of Microcapsules Injected via Renal Artery
3.3. Optoacoustic Imaging of Blood Circulation in the Target Kidney after the Microcapsules’ Administration
3.4. Capsules’ Localization and following Elimination from Target Kidney
3.5. Biodistribution Kinetics of the Fluorescent Marker after Administration to the Target Kidney in Free and Encapsulated Form
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, A.; Connolly, P.; Hakimi, A.A. Endovascular management of a large renal artery aneurysm: A case report and review of the literature. BMC Urol. 2021, 21, 121. [Google Scholar] [CrossRef]
- Smith, T.A.; Eastaway, A.; Hartt, D.; Quencer, K.B. Endovascular embolization in renal trauma: A narrative review. Ann. Transl. Med. 2021, 9, 1198. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.G. Renal interventions during endovascular aneurysm repair. Semin. Vasc. Surg. 2013, 26, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Marshalleck, F. Pediatric Arterial Interventions. Tech. Vasc. Intervent. Radiol. 2010, 13, 238–243. [Google Scholar] [CrossRef] [PubMed]
- O’Mara, J.E.; Bersin, R.M. Endovascular Management of Abdominal Aortic Aneurysms: The Year in Review. Curr. Treat. Option. Cardiovasc. Med. 2016, 18, 54. [Google Scholar] [CrossRef]
- Azizi, M.; Steichen, O.; Frank, M.; Bobrie, G.; Plouin, P.F.; Sapoval, M. Catheter-based Radiofrequency Renal-nerve Ablation in Patients with Resistant Hypertension. Eur. J. Vasc. Endovasc. 2012, 43, 293–299. [Google Scholar] [CrossRef] [Green Version]
- Weisz, G.; Filby, S.J.; Cohen, M.G.; Allie, D.E.; Weinstock, B.S.; Kyriazis, D.; Walker, C.M.; Moses, J.W.; Danna, P.; Fearon, W.F.; et al. Safety and Performance of Targeted Renal Therapy: The Be-RITe! Registry. J. Endovasc. Ther. 2009, 16, 1–12. [Google Scholar] [CrossRef]
- Fischell, T.A.; Fischell, D.R.; Ghazarossian, V.E.; Vega, F.; Ebner, A. Next generation renal denervation: Chemical “perivascular” renal denervation with alcohol using a novel drug infusion catheter. Cardiovasc. Revasc. Med. 2015, 16, 221–227. [Google Scholar] [CrossRef] [Green Version]
- Hawkins, I.F.; Wilcox, C.S.; Kerns, S.R.; Sabatelli, F.W. CO2 Digital Angiography: A Safer Contrast Agent for Renal Vascular Imaging? Am. J. Kidney Dis. 1994, 24, 685–694. [Google Scholar] [CrossRef]
- Canyigit, M.; Cetin, L.; Uguz, E.; Algin, O.; Kucuker, A.; Arslan, H.; Sener, E. Reduction of iodinated contrast load with the renal artery catheterization technique during endovascular aortic repair. Diagn. Interv. Radiol. 2012, 19, 244–250. [Google Scholar] [CrossRef]
- Hu, J.; Albadawi, H.; Zhang, Z.; Salomao, M.A.; Gunduz, S.; Rehman, S.; D’Amone, L.; Mayer, J.L.; Omenetto, F.; Oklu, R. Silk Embolic Material for Catheter-Directed Endovascular Drug Delivery. Adv. Mater. 2022, 34, 2106865. [Google Scholar] [CrossRef] [PubMed]
- Flacke, S.; Moinzadeh, A.; Libertino, J.A.; Merhige, J.; Vogel, J.M.; Lyall, K.; Khettry, U.; Bakal, C.W.; Madras, P.N. Endovascular Temporary Vessel Occlusion with a Reverse-thermosensitive Polymer for Bloodless Minimally Invasive Renal Surgery. J. Vasc. Interv. Radiol. 2010, 21, 711–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tesfamariam, B. Local arterial wall drug delivery using balloon catheter system. J. Control. Release 2016, 238, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Zaw Thin, M.; Ogunlade, O.; Comenge, J.; Patrick, P.S.; Stuckey, D.J.; David, A.L.; Lythgoe, M.F.; Beard, P.; Kalber, T.L. Stem cell delivery to kidney via minimally invasive ultrasound-guided renal artery injection in mice. Sci. Rep. 2020, 10, 7514. [Google Scholar] [CrossRef]
- Chen, S.; Agarwal, A.; Glushakova, O.Y.; Jorgensen, M.S.; Salgar, S.K.; Poirier, A.; Flotte, T.R.; Croker, B.P.; Madsen, K.M.; Atkinson, M.A.; et al. Gene Delivery in Renal Tubular Epithelial Cells Using Recombinant Adeno-Associated Viral Vectors. J. Am. Soc. Nephrol. 2003, 14, 947–958. [Google Scholar] [CrossRef] [Green Version]
- Tsujie, M.; Isaka, Y.; Nakamura, H.; Kaneda, Y.; Imai, E.; Hori, M. Prolonged transgene expression in glomeruli using an EBV replicon vector system combined with HVJ liposomes. Kidney Int. 2001, 59, 1390–1396. [Google Scholar] [CrossRef] [Green Version]
- Elmously, A.; Stern, J.R.; Greenberg, J.; Agrusa, C.J.; Schneider, D.B.; Ellozy, S.H.; Connolly, P.H. Carbon Dioxide Angiography in the Treatment of Transplant Renal Artery Stenosis. Ann. Vasc. Surg. 2020, 63, 198–203. [Google Scholar] [CrossRef]
- Jaganjac, S.; Schefe, L.; Avdagi, E.; Spahovi, H.; Hiros, M. Preoperative Kidney Tumor Embolization as Procedure for Therapy of Advanced Kidney Cancer. Acta Inform. Med. 2014, 22, 302. [Google Scholar] [CrossRef] [Green Version]
- Sauk, S.; Zuckerman, D. Renal Artery Embolization. Semin. Interv. Radiol. 2011, 28, 396–406. [Google Scholar] [CrossRef] [Green Version]
- Karalli, A.; Ghaffarpour, R.; Axelsson, R.; Lundell, L.; Bozoki, B.; Brismar, T.; Gustafsson, O. Transarterial Chemoembolization of Renal Cell Carcinoma: A Prospective Controlled Trial. J. Vasc. Interv. Radiol. 2017, 28, 1664–1672. [Google Scholar] [CrossRef] [Green Version]
- Valji, K. (Ed.) Standard Angiographic and Interventional Techniques. In Vascular and Interventional Radiology; Elsevier: Amsterdam, The Netherlands, 2006; pp. 15–48. [Google Scholar] [CrossRef]
- Prikhozhdenko, E.S.; Gusliakova, O.I.; Kulikov, O.A.; Mayorova, O.A.; Shushunova, N.A.; Abdurashitov, A.S.; Bratashov, D.N.; Pyataev, N.A.; Tuchin, V.V.; Gorin, D.A.; et al. Target delivery of drug carriers in mice kidney glomeruli via renal artery. Balance between efficiency and safety. J. Control. Release 2021, 329, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.W.; Moeckel, G.; Lien, Y.H.H. Kidney-targeted liposome-mediated gene transfer in mice. Gene Ther. 1997, 4, 426–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madry, H.; Reszka, R.; Bohlender, J.; Wagner, J. Efficacy of cationic liposome-mediated gene transfer to mesangial cells in vitro and in vivo. J. Mol. Med. 2001, 79, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Sindeeva, O.A.; Verkhovskii, R.A.; Abdurashitov, A.S.; Voronin, D.V.; Gusliakova, O.I.; Kozlova, A.A.; Mayorova, O.A.; Ermakov, A.V.; Lengert, E.V.; Navolokin, N.A.; et al. Effect of Systemic Polyelectrolyte Microcapsule Administration on the Blood Flow Dynamics of Vital Organs. ACS Biomater. Sci. Eng. 2020, 6, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Verkhovskii, R.; Ermakov, A.; Sindeeva, O.; Prikhozhdenko, E.; Kozlova, A.; Grishin, O.; Makarkin, M.; Gorin, D.; Bratashov, D. Effect of Size on Magnetic Polyelectrolyte Microcapsules Behavior: Biodistribution, Circulation Time, Interactions with Blood Cells and Immune System. Pharmaceutics 2021, 13, 2147. [Google Scholar] [CrossRef]
- Mayorova, O.A.; Sindeeva, O.A.; Lomova, M.V.; Gusliakova, O.I.; Tarakanchikova, Y.V.; Tyutyaev, E.V.; Pinyaev, S.I.; Kulikov, O.A.; German, S.V.; Pyataev, N.A.; et al. Endovascular addressing improves the effectiveness of magnetic targeting of drug carrier. Comparison with the conventional administration method. Nanomed. Nanotechnol. 2020, 28, 102184. [Google Scholar] [CrossRef]
- Voronin, D.V.; Sindeeva, O.A.; Kurochkin, M.A.; Mayorova, O.; Fedosov, I.V.; Semyachkina-Glushkovskaya, O.; Gorin, D.A.; Tuchin, V.V.; Sukhorukov, G.B. In Vitro and in Vivo Visualization and Trapping of Fluorescent Magnetic Microcapsules in a Bloodstream. ACS Appl. Mater. Inter. 2017, 9, 6885–6893. [Google Scholar] [CrossRef]
- Abdurashitov, A.S.; Prikhozhdenko, E.S.; Mayorova, O.A.; Plastun, V.O.; Gusliakova, O.I.; Shushunova, N.A.; Kulikov, O.A.; Tuchin, V.V.; Sukhorukov, G.B.; Sindeeva, O.A. Optical coherence microangiography of the mouse kidney for diagnosis of circulatory disorders. Biomed. Opt. Express 2021, 12, 4467. [Google Scholar] [CrossRef]
- Trushina, D.B.; Akasov, R.A.; Khovankina, A.V.; Borodina, T.N.; Bukreeva, T.V.; Markvicheva, E.A. Doxorubicin-loaded biodegradable capsules: Temperature induced shrinking and study of cytotoxicity in vitro. J. Mol. Liq. 2019, 284, 215–224. [Google Scholar] [CrossRef]
- Novoselova, M.V.; Loh, H.M.; Trushina, D.B.; Ketkar, A.; Abakumova, T.O.; Zatsepin, T.S.; Kakran, M.; Brzozowska, A.M.; Lau, H.H.; Gorin, D.A.; et al. Biodegradable Polymeric Multilayer Capsules for Therapy of Lung Cancer. ACS Appl. Mater. Inter. 2020, 12, 5610–5623. [Google Scholar] [CrossRef]
- Navolokin, N.; German, S.; Bucharskaya, A.; Godage, O.; Zuev, V.; Maslyakova, G.; Pyataev, N.; Zamyshliaev, P.; Zharkov, M.; Terentyuk, G.; et al. Systemic Administration of Polyelectrolyte Microcapsules: Where Do They Accumulate and When? In Vivo and Ex Vivo Study. Nanomaterials 2018, 8, 812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demina, P.A.; Sindeeva, O.A.; Abramova, A.M.; Prikhozhdenko, E.S.; Verkhovskii, R.A.; Lengert, E.V.; Sapelkin, A.V.; Goryacheva, I.Y.; Sukhorukov, G.B. Fluorescent Convertible Capsule Coding Systems for Individual Cell Labeling and Tracking. ACS Appl. Mater. Inter. 2021, 13, 19701–19709. [Google Scholar] [CrossRef] [PubMed]
- Ermakov, A.V.; Verkhovskii, R.A.; Babushkina, I.V.; Trushina, D.B.; Inozemtseva, O.A.; Lukyanets, E.A.; Ulyanov, V.J.; Gorin, D.A.; Belyakov, S.; Antipina, M.N. In Vitro Bioeffects of Polyelectrolyte Multilayer Microcapsules Post-Loaded with Water-Soluble Cationic Photosensitizer. Pharmaceutics 2020, 12, 610. [Google Scholar] [CrossRef] [PubMed]
- Angelatos, A.S.; Radt, B.; Caruso, F. Light-Responsive Polyelectrolyte/Gold Nanoparticle Microcapsules. J. Phys. Chem. B 2005, 109, 3071–3076. [Google Scholar] [CrossRef] [PubMed]
- Burmistrov, I.A.; Veselov, M.M.; Mikheev, A.V.; Borodina, T.N.; Bukreeva, T.V.; Chuev, M.A.; Starchikov, S.S.; Lyubutin, I.S.; Artemov, V.V.; Khmelenin, D.N.; et al. Permeability of the Composite Magnetic Microcapsules Triggered by a Non-Heating Low-Frequency Magnetic Field. Pharmaceutics 2021, 14, 65. [Google Scholar] [CrossRef] [PubMed]
- Novoselova, M.V.; German, S.V.; Sindeeva, O.A.; Kulikov, O.A.; Minaeva, O.V.; Brodovskaya, E.P.; Ageev, V.P.; Zharkov, M.N.; Pyataev, N.A.; Sukhorukov, G.B.; et al. Submicron-Sized Nanocomposite Magnetic-Sensitive Carriers: Controllable Organ Distribution and Biological Effects. Polymers 2019, 11, 1082. [Google Scholar] [CrossRef] [Green Version]
- Kozlova, A.A.; German, S.V.; Atkin, V.S.; Zyev, V.V.; Astle, M.A.; Bratashov, D.N.; Svenskaya, Y.I.; Gorin, D.A. Magnetic Composite Submicron Carriers with Structure-Dependent MRI Contrast. Inorganics 2020, 8, 11. [Google Scholar] [CrossRef] [Green Version]
- Svenskaya, Y.; Garello, F.; Lengert, E.; Kozlova, A.; Verkhovskii, R.; Bitonto, V.; Ruggiero, M.R.; German, S.; Gorin, D.; Terreno, E. Biodegradable polyelectrolyte/magnetite capsules for MR imaging and magnetic targeting of tumors. Nanotheranostics 2021, 5, 362–377. [Google Scholar] [CrossRef]
- Verkhovskii, R.A.; Kozlova, A.A.; Sindeeva, O.A.; Kozhevnikov, I.O.; Prikhozhdenko, E.S.; Mayorova, O.A.; Grishin, O.V.; Makarkin, M.A.; Ermakov, A.V.; Abdurashitov, A.S.; et al. Lightsheet-based flow cytometer for whole blood with the ability for the magnetic retrieval of objects from the blood flow. Biomed. Opt. Express 2021, 12, 380. [Google Scholar] [CrossRef]
- Kopach, O.; Pavlov, A.M.; Sindeeva, O.A.; Sukhorukov, G.B.; Rusakov, D.A. Biodegradable Microcapsules Loaded with Nerve Growth Factor Enable Neurite Guidance and Synapse Formation. Pharmaceutics 2020, 13, 25. [Google Scholar] [CrossRef]
- Kopach, O.; Zheng, K.; Dong, L.; Sapelkin, A.; Voitenko, N.; Sukhorukov, G.B.; Rusakov, D.A. Nano-engineered microcapsules boost the treatment of persistent pain. Drug Deliv. 2018, 25, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Tarakanchikova, Y.V.; Muslimov, A.R.; Zyuzin, M.V.; Nazarenko, I.; Timin, A.S.; Sukhorukov, G.B.; Lepik, K.V. Layer-by-Layer-Assembled Capsule Size Affects the Efficiency of Packaging and Delivery of Different Genetic Cargo. Part. Part. Syst. Char. 2021, 38, 2000228. [Google Scholar] [CrossRef]
- Emilio Dalledone Siqueira, D.; Terezinha Guillaumon, A. Angiography for Renal Artery Diseases. In Angiography; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Dahlqvist, U.; Tomic, T.T.; Söderberg, M.; Stubbe, J.; Enggaard, C.; Ericsson, A.; Zhou, A.X.; Björnson Granqvist, A.; William-Olsson, L. Direct Drug Delivery to Kidney via the Renal Artery. JOVE—J. Vis. Exp. 2021, 170, e61932. [Google Scholar] [CrossRef]
- Volodkin, D.V.; Larionova, N.I.; Sukhorukov, G.B. Protein Encapsulation via Porous CaCO3 Microparticles Templating. Biomacromolecules 2004, 5, 1962–1972. [Google Scholar] [CrossRef]
- Heeman, W.; Steenbergen, W.; van Dam, G.M.; Boerma, E.C. Clinical applications of laser speckle contrast imaging: A review. J. Biomed. Opt. 2019, 24, 1. [Google Scholar] [CrossRef] [Green Version]
- Boas, D.A.; Dunn, A.K. Laser speckle contrast imaging in biomedical optics. J. Biomed. Opt. 2010, 15, 011109. [Google Scholar] [CrossRef] [Green Version]
- Briers, D.; Duncan, D.D.; Hirst, E.; Kirkpatrick, S.J.; Larsson, M.; Steenbergen, W.; Stromberg, T.; Thompson, O.B. Laser speckle contrast imaging: Theoretical and practical limitations. J. Biomed. Opt. 2013, 18, 066018. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Mendes, B.C.; Oderich, G.S.; Reis de Souza, L.; Banga, P.; Macedo, T.A.; DeMartino, R.R.; Misra, S.; Gloviczki, P. Implications of renal artery anatomy for endovascular repair using fenestrated, branched, or parallel stent graft techniques. J. Vasc. Surg. 2016, 63, 1163–1169.e1. [Google Scholar] [CrossRef] [Green Version]
- Novoselova, M.V.; German, S.V.; Abakumova, T.O.; Perevoschikov, S.V.; Sergeeva, O.V.; Nesterchuk, M.V.; Efimova, O.I.; Petrov, K.S.; Chernyshev, V.S.; Zatsepin, T.S.; et al. Multifunctional nanostructured drug delivery carriers for cancer therapy: Multimodal imaging and ultrasound-induced drug release. Colloid. Surfaces B 2021, 200, 111576. [Google Scholar] [CrossRef]
- Parakhonskiy, B.V.; Shilyagina, N.Y.; Gusliakova, O.I.; Volovetskiy, A.B.; Kostyuk, A.B.; Balalaeva, I.V.; Klapshina, L.G.; Lermontova, S.A.; Tolmachev, V.; Orlova, A.; et al. A method of drug delivery to tumors based on rapidly biodegradable drug-loaded containers. Appl. Mater. Today 2021, 25, 101199. [Google Scholar] [CrossRef]
- Maksimova, E.A.; Barmin, R.A.; Rudakovskaya, P.G.; Sindeeva, O.A.; Prikhozhdenko, E.S.; Yashchenok, A.M.; Khlebtsov, B.N.; Solovev, A.A.; Huang, G.; Mei, Y.; et al. Air-Filled Microbubbles Based on Albumin Functionalized with Gold Nanocages and Zinc Phthalocyanine for Multimodal Imaging. Micromachines 2021, 12, 1161. [Google Scholar] [CrossRef] [PubMed]
- Kulikov, O.A.; Zharkov, M.N.; Ageev, V.P.; Yakobson, D.E.; Shlyapkina, V.I.; Zaborovskiy, A.V.; Inchina, V.I.; Balykova, L.A.; Tishin, A.M.; Sukhorukov, G.B.; et al. Magnetic Hyperthermia Nanoarchitectonics via Iron Oxide Nanoparticles Stabilised by Oleic Acid: Anti-Tumour Efficiency and Safety Evaluation in Animals with Transplanted Carcinoma. Int. J. Mol. Sci. 2022, 23, 4234. [Google Scholar] [CrossRef]
- Pan, B.; Zhang, H.; Hong, Y.; Ma, M.; Wan, X.; Cao, C. Indoleamine-2,3-Dioxygenase Activates Wnt/β-Catenin Inducing Kidney Fibrosis after Acute Kidney Injury. Gerontology 2021, 67, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, E.; Germann, G.; Mathur, A. Microcirculation in Muscle. Ann. Plas. Surg. 1986, 17, 13–16. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, G.; Yan, J.; Park, B.; Hoffman, A.; Tie, G.; Wang, R.; Messina, L.M. Cellular and molecular mechanism regulating blood flow recovery in acute versus gradual femoral artery occlusion are distinct in the mouse. J. Vasc. Surg. 2008, 48, 1546–1558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindsey, P.; Echeverria, A.; Poi, M.J.; Matos, J.; Bechara, C.F.; Cheung, M.; Lin, P.H. Thromboembolic Risk of Endovascular Intervention for Lower Extremity Deep Venous Thrombosis. Ann. Vasc. Surg. 2018, 49, 247–254. [Google Scholar] [CrossRef]
- Matthay, Z.A.; Flanagan, C.P.; Sanders, K.; Smith, E.J.; Lancaster, E.M.; Gasper, W.J.; Kornblith, L.Z.; Hiramoto, J.S.; Conte, M.S.; Iannuzzi, J.C. Risk factors for venous thromboembolism after vascular surgery and implications for chemoprophylaxis strategies. J. Vasc. Surg.–Venous Lymphat. Disord. 2022, 10, 585–593.e2. [Google Scholar] [CrossRef]
- Omar, M.; Schwarz, M.; Soliman, D.; Symvoulidis, P.; Ntziachristos, V. Pushing the Optical Imaging Limits of Cancer with Multi-Frequency-Band Raster-Scan Optoacoustic Mesoscopy (RSOM). Neoplasia 2015, 17, 208–214. [Google Scholar] [CrossRef] [Green Version]
- Haedicke, K.; Agemy, L.; Omar, M.; Berezhnoi, A.; Roberts, S.; Longo-Machado, C.; Skubal, M.; Nagar, K.; Hsu, H.T.; Kim, K.; et al. High-resolution optoacoustic imaging of tissue responses to vascular-targeted therapies. Nat. Biomed. Eng. 2020, 4, 286–297. [Google Scholar] [CrossRef]
- Laufer, J.G.; Zhang, E.Z.; Treeby, B.E.; Cox, B.T.; Beard, P.C.; Johnson, P.; Pedley, B. In vivo preclinical photoacoustic imaging of tumor vasculature development and therapy. J. Biomed. Opt. 2012, 17, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogunlade, O.; Connell, J.J.; Huang, J.L.; Zhang, E.; Lythgoe, M.F.; Long, D.A.; Beard, P. In vivo three-dimensional photoacoustic imaging of the renal vasculature in preclinical rodent models. Am. J. Physiol.–Renal 2018, 314, F1145–F1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monici, M. Cell and tissue autofluorescence research and diagnostic applications. Biotechnol. Annu. Rev. 2005, 11, 227–256. [Google Scholar] [CrossRef] [PubMed]
- Croce, A.; Bottiroli, G. Autofluorescence spectroscopy and imaging: A tool for biomedical research and diagnosis. Eur. J. Histochem. 2014, 58, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croce, A.C.; Ferrigno, A.; Bottiroli, G.; Vairetti, M. Autofluorescence-based optical biopsy: An effective diagnostic tool in hepatology. Liver Int. 2018, 38, 1160–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tirapelli, L.F.; Trazzi, B.F.M.; Bagnato, V.S.; Tirapelli, D.P.C.; Kurachi, C.; da Costa, M.M.; Tucci, S.; Cologna, A.J.; Martins, A.C.P. Histopathology and laser autofluorescence of ischemic kidneys of rats. Laser. Med. Sci. 2009, 24, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, J.T.; Demos, S.; Michalopoulou, A.; Pierce, J.L.; Troppmann, C. Assessment of renal ischemia by optical spectroscopy. J. Surg. Res. 2004, 122, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.H.; Wu, Y.M.; Chang, W.T.; Luo, T.; Yang, Y.C.; Cho, H.D.; Liau, I. Molecular Imaging of Ischemia and Reperfusion in Vivo with Mitochondrial Autofluorescence. Anal. Chem. 2014, 86, 5024–5031. [Google Scholar] [CrossRef] [Green Version]
- Schwinn, K.; Ferré, N.; Huix-Rotllant, M. UV-visible absorption spectrum of FAD and its reduced forms embedded in a cryptochrome protein. Phys. Chem. Chem. Phys. 2020, 22, 12447–12455. [Google Scholar] [CrossRef]
- Tercan, M.; Yılmaz İnal, F.; Seneldir, H.; Kocoglu, H. Nephroprotective Efficacy of Sugammadex in Ischemia-Reperfusion Injury: An Experimental Study in a Rat Model. Cureus 2021, 13, e15726. [Google Scholar] [CrossRef]
- Fischer, R.S.; Vangala, C.; Truong, L.; Mandayam, S.; Chavarria, D.; Granera Llanes, O.M.; Fonseca Laguna, M.U.; Guerra Baez, A.; Garcia, F.; García-Trabanino, R.; et al. Early detection of acute tubulointerstitial nephritis in the genesis of Mesoamerican nephropathy. Kidney Int. 2018, 93, 681–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novoselova, M.V.; Voronin, D.V.; Abakumova, T.O.; Demina, P.A.; Petrov, A.V.; Petrov, V.V.; Zatsepin, T.S.; Sukhorukov, G.B.; Gorin, D.A. Focused ultrasound-mediated fluorescence of composite microcapsules loaded with magnetite nanoparticles: In vitro and in vivo study. Colloid. Surfaces B 2019, 181, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Yang, Q.F.; Du, H.Y.; Tang, Y.L.; Xu, G.Z.; Yan, W.P. Spectroscopic Investigation on the Interaction of a Cyanine Dye with Serum Albumins. Chin. J. Chem. 2008, 26, 397–401. [Google Scholar] [CrossRef]
- Kurutos, A.; Ryzhova, O.; Trusova, V.; Gorbenko, G.; Gadjev, N.; Deligeorgiev, T. Symmetric Meso-Chloro-Substituted Pentamethine Cyanine Dyes Containing Benzothiazolyl/Benzoselenazolyl Chromophores Novel Synthetic Approach and Studies on Photophysical Properties upon Interaction with bio-Objects. J. Fluoresc. 2016, 26, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Saikiran, M.; Sato, D.; Pandey, S.S.; Ohta, T.; Hayase, S.; Kato, T. Photophysical characterization and BSA interaction of the direct ring carboxy functionalized unsymmetrical NIR cyanine dyes. Dyes Pigments 2017, 140, 6–13. [Google Scholar] [CrossRef]
- Bidwell, G.L.; Mahdi, F.; Shao, Q.; Logue, O.C.; Waller, J.P.; Reese, C.; Chade, A.R. A kidney-selective biopolymer for targeted drug delivery. Am. J. Physiol.–Renal 2017, 312, F54–F64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenhard, S.C.; McAlexander, A.; Virtue, A.; Fieles, W.; Skedzielewski, T.; Rambo, M.; Trinh, H.; Cheng, S.H.; Hong, H.; Isidro-Llobet, A.; et al. In Vivo Imaging of Small Molecular Weight Peptides for Targeted Renal Drug Delivery: A Study in Normal and Polycystic Kidney Diseased Mice. J. Pharmacol. Exp. Ther. 2019, 370, 786–795. [Google Scholar] [CrossRef]
- Xu, Y.; Qin, S.; Niu, Y.; Gong, T.; Zhang, Z.; Fu, Y. Effect of fluid shear stress on the internalization of kidney-targeted delivery systems in renal tubular epithelial cells. Acta Pharm. Sinica B 2020, 10, 680–692. [Google Scholar] [CrossRef]
- Liu, G.W.; Pippin, J.W.; Eng, D.G.; Lv, S.; Shankland, S.J.; Pun, S.H. Nanoparticles exhibit greater accumulation in kidney glomeruli during experimental glomerular kidney disease. Physiol. Rep. 2020, 8, e14545. [Google Scholar] [CrossRef]
- Chen, D.; Han, S.; Zhu, Y.; Hu, F.; Wei, Y.; Wang, G. Kidney-targeted drug delivery via rhein-loaded polyethyleneglycol-co-polycaprolactone-co-polyethyleneimine nanoparticles for diabetic nephropathy therapy. Int. J. Nanomed. 2018, 13, 3507–3527. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Poon, C.; Chin, D.; Milkowski, S.; Lu, V.; Hallows, K.R.; Chung, E.J. Design and in vivo characterization of kidney-targeting multimodal micelles for renal drug delivery. Nano Res. 2018, 11, 5584–5595. [Google Scholar] [CrossRef]
- Tang, T.T.; Lv, L.L.; Wang, B.; Cao, J.Y.; Feng, Y.; Li, Z.L.; Wu, M.; Wang, F.M.; Wen, Y.; Zhou, L.T.; et al. Employing Macrophage-Derived Microvesicle for Kidney-Targeted Delivery of Dexamethasone: An Efficient Therapeutic Strategy against Renal Inflammation and Fibrosis. Theranostics 2019, 9, 4740–4755. [Google Scholar] [CrossRef] [PubMed]
- Herman, T.F.; Santos, C. First Pass Effect; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- She, Z.; Antipina, M.N.; Li, J.; Sukhorukov, G.B. Mechanism of Protein Release from Polyelectrolyte Multilayer Microcapsules. Biomacromolecules 2010, 11, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Xu, Y.; Zhou, X.; Gong, T.; Zhang, Z.R.; Fu, Y. An injectable micelle-hydrogel hybrid for localized and prolonged drug delivery in the management of renal fibrosis. Acta Pharm. Sinica B 2021, 11, 835–847. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gusliakova, O.I.; Prikhozhdenko, E.S.; Plastun, V.O.; Mayorova, O.A.; Shushunova, N.A.; Abdurashitov, A.S.; Kulikov, O.A.; Abakumov, M.A.; Gorin, D.A.; Sukhorukov, G.B.; et al. Renal Artery Catheterization for Microcapsules’ Targeted Delivery to the Mouse Kidney. Pharmaceutics 2022, 14, 1056. https://doi.org/10.3390/pharmaceutics14051056
Gusliakova OI, Prikhozhdenko ES, Plastun VO, Mayorova OA, Shushunova NA, Abdurashitov AS, Kulikov OA, Abakumov MA, Gorin DA, Sukhorukov GB, et al. Renal Artery Catheterization for Microcapsules’ Targeted Delivery to the Mouse Kidney. Pharmaceutics. 2022; 14(5):1056. https://doi.org/10.3390/pharmaceutics14051056
Chicago/Turabian StyleGusliakova, Olga I., Ekaterina S. Prikhozhdenko, Valentina O. Plastun, Oksana A. Mayorova, Natalia A. Shushunova, Arkady S. Abdurashitov, Oleg A. Kulikov, Maxim A. Abakumov, Dmitry A. Gorin, Gleb B. Sukhorukov, and et al. 2022. "Renal Artery Catheterization for Microcapsules’ Targeted Delivery to the Mouse Kidney" Pharmaceutics 14, no. 5: 1056. https://doi.org/10.3390/pharmaceutics14051056