Abstract
Background
Persistent viral RNA shedding of SARS-CoV-2 following COVID-19 has increasingly been recognized, with limited understanding of its implications on outcomes in hospitalized COVID-19 patients.
Methods
We retrospectively assessed for persistent viral shedding across Northwestern Medicine Healthcare (NMHC) patients between March and August 2020. We assessed for predictors of persistent viral shedding, in-hospital delirium, and six-month mortality using binary logistic regression.
Results
Of the 2,518 hospitalized patients with an RT-PCR-confirmed diagnosis of COVID-19, 959 underwent repeat SARS-CoV-2 RT-PCR at least fourteen days from initial positive testing. Of those, 405 (42.2%) patients were found to have persistent viral shedding. Persistent viral shedding was associated with male sex, increased BMI, diabetes mellitus, chronic kidney disease, and exposure to corticosteroids during initial COVID-19 hospitalization. Persistent viral shedding was independently associated with incidence of in-hospital delirium after adjusting for factors including severity of respiratory dysfunction (OR 2.45; 95% CI 1.75, 3.45). Even after adjusting for age, severity of respiratory dysfunction, and occurrence of in-hospital delirium, persistent viral shedding remained significantly associated with increased six-month mortality (OR 2.43; 95% CI 1.42, 4.29).
Conclusions
Persistent viral shedding occurs frequently in hospitalized COVID-19 patients and is associated with in-hospital delirium and increased six-month mortality.
Similar content being viewed by others
Introduction
SARS-CoV-2 is the virus responsible for the Coronavirus Disease 2019 (COVID-19) global pandemic beginning March 2020, as declared by the World Health Organization [1]. As of February 2022, COVID-19 has affected over 429 million people worldwide and is responsible for over 5.9 million deaths [2]. A majority of patients infected with SARS-CoV-2 will have mild clinical symptoms, yet some develop severe disease requiring hospitalization and invasive mechanical ventilation [3, 4]. These patients face the highest risk of mortality [5]. Nucleic acid amplification tests, specifically real-time reverse transcription polymerase chain reaction (RT-PCR) assays, constitute the clinical standard for diagnosing infection with SARS-CoV-2 in hospitalized patients [6]. However, patients with persistently or intermittently positive RT-PCR assays present a clinical conundrum for health experts [7].
Evaluation for persistent viral RNA shedding is one method to track recovery and long-term response to severe viral infections. Detection of persistent viral shedding is influenced by initial disease severity, viral load, and sample source [8,9,10]. The phenomenon of persistent viral shedding is well-studied and demonstrated in numerous other viruses with broad public health impact, including avian influenza A [11], Zika [12], and Ebola [13]. It is unclear whether persistent detection of SARS-CoV-2 RNA in routine nasopharyngeal swabs of hospitalized patients represents replicating virus or simply viral nucleic acid in cell debris. Additionally, it is unresolved whether persistent RNA detection provides any prognostic or mechanistic information regarding a hospitalized patient’s clinical course or potential for developing long-term post-viral syndromes. Therefore, it becomes challenging to counsel individuals appropriately regarding the interpretation and risks of a persistently positive SARS-CoV-2 RT-PCR assay.
Moreover, numerous studies with other viruses have suggested that those patients with persistent viral shedding demonstrate a dysfunctional T-cell response [14, 15], and hospitalized patients with severe disease have more active and persistent viral replication [16]. In this setting, weakened host immune responses may slow viral clearance and contribute to persistent viral shedding [17]. Severe COVID-19 disease, male sex, and delayed presentation for treatment are all recognized risk factors for prolonged viral shedding [18].
To further inform ongoing clinical care of hospitalized COVID-19 patients and explore disease mechanisms, we sought to determine the prevalence and risk factors for persistent viral RNA shedding and evaluate its association with delirium, as the primary neurologic complication of acute COVID-19 [19, 20], and six-month mortality in hospitalized COVID-19 patients.
Methods
This study was reviewed and approved by the Northwestern University Institutional Review Board, and given the retrospective nature of the study, a waiver of the need for informed consent was granted (STU00212627).
Patient Identification
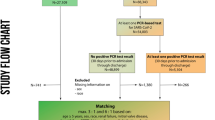
We used an automated query of the Northwestern Medicine Healthcare (NMHC) electronic medical record (EMR) to identify all patients admitted to an NMHC hospital for a diagnosis of COVID-19 between March 5th and August 9th 2020. NMHC consists of a Chicago (Illinois, USA) area network of ten hospitals, including an academic medical center, and affiliated outpatient centers serviced by a central EMR system. COVID-19 diagnosis was confirmed by a documented positive SARS-CoV-2 RT-PCR assay of nasopharyngeal swab or bronchoalveolar lavage fluid. In order to assess for evidence of persistent shedding of SARS-CoV-2 nucleic acid, inclusion in the study cohort also required availability of a repeat SARS-CoV-2 RT-PCR assay 14 days or longer after the initial diagnostic RT-PCR. A 14-day time period from initial confirmed positive RT-PCR (rather than symptom onset) was used to define persistent shedding as a conservative interpretation of the Centers for Disease Control and Prevention’s (CDC) 14-day COVID-19 quarantine/isolation endorsement [21]. Identification of a repeat nasopharyngeal SARS-CoV-2 RT-PCR assay was determined using query of the NMHC EMR, including query of the 20 Illinois hospital systems participating in the EPIC (EPIC Systems Corporation, Verona, WI) Care Everywhere Network. All RT-PCR assays were collected for clinical indications. The resulting cohort of patients were dichotomized into those whose repeat RT-PCR assay(s) were never positive after 14 days (Negative Shedding) and those who had at least one positive repeat RT-PCR documented after 14 days (Persistent Shedding).
Data Collection Procedures
Demographic, medical comorbidity and COVID-19 hospital course data were collected by EMR review. Laboratory and hospital medication administration data were collected by automated electronic query. Acute respiratory failure and acute respiratory distress syndrome (ARDS) were identified from the documentation of each patient’s attending intensive care physician, and the severity of COVID-19 pulmonary disease was categorized as no acute respiratory failure, acute respiratory failure, and ARDS for subsequent analysis. The presence of the delirium-encephalopathy syndrome during the COVID-19 hospitalization was identified using results from Confusion Assessment Method (CAM) evaluations that were serially performed and documented contemporaneously by each patient’s nurse. The CAM is a well-validated and widely used clinical and research tool for the identification of the delirium-encephalopathy syndrome and has been in routine clinical use at NMHC since 2008 [22]. A broader syndrome of encephalopathy during the COVID-19 hospitalization (subsequently referred to as acute brain dysfunction) was also identified as ever having a positive CAM evaluation for delirium or ever being in coma, as assessed by having a Richmond Agitation and Sedation Score (RASS) of -4 or -5 (-4: no response to voice but movement or eye opening to physical stimulation; -5: no response to voice or physical stimulation). We also documented if patients ever experienced agitation during COVID-19 hospitalization as assessed by the RASS (+ 2 to + 4). Similar to the CAM, the RASS has been in routine clinical use at NMHC since 2008 and is a widely used standardized scale throughout intensive care units [23].
Discharge disposition was extracted from the EMR. Six-month post-hospital admission mortality status was available for each patient using an NMHC-wide COVID-19 mortality monitoring initiative. We also identified all acute-care hospital reencounters (hospitalizations, emergency room visits, or observations stays) that occurred within four months of discharge from the initial COVID-19 hospitalization in any of the 20 hospital systems participating in the EPIC Care Everywhere Network.
Statistical analysis
All statistical analysis was performed using R version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria) with two-sided P ≤ 0.05 considered statistically significant. Data were summarized as number of patients (frequency), mean (standard deviation) for normally distributed variables, and median (interquartile range [IQR]) for non-normally distributed variables. Associations were assessed using Fisher’s exact test, Spearman’s rank correlation test, and Wilcoxon rank-sum test. A priori adjusted binary logistic regression models were developed for: 1) variables associated with persistent shedding, 2) association of persistent shedding with experiencing delirium during the COVID-19 hospitalization, and 3) association of persistent shedding with 6-month mortality. All models were adjusted for a priori variables of age, age2 (to consider greater effects at the extremes of age), and sex. The a priori model for persistent viral shedding also included administration of corticosteroids during the hospitalization—as this might be expected to modulate inflammation and viral clearance—and common comorbidities widely reported to predict more severe COVID-19, including: body mass index (BMI), diabetes mellitus, hypertension, vascular disease, and chronic kidney disease (CKD) [24]. The a priori model of delirium was adjusted for corticosteroid administration—as corticosteroids have previously been described as a potential risk factor for delirium [25]—and for factors our group has previously described as associated with delirium in COVID-19, including: prior history of any central nervous system disease, CKD, and respiratory dysfunction severity [26]. Sedative medication exposure was not considered in the delirium model, as sedative exposure was highly collinear with respiratory dysfunction severity, and existing delirium literature suggests that delirium related to sedative exposure is equally detrimental as delirium due to other causes, such as sepsis and hypoxia [27]. The a priori model of six-month mortality was adjusted for respiratory dysfunction severity and delirium occurrence since our group has previously shown these factors to be associated with mortality after COVID-19. To avoid overfitting models to the data, we also used a backward variable selection algorithm based on Akaike Information Criteria optimization to compare effect estimates in a priori and parsimoniously adjusted models.
In addition, we performed a mediation analysis in the persistent shedding model. Mediation analysis can provide insight into potential disease mechanisms by identifying factors that mediate the association between exposure and outcome variables and therefore may lie along a causal pathway between exposure and outcome. Given the prominence of respiratory failure in COVID-19 and reports of inflammation and coagulopathy as potential contributors to COVID-19 pathophysiology, we considered respiratory dysfunction severity, maximum C-reactive protein (non-specific marker of inflammation), and maximum D-dimer (non-specific marker of coagulopathy) as potential mediator variables. We assessed for mediation qualitatively as described by Baron and Kenny [28] and approximated the magnitude of mediation using the R package MMA (Multiple Mediation Analysis). We considered no meaningful mediation to occur if < 20% of an association was explained by mediators, partial mediation to occur if 20–80% of an association was explained by mediators, and complete mediation to occur if > 80% of an association was explained by mediators. In the event of identifying mediation, the mediator variables were included in the development of the parsimoniously adjusted model of persistent shedding while any completely mediated variables were removed.
Results
A total of 2518 patients were admitted to an NMHC hospital for COVID-19 between March 5th and August 9th 2020 of whom 959 (38.1%, mean age 59.4 (17.1) years, 480 (50.1%) females) were identified as having a repeat SARS-CoV-2 RT-PCR 14 days or later from their initial diagnostic SARS CoV-2 RT-PCR. The Negative Shedding group included 554 (57.8%) patients while the Persistent Shedding group included 405 (42.2%) patients, and Table 1 summarizes the demographics, comorbidities, hospital events, and outcome endpoints of the cohort. These groups did not differ in age, race, or ethnicity but the Persistent Shedding group had a higher proportion of males (53.8% versus 47.1%, p = 0.047). The Persistent Shedding group had more repeat RT-PCR tests performed at 14 days or later (median 4 [3, 7] versus 3 [2, 5], p < 0.001) but the Negative Shedding group had RT-PCR assays performed over a longer period of time (median 131.49 [62.79, 196.23] versus 63.97 [30.46, 149.19] days from initial diagnostic RT-PCR, p < 0.001). Overall, the last positive RT-PCR in the Persistent Shedding group occurred at median 34.56 [21.40, 56.66] days from the initial RT-PCR. However, 49 (12.1%) of the Persistent Shedding patients had positive RT-PCR results at 90 days or later, and the longest observed positive RT-PCR test was at 269 days from initial positive RT-PCR test. Of the Persistent Shedding patients, 111 (27.4%) had a positive repeat RT-PCR after having had a prior negative repeat RT-PCR, and the positive-after-negative repeat RT-PCR occurred at median 53.40 [33.04, 88.10] days from the initial positive RT-PCR.
Clinical Factors Associated with Persistent Shedding
In unadjusted analysis (Table 1) and the a priori model (Table 2), occurrence of Persistent Shedding was associated with male sex, BMI, diabetes mellitus, CKD, and corticosteroid exposure during initial COVID-19 hospitalization. However, consideration of the mediators respiratory dysfunction severity, maximum C-reactive protein, and maximum D-dimer (Table 2) jointly resulted in complete mediation of the BMI association and partial mediation of the male sex, diabetes mellitus, and corticosteroid exposure associations with persistent shedding. Of note, no mediation occurred for CKD. As individual mediators, the strongest mediation effects resulted from respiratory dysfunction severity (mediation for: male sex 28%, BMI 100%, diabetes mellitus 32%, corticosteroids 35%) followed by maximum C-reactive protein (mediation for: BMI 28% [suggesting some shared mediation with respiratory dysfunction severity], corticosteroids 26%), supporting a causal role of these variables in a priori associations of male sex, BMI, diabetes mellitus, and corticosteroid exposure. Maximum D-dimer did not contribute to > 20% mediation for any variable. After consideration of mediation and parsimonious model development, CKD (adjusted OR 1.51, 95% CI [1.06, 2.15]), respiratory dysfunction severity (respiratory failure OR 1.48, 95% CI [1.03, 2.13]; ARDS OR 3.06, 95% CI [2.02, 4.65]), and maximum C-reactive protein (OR 1.004 per mg/dL, 95% CI [1.002, 1.006]) were independently associated with persistent shedding.
Associations with Delirium and Acute Brain Dysfunction
Persistent shedding was associated with both incidence of in-hospital delirium (ever CAM positive; unadjusted OR 3.21, 95% CI [2.45, 4.22], p < 0.001) and acute brain dysfunction (ever CAM positive or coma by RASS; unadjusted OR 3.18, 95% CI [2.43, 4.18], p < 0.001). Furthermore, persistent shedding remained significantly and independently associated with incidence of delirium (adjusted OR 2.45, 95% CI [1.75, 3.45], p < 0.001) and acute brain dysfunction (adjusted OR 2.40, 95% CI [1.71, 3.38], p < 0.001) during COVID-19 hospitalization, even after adjusting for factors including respiratory failure severity (Table 3).
Associations with Discharge Disposition and Mortality
Persistent Shedding patients experienced significantly longer COVID-19 hospitalization lengths of stay (20.08 (20.59) versus 10.83 (11.89) days, p < 0.001) and were less likely to be discharged to home (50.1% versus 76.4%, p < 0.001; unadjusted OR 0.31, 95% CI [0.24, 0.41], p < 0.001). Overall, Persistent Shedding patients were not more likely than Negative Shedding patients to experience an acute-care hospital reencounter within four months [29]; however, amongst patients who had at least one reencounter, Persistent Shedding patients had more reencounters in four months than Negative Shedding patients (median 2 [1, 4] versus 2 [1, 3], p = 0.011). Persistent Shedding patients also had greater 6-month mortality (14.8% versus 3.8%, p < 0.001; unadjusted OR 4.41, 95% CI [2.68, 7.55], p < 0.001). Persistent shedding remained significantly associated with increased six-month mortality even after adjustment for age, respiratory dysfunction severity, and occurrence of delirium (Table 4; adjusted OR 2.43, 95% CI [1.42, 4.29], p = 0.002).
Discussion
Persistent viral shedding is a known marker of initial disease severity and varies extensively based on viral infection type, corticosteroid/antiretroviral treatment, and host immunity [8, 30]. However, SARS-CoV-2 viral load has not necessarily correlated with disease severity, though current literature remains conflicting [31, 32]. SARS-CoV-2 is unique in its increased duration of persistent shedding of viral RNA, even in comparison to other coronaviruses such as SARS-CoV and MERS-CoV (mean 17 versus 15 days) [33]. Understanding these differences in viral shedding, infectious potential, and impact are critical to public health treatment and containment policies. While viral nucleic acid detection by RT-PCR is unable to discern between active or inactive virus, nor assess response to therapy, the presence of a positive SARS-CoV-2 RT-PCR test may provide other insights into the host’s viral clearance capabilities or severity of the initial viral infection itself. Given the ongoing widespread clinical use of the nasopharyngeal SARS-CoV-2 RT-PCR test, the implications of persistent viral shedding have never been more relevant to public health.
The host immune response plays a pivotal role in viral clearance efficiency. Specifically, the T-cell mediated response is necessary for SARS-CoV-2 clearance [34]. Robust activation of the T-cell response following SARS-CoV-2 infection may also lead to exhaustion, impairing ongoing host T-cell function [35]. Persistent viral shedding in this setting may suggest an inadequate or ineffective T-cell response. However, despite detectable T-cell responses, some infected individuals continue to shed virus during recovery [34]. This failure to clear the virus may be secondary to dysregulation of the host immune response, or evasion of the immune system by the viral pathogen. The existence of viral reservoirs capable of evading host immune responses and leading to chronic states of infection are known, and human immunodeficiency virus (HIV) [36] and hepatitis B (HBV) [37] are well established examples of this phenomenon. A viral reservoir mechanism may play a role in SARS-CoV-2 infection, with the nasopharynx serving as a potential viral reservoir in those patients with persistently positive nasopharyngeal SARS-CoV-2 RT PCR tests. [38, 39].
The dysfunctional immune response influencing viral clearance may also contribute to the higher incidence of in-hospital delirium recognized in Persistent Shedding patients. Delirium is a complex, broadly encompassing phenotype, within the encephalopathy spectrum, that has numerous triggers and predisposing medical comorbidities [40]. The link between inflammation and delirium has only recently gained significant recognition. Though the exact mechanisms by which a systemic inflammatory response contributes to delirium remain elusive, numerous studies have established the foundations for pursuing these mechanisms [41, 42]. Given that COVID-19 is an inflammatory disease at onset, it is possible that the same mechanisms leading to persistent viral shedding also contribute to in-hospital delirium, further supporting the inflammation-mediated etiology of delirium hypothesis. Improved understanding of the mechanisms leading to delirium in COVID-19 is greatly needed given the likely contribution of delirium to early death and accelerated aging through chronic cognitive and functional impairment and earlier onset dementia. [19, 43, 44].
The relationship between inflammation and steroid exposure during COVID-19 is not straightforward. Steroid exposure during incident COVID-19 was associated with increased risk of persistent viral shedding. Similar findings have been reported with other viral infections, including influenza infections requiring hospitalization [45]. However, treatment with dexamethasone in COVID-19 requiring hospitalization has demonstrated improved outcomes, reducing short-term mortality and indications for mechanical ventilation [46]. Long-term implications of dexamethasone in COVID-19 on morbidity and secondary infections remain unknown and may be influenced by overall duration of steroid exposure. Our data suggest that prolonged steroid administration might be expected to increase the incidence of persistent viral shedding, but how it may impact outcomes in this setting remains unknown.
There is also strong reason to consider the role of microvascular endothelial cells in the pathogenesis of delirium in COVID-19 patients [47]. First, SARS-CoV-2 infects endothelial cells, causing endothelial cell injury (endotheliitis), promoting the genesis of microthrombi and disrupting the blood–brain barrier [47]. Second, both SARS-CoV-2 infection [48,49,50,51,52,53,54,55,56,57,58,59,60] and the vascular risk factors associated with increased COVID-19 mortality (e.g. obesity) [61] are known to be associated with endothelial dysfunction. Third, there is increasing evidence that microvascular dysfunction critically contributes to the pathogenesis of delirium, by impairing regional cerebral blood flow and neurovascular coupling and disrupting the blood–brain barrier [62]. It is presently unknown whether persistent viral shedding in the nasopharynx is also associated with persistent viral replication in the microvascular endothelial cells of the cerebral circulation. In that regard, it is important that many other viruses, including the human cytomegalovirus (CMV), also exhibit endothelial tropism and CMV replication in endothelial cells can persist for decades [63]. The hypothesis that SARS-CoV-2-induced persistent microvascular dysfunction contributes to the long-term sequelae of COVID-19 warrants further experimental testing.
Along with the retrospective design, incomplete capture of patients returning to the hospital remains a limitation of our study. This may have inadvertently biased the study in a conservative manner, as patients presenting to hospitals outside the NMHC and Care Everywhere systems would not have been captured through our study methods. Given the broad catchment area of NMHC in conjunction with the EPIC Care Everywhere Network and likelihood of patients to return to similar treatment facilities, we suspect this effect would have been minimal in our cohort and not significantly altered our results. The frequency and timing of respiratory specimen collection was not standardized across all patients, and some studies have suggested a circadian association between positive SARS-CoV-2 RNA detection in nasopharyngeal swabs [64]. However, since all samples were tested for clinical indications, this would have preferentially biased for sample collection during clinical symptoms (though perhaps not symptoms specific to COVID-19). A subsequent study with standardized evaluation of follow-up RT-PCR testing would rectify this limitation. Additionally, the CDC’s evolving definition of persistent infection versus re-infection was not initially addressed in our methods. A total of 49 patients in our study (12.1%) were noted to have a positive persistent RT-PCR result more than 90 days from initial positive test, which would now classify them as warranting evaluation for re-infection before they could be classified as persistent shedding. Furthermore, not all historical risk factors for developing the delirium-encephalopathy syndrome were available for analysis. For example, while data were available on a medical history of nervous system disease as a risk factor for developing delirium, data on whether patients had ever previously experienced delirium during a hospitalization were not available, which would be expected to predict future episodes of delirium during subsequent hospitalizations. An additional limitation is that the time from symptom onset to diagnosis of COVID-19 was not available for this entire cohort, which prevented investigating if the duration of symptoms experienced prior to initial positive SARS-CoV-2 RT-PCR was associated with the occurrence of persistent viral shedding. However, a separately published study encompassing the first month of NMHC’s pandemic experience found that patients who developed encephalopathy were typically hospitalized one day sooner after symptom onset than those who did not develop encephalopathy (median 6 [3, 9] versus 7 [4, 10] days, p = 0.014); although, the relationship between duration of symptoms before hospitalization and hospital discharge outcome was not statistically significant after accounting for other factors associated with outcome [19]. This study hypothesized that earlier presentation to hospital resulted from those with encephalopathy having an overall more severe disease trajectory.
Overall, the findings from the current study have broad implications on clinical decisions regarding treatment and follow-up evaluation of hospitalized COVID-19 patients. Persistent viral shedding as defined by positive nasopharyngeal RT-PCR testing is a frequent event, occurring in as many as 42.2% of re-tested hospitalized patients. Persistent viral shedding likely depends on the severity of the infection and the immune status of the patients [65]. In previous studies the reported prevalence of persistent viral shedding ranged from 12.8% to 38% [65, 66]. This phenomenon may warrant closer observation and follow-up in high-risk patients (those with severe disease, male gender, obesity, and exposure to corticosteroids as identified by our study). It is unknown if patients with persistent viral shedding may require longer periods of isolation. Additional studies are needed to evaluate the specific implications of persistent viral shedding in patients infected with SARS-CoV-2, including the risk of disease transmission and the duration of isolation needed for a safe resumption of social activities and return to the workplace. Further immunologic analyses and T-cell phenotyping may help elucidate the cause of persistent viral shedding. Whether post-acute sequelae of SARS-CoV-2 infection occur more frequently in previously hospitalized patients with persistent viral shedding remains to be determined. This may be a reasonable hypothesis, given our findings of worse discharge disposition and greater delirium incidence and mortality in those experiencing persistent viral shedding. As novel variants of SARS-CoV-2 emerge, a deeper immunologic understanding of host response and association with outcomes with specific variants may help identify “at-risk” hospitalized patients and better inform clinicians on management and treatment.
References
Cucinotta D, Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020;91:157–60.
Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–4.
Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–42.
Paules CI, Marston HD, Fauci AS. Coronavirus Infections-More Than Just the Common Cold. JAMA. 2020;323:707–8.
Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–81.
Hanson KE, Caliendo AM, Arias CA, Hayden MK, Englund JA, Lee MJ, Loeb M, Patel R, El Alayli A, Altayar O, Patel P, Falck-Ytter Y, Lavergne V, Morgan RL, Murad MH, Sultan S, Bhimraj A and Mustafa RA. The Infectious Diseases Society of America Guidelines on the Diagnosis of COVID-19: Molecular Diagnostic Testing. Clin Infect Dis. 2021.
Widders A, Broom A, Broom J. SARS-CoV-2: The viral shedding vs infectivity dilemma. Infect Dis Health. 2020;25:210–5.
Joukar F, Yaghubi Kalurazi T, Khoshsorour M, Taramian S, Mahfoozi L, Balou HA, Jafarinezhad A, Pourkazemi A, Hesni E, Asgharnezhad M, Shenagari M, Jahanzad I, Naghipour M, Maroufizadeh S, Mansour-Ghanaei F. Persistence of SARS-CoV-2 RNA in the nasopharyngeal, blood, urine, and stool samples of patients with COVID-19: a hospital-based longitudinal study. Virol J. 2021;18:134.
Owusu D, Pomeroy MA, Lewis NM, Wadhwa A, Yousaf AR, Whitaker B, Dietrich E, Hall AJ, Chu V, Thornburg N, Christensen K, Kiphibane T, Willardson S, Westergaard R, Dasu T, Pray IW, Bhattacharyya S, Dunn A, Tate JE, Kirking HL, Matanock A and Household Transmission Study T. Persistent SARS-CoV-2 RNA Shedding without Evidence of Infectiousness: A Cohort Study of Individuals with COVID-19. J Infect Dis. 2021.
Bjorkman KK, Saldi TK, Lasda E, Bauer LC, Kovarik J, Gonzales PK, Fink MR, Tat KL, Hager CR, Davis JC, Ozeroff CD, Brisson GR, Larremore DB, Leinwand LA, McQueen MB and Parker R. Higher viral load drives infrequent SARS-CoV-2 transmission between asymptomatic residence hall roommates. J Infect Dis. 2021.
Wang Y, Guo Q, Yan Z, Zhou D, Zhang W, Zhou S, Li YP, Yuan J, Uyeki TM, Shen X, Wu W, Zhao H, Wu YF, Shang J, He Z, Yang Y, Zhao H, Hong Y, Zhang Z, Wu M, Wei T, Deng X, Deng Y, Cai LH, Lu W, Shu H, Zhang L, Luo H, Ing Zhou Y, Weng H, Song K, Yao L, Jiang M, Zhao B, Chi R, Guo B, Fu L, Yu L, Min H, Chen P, Chen S, Hong L, Mao W, Huang X, Gu L, Li H, Wang C, Cao B and Network CA-C. Factors Associated With Prolonged Viral Shedding in Patients With Avian Influenza A(H7N9) Virus Infection. J Infect Dis. 2018;217:1708–1717.
Paz-Bailey G, Rosenberg ES, Doyle K, Munoz-Jordan J, Santiago GA, Klein L, Perez-Padilla J, Medina FA, Waterman SH, Gubern CG, Alvarado LI, Sharp TM. Persistence of Zika Virus in Body Fluids - Final Report. N Engl J Med. 2018;379:1234–43.
Subissi L, Keita M, Mesfin S, Rezza G, Diallo B, Van Gucht S, Musa EO, Yoti Z, Keita S, Djingarey MH, Diallo AB, Fall IS. Ebola Virus Transmission Caused by Persistently Infected Survivors of the 2014–2016 Outbreak in West Africa. J Infect Dis. 2018;218:S287–91.
Brooks DG, Teyton L, Oldstone MB, McGavern DB. Intrinsic functional dysregulation of CD4 T cells occurs rapidly following persistent viral infection. J Virol. 2005;79:10514–27.
Zheng HY, Zhang M, Yang CX, Zhang N, Wang XC, Yang XP, Dong XQ, Zheng YT. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell Mol Immunol. 2020;17:541–3.
van Kampen JJA, van de Vijver D, Fraaij PLA, Haagmans BL, Lamers MM, Okba N, van den Akker JPC, Endeman H, Gommers D, Cornelissen JJ, Hoek RAS, van der Eerden MM, Hesselink DA, Metselaar HJ, Verbon A, de Steenwinkel JEM, Aron GI, van Gorp ECM, van Boheemen S, Voermans JC, Boucher CAB, Molenkamp R, Koopmans MPG, Geurtsvankessel C, van der Eijk AA. Duration and key determinants of infectious virus shedding in hospitalized patients with coronavirus disease-2019 (COVID-19). Nat Commun. 2021;12:267.
Hu F, Chen F, Ou Z, Fan Q, Tan X, Wang Y, Pan Y, Ke B, Li L, Guan Y, Mo X, Wang J, Wang J, Luo C, Wen X, Li M, Ren P, Ke C, Li J, Lei C, Tang X, Li F. A compromised specific humoral immune response against the SARS-CoV-2 receptor-binding domain is related to viral persistence and periodic shedding in the gastrointestinal tract. Cell Mol Immunol. 2020;17:1119–25.
Xu K, Chen Y, Yuan J, Yi P, Ding C, Wu W, Li Y, Ni Q, Zou R, Li X, Xu M, Zhang Y, Zhao H, Zhang X, Yu L, Su J, Lang G, Liu J, Wu X, Guo Y, Tao J, Shi D, Yu L, Cao Q, Ruan B, Liu L, Wang Z, Xu Y, Liu Y, Sheng J, Li L. Factors Associated With Prolonged Viral RNA Shedding in Patients with Coronavirus Disease 2019 (COVID-19). Clin Infect Dis. 2020;71:799–806.
Liotta EM, Batra A, Clark JR, Shlobin NA, Hoffman SC, Orban ZS and Koralnik IJ. Frequent neurologic manifestations and encephalopathy-associated morbidity in Covid-19 patients. Ann Clin Transl Neurol. 2020.
Chou SH, Beghi E, Helbok R, Moro E, Sampson J, Altamirano V, Mainali S, Bassetti C, Suarez JI, McNett M, Consortium GC-N and Consortium E. Global Incidence of Neurological Manifestations Among Patients Hospitalized With COVID-19-A Report for the GCS-NeuroCOVID Consortium and the ENERGY Consortium. JAMA Netw Open. 2021;4:e2112131.
Centers for Disease Control and Prevention. COVID-19: When to Quarantine. https://www.cdc.gov/coronavirus/2019-ncov/your-health/quarantine-isolation.html. Accessed 11 Feb 2021.
Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP and Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–8.
Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, Tesoro EP, Elswick RK. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–44.
Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, the Northwell C-RC, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M and Zanos TP. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059.
Schreiber MP, Colantuoni E, Bienvenu OJ, Neufeld KJ, Chen KF, Shanholtz C, Mendez-Tellez PA, Needham DM. Corticosteroids and transition to delirium in patients with acute lung injury. Crit Care Med. 2014;42:1480–6.
Liotta EM, Batra A, Clark JR, Shlobin NA, Hoffman SC, Orban ZS, Koralnik IJ. Frequent neurologic manifestations and encephalopathy-associated morbidity in Covid-19 patients. Ann Clin Transl Neurol. 2020;7:2221–30.
Girard TD, Thompson JL, Pandharipande PP, Brummel NE, Jackson JC, Patel MB, Hughes CG, Chandrasekhar R, Pun BT, Boehm LM, Elstad MR, Goodman RB, Bernard GR, Dittus RS, Ely EW. Clinical phenotypes of delirium during critical illness and severity of subsequent long-term cognitive impairment: a prospective cohort study. Lancet Respir Med. 2018;6:213–22.
Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–82.
Clark JR, Batra A, Shlobin NA, Hoffman SC, Orban ZS, Koralnik IJ, Liotta EM. Acute-care hospital reencounters in COVID-19 patients. Geroscience. 2021.
Chen WJ, Yang JY, Lin JH, Fann CS, Osyetrov V, King CC, Chen YM, Chang HL, Kuo HW, Liao F, Ho MS. Nasopharyngeal shedding of severe acute respiratory syndrome-associated coronavirus is associated with genetic polymorphisms. Clin Infect Dis. 2006;42:1561–9.
Argyropoulos KV, Serrano A, Hu J, Black M, Feng X, Shen G, Call M, Kim MJ, Lytle A, Belovarac B, Vougiouklakis T, Lin LH, Moran U, Heguy A, Troxel A, Snuderl M, Osman I, Cotzia P, Jour G. Association of Initial Viral Load in Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Patients with Outcome and Symptoms. Am J Pathol. 2020;190:1881–7.
Maltezou HC, Raftopoulos V, Vorou R, Papadima K, Mellou K, Spanakis N, Kossyvakis A, Gioula G, Exindari M, Froukala E, Martinez-Gonzalez B, Panayiotakopoulos G, Papa A, Mentis A, Tsakris A. Association Between Upper Respiratory Tract Viral Load, Comorbidities, Disease Severity, and Outcome of Patients With SARS-CoV-2 Infection. J Infect Dis. 2021;223:1132–8.
Cevik M, Tate M, Lloyd O, Maraolo AE, Schafers J, Ho A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe. 2021;2:e13–22.
Le Bert N, Tan AT, Kunasegaran K, Tham CYL, Hafezi M, Chia A, Chng MHY, Lin M, Tan N, Linster M, Chia WN, Chen MI, Wang LF, Ooi EE, Kalimuddin S, Tambyah PA, Low JG, Tan YJ, Bertoletti A. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584:457–62.
De Biasi S, Meschiari M, Gibellini L, Bellinazzi C, Borella R, Fidanza L, Gozzi L, Iannone A, Lo Tartaro D, Mattioli M, Paolini A, Menozzi M, Milic J, Franceschi G, Fantini R, Tonelli R, Sita M, Sarti M, Trenti T, Brugioni L, Cicchetti L, Facchinetti F, Pietrangelo A, Clini E, Girardis M, Guaraldi G, Mussini C, Cossarizza A. Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat Commun. 2020;11:3434.
Pomerantz RJ. Reservoirs of human immunodeficiency virus type 1: the main obstacles to viral eradication. Clin Infect Dis. 2002;34:91–7.
Nassal M. HBV cccDNA: viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut. 2015;64:1972–84.
Chertow D, Stein S, Ramelli S, Grazioli A, Chung J-Y, Singh M, Yinda CK, Winkler C, Dickey J, Ylaya K, Ko SH, Platt A, Burbelo P, Quezado M, Pittaluga S, Purcell M, Munster V, Belinky F, Ramos-Benitez M, Boritz E, Herr D, Rabin J, Saharia K, Madathil R, Tabatabai A, Soherwardi S, McCurdy M, Peterson K, Cohen J, de Wit E, Vannella K, Hewitt S and Kleiner D. SARS-CoV-2 infection and persistence throughout the human body and brain. 2021.
Goh D, Lim JCT, Fernández SB, Lee JN, Joseph CR, Neo ZW, Guerrero S, Lau MC, Sheng JYP. Persistence of residual SARS-CoV-2 viral antigen and RNA in tissues of patients with long COVID-19. 2022.
Davis DH, Kreisel SH, Muniz Terrera G, Hall AJ, Morandi A, Boustani M, Neufeld KJ, Lee HB, Maclullich AM, Brayne C. The epidemiology of delirium: challenges and opportunities for population studies. Am J Geriatr Psychiatry. 2013;21:1173–89.
Cerejeira J, Firmino H, Vaz-Serra A, Mukaetova-Ladinska EB. The neuroinflammatory hypothesis of delirium. Acta Neuropathol. 2010;119:737–54.
van Gool WA, van de Beek D, Eikelenboom P. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet. 2010;375:773–5.
Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, Brummel NE, Hughes CG, Vasilevskis EE, Shintani AK, Moons KG, Geevarghese SK, Canonico A, Hopkins RO, Bernard GR, Dittus RS, Ely EW and Investigators B-IS. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369:1306–16.
Davis DH, Muniz Terrera G, Keage H, Rahkonen T, Oinas M, Matthews FE, Cunningham C, Polvikoski T, Sulkava R, MacLullich AM, Brayne C. Delirium is a strong risk factor for dementia in the oldest-old: a population-based cohort study. Brain. 2012;135:2809–16.
Lee N, Chan PK, Hui DS, Rainer TH, Wong E, Choi KW, Lui GC, Wong BC, Wong RY, Lam WY, Chu IM, Lai RW, Cockram CS, Sung JJ. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis. 2009;200:492–500.
Group RC, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384:693–704.
Ostergaard L. SARS CoV-2 related microvascular damage and symptoms during and after COVID-19: Consequences of capillary transit-time changes, tissue hypoxia and inflammation. Physiol Rep. 2021;9:e14726.
Ambrosino P, Calcaterra I, Molino A, Moretta P, Lupoli R, Spedicato GA, Papa A, Motta A, Maniscalco M and Di Minno MND. Persistent Endothelial Dysfunction in Post-Acute COVID-19 Syndrome: A Case-Control Study. Biomedicines. 2021;9.
Andrianto, Al-Farabi MJ, Nugraha RA, Marsudi BA and Azmi Y. Biomarkers of endothelial dysfunction and outcomes in coronavirus disease 2019 (COVID-19) patients: A systematic review and meta-analysis. Microvasc Res. 2021;138:104224.
Charfeddine S, Ibn Hadj Amor H, Jdidi J, Torjmen S, Kraiem S, Hammami R, Bahloul A, Kallel N, Moussa N, Touil I, Ghrab A, Elghoul J, Meddeb Z, Thabet Y, Kammoun S, Bouslama K, Milouchi S, Abdessalem S and Abid L. Long COVID 19 Syndrome: Is It Related to Microcirculation and Endothelial Dysfunction? Insights From TUN-EndCOV Study. Front Cardiovasc Med. 2021;8:745758.
Jud P, Gressenberger P, Muster V, Avian A, Meinitzer A, Strohmaier H, Sourij H, Raggam RB, Stradner MH, Demel U, Kessler HH, Eller K, Brodmann M. Evaluation of Endothelial Dysfunction and Inflammatory Vasculopathy After SARS-CoV-2 Infection-A Cross-Sectional Study. Front Cardiovasc Med. 2021;8:750887.
Mejia-Renteria H, Travieso A, Sagir A, Martinez-Gomez E, Carrascosa-Granada A, Toya T, Nunez-Gil IJ, Estrada V, Lerman A, Escaned J. In-vivo evidence of systemic endothelial vascular dysfunction in COVID-19. Int J Cardiol. 2021;345:153–5.
Mezoh G, Crowther NJ. Endothelial Dysfunction as a Primary Consequence of SARS-CoV-2 Infection. Adv Exp Med Biol. 2021;1321:33–43.
Moretta P, Maniscalco M, Papa A, Lanzillo A, Trojano L, Ambrosino P. Cognitive impairment and endothelial dysfunction in convalescent COVID-19 patients undergoing rehabilitation. Eur J Clin Invest. 2022;52:e13726.
Prasad M, Leon M, Lerman LO, Lerman A. Viral Endothelial Dysfunction: A Unifying Mechanism for COVID-19. Mayo Clin Proc. 2021;96:3099–108.
Ruhl L, Pink I, Kuhne JF, Beushausen K, Keil J, Christoph S, Sauer A, Boblitz L, Schmidt J, David S, Jack HM, Roth E, Cornberg M, Schulz TF, Welte T, Hoper MM, Falk CS. Endothelial dysfunction contributes to severe COVID-19 in combination with dysregulated lymphocyte responses and cytokine networks. Signal Transduct Target Ther. 2021;6:418.
Sabioni L, De Lorenzo A, Lamas C, Muccillo F, Castro-Faria-Neto HC, Estato V, Tibirica E. Systemic microvascular endothelial dysfunction and disease severity in COVID-19 patients: Evaluation by laser Doppler perfusion monitoring and cytokine/chemokine analysis. Microvasc Res. 2020;134:104119.
Sashindranath M, Nandurkar HH. Endothelial Dysfunction in the Brain: Setting the Stage for Stroke and Other Cerebrovascular Complications of COVID-19. Stroke. 2021;52:1895–904.
Seitz A, Ong P. Endothelial dysfunction in COVID-19: A potential predictor of long-COVID? Int J Cardiol. 2022;349:155–6.
Wagner JUG, Bojkova D, Shumliakivska M, Luxan G, Nicin L, Aslan GS, Milting H, Kandler JD, Dendorfer A, Heumueller AW, Fleming I, Bibli SI, Jakobi T, Dieterich C, Zeiher AM, Ciesek S, Cinatl J, Dimmeler S. Increased susceptibility of human endothelial cells to infections by SARS-CoV-2 variants. Basic Res Cardiol. 2021;116:42.
Balasubramanian P, Kiss T, Tarantini S, Nyul-Toth A, Ahire C, Yabluchanskiy A, Csipo T, Lipecz A, Tabak A, Institoris A, Csiszar A, Ungvari Z. Obesity-induced cognitive impairment in older adults: a microvascular perspective. Am J Physiol Heart Circ Physiol. 2021;320:H740–61.
Wilson JE, Mart MF, Cunningham C, Shehabi Y, Girard TD, MacLullich AMJ, Slooter AJC, Ely EW. Delirium. Nat Rev Dis Primers. 2020;6:90
Revello MG, Gerna G. Human cytomegalovirus tropism for endothelial/epithelial cells: scientific background and clinical implications. Rev Med Virol. 2010;20:136–55.
McNaughton CD, Adams NM, Johnson CH, Ward MJ, Lasko TA. Diurnal variation in SARS-CoV-2 PCR test results: Test accuracy may vary by time of day. medRxiv. 2021:2021.03.12.21253015.
Vena A, Taramasso L, Di Biagio A, Mikulska M, Dentone C, De Maria A, Magnasco L, Nicolini LA, Bruzzone B, Icardi G, Orsi A, Pelosi P, Ball L, Battaglini D, Brunetti I, Loconte M, Patroniti NA, Robba C, Bavastro M, Cerchiaro M, Giacobbe DR, Schiavetti I, Berruti M, Bassetti M and group Gs. Prevalence and Clinical Significance of Persistent Viral Shedding in Hospitalized Adult Patients with SARS-CoV-2 Infection: A Prospective Observational Study. Infect Dis Ther. 2021;10:387–398.
Yu F, Yan L, Wang N, Yang S, Wang L, Tang Y, Gao G, Wang S, Ma C, Xie R, Wang F, Tan C, Zhu L, Guo Y, Zhang F. Quantitative Detection and Viral Load Analysis of SARS-CoV-2 in Infected Patients. Clin Infect Dis. 2020;71:793–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare related to the manuscript content.
Financial Support
Dr. Liotta is supported by National Institutes of Health grant L30 NS098427.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Preliminary information from this study was presented in abstract form at the October 2021 Neurocritical Care Society meeting in Chicago, IL USA.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Batra, A., Clark, J.R., Kang, A.K. et al. Persistent viral RNA shedding of SARS-CoV-2 is associated with delirium incidence and six-month mortality in hospitalized COVID-19 patients. GeroScience 44, 1241–1254 (2022). https://doi.org/10.1007/s11357-022-00561-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-022-00561-z