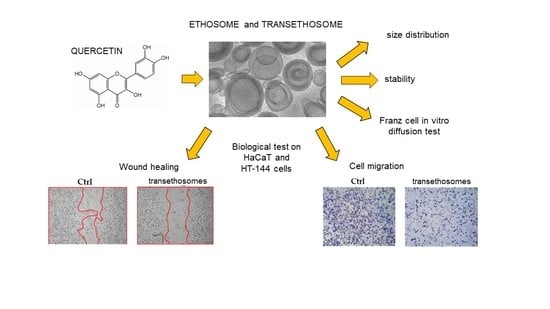
Ethosomes and Transethosomes as Cutaneous Delivery Systems for Quercetin: A Preliminary Study on Melanoma Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Ethosome Preparation
2.3. Transethosome Preparation
2.4. Cryo-Transmission Electron Microscopy (Cryo-TEM)
2.5. Photon Correlation Spectroscopy (PCS)
2.6. QT Entrapment Capacity Evaluation
2.7. Franz Cell Diffusion Experiments
2.7.1. In Vitro Release Test (IVRT)
2.7.2. In Vitro Skin Permeation Test (IVPT)
2.8. HPLC Analysis
2.9. Photochemiluminescence (PCL) Test
2.10. Biological Activity Studies
2.10.1. Cytotoxicity Study
2.10.2. Wound Healing Assay
2.10.3. Cell Migration Assay
2.11. Statistical Analysis
3. Results
3.1. Preparation of Ethosomes and Transethosomes
3.2. Characterization of Ethosomes and Transethosomes
3.2.1. Morphology and Size
3.2.2. QT Entrapment Capacity (EC)
3.3. Evaluation of QT Antioxidant Activity
3.4. Stability Studies
3.5. IVRT
3.6. IVPT
3.7. Biological Activity Studies
3.7.1. Cell Viability
3.7.2. Wound Healing Assay
3.7.3. Migration Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 16275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Sun, C.; Mao, L.; Ma, P.; Liu, F.; Yang, J.; Gao, Y. The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends Food Sci. Technol. 2016, 56, 21–38. [Google Scholar] [CrossRef]
- Tang, J.; Diao, P.; Shu, X.; Li, L.; Xiong, L. Quercetin and Quercitrin Attenuates the Inflammatory Response and Oxidative Stress in LPS-Induced RAW264.7 Cells: In Vitro Assessment and a Theoretical Model. Biomed Res. Int. 2019, 2019, 7039802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, D.D.; Zhang, W.; Gao, Y.L.; Sun, Y.Z.; Wang, H.X.; Qi, R.Q.; Chen, H.D.; Gao, X.H. Anti-inflammatory effects of quercetin in a mouse model of MC903-induced atopic dermatitis. Int. Immunopharmacol. 2019, 74, 105676. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [Green Version]
- Sajadimajd, S.; Bahramsoltani, R.; Iranpanah, A.; Kumar Patra, J.; Das, G.; Gouda, S.; Rahimi, R.; Rezaeiamiri, E.; Cao, H.; Giampieri, F.; et al. Advances on Natural Polyphenols as Anticancer Agents for Skin Cancer. Pharmacol. Res. 2020, 151, 104584. [Google Scholar] [CrossRef]
- Leonardi, G.C.; Falzone, L.; Salemi, R.; Zanghì, A.; Spandidos, D.A.; Mccubrey, J.A.; Candido, S.; Libra, M. Cutaneous melanoma: From pathogenesis to therapy (Review). Int. J. Oncol. 2018, 52, 1071–1080. [Google Scholar] [CrossRef] [Green Version]
- Nogueira, J.A.M.; Arbizu, M.V.; Rey, C.M. Adjuvant treatment of melanoma. Cancer Chemother. Rev. 2008, 3, 10–22. [Google Scholar] [CrossRef]
- Moser, J.C.; Grossmann, K.F. Adjuvant therapy for resected high-risk melanoma. Semin. Cutan. Med. Surg. 2018, 37, 109–111. [Google Scholar] [CrossRef]
- Fan, M.; Zhang, G.; Hu, X.; Xu, X.; Gong, D. Quercetin as a tyrosinase inhibitor: Inhibitory activity, conformational change and mechanism. Food Res. Int. 2017, 100, 226–233. [Google Scholar] [CrossRef]
- Cai, X.; Fang, Z.; Dou, J.; Yu, A.; Zhai, G. Bioavailability of quercetin: Problems and promises. Curr. Med. Chem. 2013, 20, 2572–2582. [Google Scholar] [CrossRef] [PubMed]
- Hatahet, T.; Morille, M.; Hommoss, A.; Devoisselle, J.M.; Müller, R.H.; Bégu, S. Quercetin topical application, from conventional dosage forms to nanodosage forms. Eur. J. Pharm. Biopharm. 2016, 108, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Park, S.N.; Lee, H.J.; Kim, H.S.; Park, M.A.; Gu, H.A. Enhanced transdermal deposition and characterization of quercetin-loaded ethosomes. Korean J. Chem. Eng. 2013, 30, 688–692. [Google Scholar] [CrossRef]
- Ramadon, D.; Anwar, E.; Harahap, Y. In vitro penetration and bioavailability of novel transdermal quercetin-loaded ethosomal gel. Indian J. Pharm. Sci. 2017, 79, 948–956. [Google Scholar] [CrossRef]
- Harris, Z.; Donovan, M.G.; Branco, G.M.; Limesand, K.H.; Burd, R. Quercetin as an Emerging Anti-Melanoma Agent: A Four-Focus Area Therapeutic Development Strategy. Front. Nutr. 2016, 3, 48. [Google Scholar] [CrossRef] [PubMed]
- Touitou, E.; Dayan, N.; Bergelson, L.; Godin, B.; Eliaz, M. Ethosomes—Novel vesicular carriers for enhanced delivery: Characterization and skin penetration properties. J. Control. Release 2000, 65, 403–418. [Google Scholar] [CrossRef]
- Abdulbaqi, I.M.; Darwis, Y.; Khan, N.A.K.; Assi, R.A.; Khan, A.A. Ethosomal nanocarriers: The impact of constituents and formulation techniques on ethosomal properties, in vivo studies, and clinical trials. Int. J. Nanomed. 2016, 11, 2279–2304. [Google Scholar] [CrossRef] [Green Version]
- Natsheh, H.; Vettorato, E.; Touitou, E. Ethosomes for Dermal Administration of Natural Active Molecules. Curr. Pharm. Des. 2019, 25, 2338–2348. [Google Scholar] [CrossRef]
- Godin, B.; Touitou, E. Ethosomes: New prospects in transdermal delivery. Crit. Rev. Ther. Drug Carr. Syst. 2003, 20, 63–102. [Google Scholar] [CrossRef]
- Costanzo, M.; Esposito, E.; Sguizzato, M.; Lacavalla, M.A.; Drechsler, M.; Valacchi, G.; Zancanaro, C.; Malatesta, M. Formulative study and intracellular fate evaluation of ethosomes and transethosomes for vitamin D3 delivery. Int. J. Mol. Sci. 2021, 22, 5341. [Google Scholar] [CrossRef]
- Song, C.K.; Balakrishnan, P.; Shim, C.K.; Chung, S.J.; Chong, S.; Kim, D.D. A novel vesicular carrier, transethosome, for enhanced skin delivery of voriconazole: Characterization and in vitro/in vivo evaluation. Colloids Surf. B Biointerfaces 2012, 92, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Ascenso, A.; Raposo, S.; Batista, C.; Cardoso, P.; Mendes, T.; Praça, F.G.; Bentley, M.V.L.B.; Simões, S. Development, characterization, and skin delivery studies of related ultradeformable vesicles: Transfersomes, ethosomes, and transethosomes. Int. J. Nanomed. 2015, 10, 5837–5851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, H.; Wen, J.; Li, H.; Meng, Y.; Zhang, Y.; Zhang, N.; Zheng, W. Enhanced transdermal permeability and drug deposition of rheumatoid arthritis via sinomenine hydrochloride-loaded antioxidant surface transethosome. Int. J. Nanomed. 2019, 14, 3177–3188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, N.R.; Wong, T.W. 5-Fluorouracil ethosomes–skin deposition and melanoma permeation synergism with microwave. Artif. Cells Nanomed. Biotechnol. 2018, 46, 568–577. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Wang, X.; Wu, J.; Zhang, D.; Zhang, L.; Song, X.; Hong, H.; He, C.; Mo, X.; Wu, S.; et al. Polyethylenimine and sodium cholate-modified ethosomes complex as multidrug carrier for the treatment of melanoma through transdermal delivery. Nanomedicine 2019, 14, 239. [Google Scholar] [CrossRef]
- U.S. Department of Health; Human Services Food; Drug Administration; Center for Drug Evaluation and Research (CDER). Transdermal and Topical Delivery Systems—Product Development and Quality Considerations. Guidance for Industry. Food Drug Adm. 2019, 84, 64319–64321. [Google Scholar]
- European Medicines Agency. Draft Guideline on Quality and Equivalence of Topical Products; European Medicines Agency: Amsterdam, The Netherlands, 2018; pp. 1–36.
- The United States Pharmacopeial Convention. 〈1724〉 Semisolid Drug Products—Performance Tests. Usp 2014, 37, 1273–1284. [Google Scholar]
- Ng, S.F.; Rouse, J.; Sanderson, D.; Eccleston, G. A Comparative study of transmembrane diffusion and permeation of ibuprofen across synthetic membranes using franz diffusion cells. Pharmaceutics 2010, 2, 209. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, S.J.; Trottet, L.; Carter, T.P.; Heard, C.M. Effects of Membrane Type and Liquid/Liquid Phase Boundary on In Vitro Release of Ketoprofen from Gel Formulations. J. Drug Target. 2003, 11, 373–379. [Google Scholar] [CrossRef]
- Van Smeden, J.; Bouwstra, J.A. Stratum Corneum Lipids: Their Role for the Skin Barrier Function in Healthy Subjects and Atopic Dermatitis Patients. Curr. Probl. Dermatol. 2016, 49, 8–26. [Google Scholar] [CrossRef]
- Haq, A.; Goodyear, B.; Ameen, D.; Joshi, V.; Michniak-Kohn, B. Strat-M® synthetic membrane: Permeability comparison to human cadaver skin. Int. J. Pharm. 2018, 547, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Pecora, R. Dynamic light scattering measurement of nanometer particles in liquids. J. Nanopart. Res. 2000, 2, 123–131. [Google Scholar] [CrossRef]
- Costa, P.; Lobo, J.M.S. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Le Guyader, G.; Do, B.; Vieillard, V.; Andrieux, K.; Paul, M. Comparison of the in vitro and ex vivo permeation of existing topical formulations used in the treatment of facial angiofibroma and characterization of the variations observed. Pharmaceutics 2020, 12, 1060. [Google Scholar] [CrossRef] [PubMed]
- Popov, I.; Levin, G. Antioxidative homeostasis: Characterization by means of chemiluminescent technique. Methods Enzym. 1999, 300, 437–456. [Google Scholar] [CrossRef]
- Sguizzato, M.; Mariani, P.; Spinozzi, F.; Benedusi, M.; Cervellati, F.; Cortesi, R.; Drechsler, M.; Prieux, R.; Valacchi, G.; Esposito, E. Ethosomes for coenzyme Q10 cutaneous administration: From design to 3D skin tissue evaluation. Antioxidants 2020, 9, 485. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; Pecorelli, A.; Mencarelli, M.; Carbotti, P.; Fortino, V. Rottlerin: A multifaced regulator of keratinocyte cell cycle. Exp. Dermatol. 2009, 18, 516–521. [Google Scholar] [CrossRef]
- Maria, M.X.; Claudia, S.; Giuseppe, B.; Franco, C.; Francesca, F.; Lila, M.A.; Giuseppe, V. SR-B1 involvement in keratinocytes in vitro wound closure. Arch. Biochem. Biophys. 2018, 658, 1–6. [Google Scholar] [CrossRef]
- Toniazzo, T.; Peres, M.S.; Ramos, A.P.; Pinho, S.C. Encapsulation of quercetin in liposomes by ethanol injection and physicochemical characterization of dispersions and lyophilized vesicles. Food Biosci. 2017, 19, 17–25. [Google Scholar] [CrossRef]
- Sguizzato, M.; Ferrara, F.; Hallan, S.S.; Baldisserotto, A.; Drechsler, M.; Malatesta, M.; Costanzo, M.; Cortesi, R.; Puglia, C.; Valacchi, G.; et al. Ethosomes and transethosomes for mangiferin transdermal delivery. Antioxidants 2021, 10, 768. [Google Scholar] [CrossRef]
- Monica, L.L.; Jordi, G.; Francisco, F.C. In situ bioadhesive film-forming system for topical delivery of mometasone furoate: Characterization and biopharmaceutical properties. J. Drug Deliv. Sci. Technol. 2020, 59, 101852. [Google Scholar] [CrossRef]
- El-Shenawy, A.A.; Abdelhafez, W.A.; Ismail, A.; Kassem, A.A. Formulation and Characterization of Nanosized Ethosomal Formulations of Antigout Model Drug (Febuxostat) Prepared by Cold Method: In Vitro/Ex Vivo and In Vivo Assessment. AAPS PharmSciTech 2020, 21, 31. [Google Scholar] [CrossRef]
- Heylings, J.R. Diffusion cell design. In Topical Drug Bioavailability, Bioequivalence, and Penetration; Howard, I., Maibach, J., Jenner, V.P.S., Eds.; Springer: New York, NY, USA, 2015; pp. 69–80. [Google Scholar] [CrossRef]
- Neuwirthova, J.; Gal, B.; Smilek, P.; Urbankova, P. Potential of the Flavonoid Quercetin to Prevent and Treat Cancer—Current Status of Research. Klin. Onkol. 2018, 31, 184. [Google Scholar] [CrossRef] [Green Version]
- Soll, F.; Ternent, C.; Berry, I.M.; Kumari, D.; Moore, T. Quercetin Inhibits Proliferation and Induces Apoptosis of B16 Melanoma. Assay Drug Dev. Technol. 2020, 18, 261. [Google Scholar] [CrossRef]
- Kim, S.H.; Yoo, E.S.; Woo, J.S.; Han, S.H.; Lee, J.H.; Jung, S.H.; Kim, H.J.; Jung, J.Y. Antitumor and apoptotic effects of quercetin on human melanoma cells involving JNK/P38 MAPK signaling activation. Eur. J. Pharmacol. 2019, 860, 172568. [Google Scholar] [CrossRef]
- Cao, H.H.; Cheng, C.Y.; Su, T.; Fu, X.Q.; Guo, H.; Li, T.; Tse, A.K.W.; Kwan, H.Y.; Yu, H.; Yu, Z.L. Quercetin inhibits HGF/c-Met signaling and HGFstimulated melanoma cell migration and invasion. Mol. Cancer 2015, 14, 103. [Google Scholar] [CrossRef] [Green Version]
- Tian, W.; Hu, Q.; Xu, Y.; Xuu, Y. Effect of soybean-lecithin as an enhancer of buccal mucosa absorption of insulin. Biomed. Mater. Eng. 2012, 22, 171–178. [Google Scholar] [CrossRef]
- McIntosh, T.J. Hydration properties of lamellar and non-lamellar phases of phosphatidylcholine and phospatidylethanolamine. Chem. Phys. Lipids 1996, 81, 117–131. [Google Scholar] [CrossRef]
- Wohlrab, J.; Klapperstück, T.; Reinhardt, H.W.; Albrecht, M. Interaction of epicutaneously applied lipids with stratum corneum depends on the presence of either emulsifiers or hydrogenated phosphatidylcholine. Ski. Pharmacol. Physiol. 2010, 23, 298. [Google Scholar] [CrossRef]
- Sguizzato, M.; Ferrara, F.; Mariani, P.; Pepe, A.; Cortesi, R.; Huang, N.; Simelière, F.; Boldrini, P.; Baldisserotto, A.; Valacchi, G.; et al. “Plurethosome” as vesicular system for cutaneous administration of mangiferin: Formulative study and 3D skin tissue evaluation. Pharmaceutics 2021, 13, 1124. [Google Scholar] [CrossRef]
- Eid, J.; Jraij, A.; Greige-Gerges, H.; Monticelli, L. Effect of quercetin on lipid membrane rigidity: Assessment by atomic force microscopy and molecular dynamics simulations. BBA Adv. 2021, 1, 100018. [Google Scholar] [CrossRef]
- Han, J.; Amau, M.; Okamoto, Y.; Suga, K.; Umakoshi, H. Investigation of Quercetin interaction behaviors with lipid bilayers: Toward understanding its antioxidative effect within biomembrane. J. Biosci. Bioeng. 2021, 132, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.J.; Yoon, J.H.; Kang, N.G.; Park, S.G.; Jeong, S.H. Diffusion properties of different compounds across various synthetic membranes using Franz-type diffusion cells. J. Pharm. Investig. 2012, 42, 271–277. [Google Scholar] [CrossRef]
- Sguizzato, M.; Valacchi, G.; Pecorelli, A.; Boldrini, P.; Simelière, F.; Huang, N.; Cortesi, R.; Esposito, E. Gallic acid loaded poloxamer gel as new adjuvant strategy for melanoma: A preliminary study. Colloids Surf. B Biointerfaces 2020, 185, 110613. [Google Scholar] [CrossRef]
- Sen, C.K.; Roy, S. Redox signals in wound healing. Biochim. Biophys. Acta Gen. Subj. 2008, 1780, 1348–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef] [Green Version]








| Formulation Code | PC 1 % w/w | Ethanol % w/w | T80 2 % w/w | Water % w/w | QT 3 % w/w |
|---|---|---|---|---|---|
| ETO0.9 | 0.9 | 29.10 | - | 70.0 | - |
| ETO0.9-QT | 0.9 | 29.05 | - | 70.0 | 0.05 |
| T-ETO0.9 | 0.9 | 29.10 | 0.3 | 69.7 | - |
| T-ETO0.9-QT | 0.9 | 29.05 | 0.3 | 69.7 | 0.05 |
| T-ETO2.7 | 2.7 | 27.00 | 0.3 | 69.7 | - |
| T-ETO2.7-QT | 2.7 | 26.95 | 0.3 | 69.7 | 0.05 |
| Formulation Code | Time (Days) | Z Average (nm) ± s.d. | Typical Intensity Distribution | Dispersity Index ± s.d. | |
|---|---|---|---|---|---|
| nm * | Area (%) * | ||||
| ETO0.9 | 1 | 212.25 ± 14.20 | 227.4 | 100 | 0.12 ± 0.01 |
| 90 | 230.52 ± 25.51 | 245.5 | 100 | 0.14 ± 0.03 | |
| ETO0.9-QT | 1 | 258.18 ± 21.42 | 230.4 | 96 | 0.24 ± 0.02 |
| 90 | 235.45 ± 20.20 | 220.7 | 97 | 0.22 ± 0.03 | |
| T-ETO0.9 | 1 | 161.90 ± 11.32 | 174.5 | 100 | 0.13 ± 0.02 |
| 90 | 229.15 ± 42.42 | 201.3 | 100 | 0.15 ± 0.03 | |
| T-ETO0.9-QT | 1 | 240.12 ± 21.30 | 230.4 | 91 | 0.24 ± 0.01 |
| 90 | 383.25 ± 47.61 | 290.2 | 87 | 0.26 ± 0.03 | |
| T-ETO2.7 | 1 | 354.00 ± 35.53 | 322.6 | 92 | 0.25 ± 0.02 |
| 90 | 362.00 ± 24.32 | 330.5 | 90 | 0.24 ± 0.01 | |
| T-ETO2.7-QT | 1 | 394.00 ± 32.21 | 350.2 | 91 | 0.23 ± 0.02 |
| 90 | 412.21 ± 27.40 | 320.8 | 88 | 0.26 ± 0.01 | |
| Formulation Code | EC 1 (%) | ACL 2 (μmol TE/g) |
|---|---|---|
| ETO0.9-QT | 56.44 ± 1.00 | 3.26 ± 0.17 |
| T-ETO0.9-QT | 59.19 ± 1.04 | 3.16 ± 0.10 |
| T-ETO2.7-QT | 64.10 ± 0.55 | 5.06 ± 0.25 |
| SOL-QT | - | 3.82 ± 0.05 |
| Formulation Code | RQT 1 ± s.d. (μg/cm2/h) | Tlag 2± s.d. (h) | AQT 3 ± s.d. (μg/cm2) | MQT 4 ± s.d. (μg) |
|---|---|---|---|---|
| ETO0.9-QT | 18.84 ± 0.91 N | 1.00 ± 0.10 N | 35.4 ± 1.2 N | 18.0 ± 1.1 N |
| 23.73 ± 0.22 P | 0.70 ± 0.00 P | 48.0 ± 2.4 P | 1.2 ± 0.1 P | |
| T-ETO0.9-QT | 24.63 ± 0.93 N | 1.04 ± 0.12 N | 40.4 ± 2.0 N | 14.0 ± 1.2 N |
| 24.68 ± 0.92 P | 0.50 ± 0.00 P | 54.0 ± 2.2 P | 3.0 ± 0.5 P | |
| T-ETO2.7-QT | 8.99 ± 0.91 N | 1.05 ± 0.33 N | 16.1 ± 0.4 N | 22.0 ± 2.2 N |
| 30.00 ± 1.20 P | 0.62 ± 0.00 P | 64.0 ± 2.4 P | 1.2 ± 0.1 P | |
| SOL-QT | 49.13 ± 2.52 N | 0.90 ± 0.01 N | 99.6 ± 3.4 N | 34.0 ± 2.0 N |
| 51.77 ± 1.91 N | 0.56 ± 0.02 P | 104.0 ± 2.8 P | 9.0 ± 0.1 P |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrara, F.; Benedusi, M.; Sguizzato, M.; Cortesi, R.; Baldisserotto, A.; Buzzi, R.; Valacchi, G.; Esposito, E. Ethosomes and Transethosomes as Cutaneous Delivery Systems for Quercetin: A Preliminary Study on Melanoma Cells. Pharmaceutics 2022, 14, 1038. https://doi.org/10.3390/pharmaceutics14051038
Ferrara F, Benedusi M, Sguizzato M, Cortesi R, Baldisserotto A, Buzzi R, Valacchi G, Esposito E. Ethosomes and Transethosomes as Cutaneous Delivery Systems for Quercetin: A Preliminary Study on Melanoma Cells. Pharmaceutics. 2022; 14(5):1038. https://doi.org/10.3390/pharmaceutics14051038
Chicago/Turabian StyleFerrara, Francesca, Mascia Benedusi, Maddalena Sguizzato, Rita Cortesi, Anna Baldisserotto, Raissa Buzzi, Giuseppe Valacchi, and Elisabetta Esposito. 2022. "Ethosomes and Transethosomes as Cutaneous Delivery Systems for Quercetin: A Preliminary Study on Melanoma Cells" Pharmaceutics 14, no. 5: 1038. https://doi.org/10.3390/pharmaceutics14051038