Abstract
Starch-polyacrylic acid-polyvinylsulfonic acid (St-g-PAA-PVSA) graft copoymer was synthesized by gamma radiation as an initiator. The chemical structure, morphology, and thermal stability of the graft copolymer were investigated using FTIR, SEM, and TGA. The St-g-PAA-PVSA graft copolymer was employed as an adsorbent for the removal of Co(II) and Eu(III) radionuclides from their aqueous solutions by batch adsorption method. Several experimental factors such as pH, contact time, initial concentration of adsorbate, and temperature were used to find the best conditions for the removal of Co(II) and Eu(III) radionuclides. The pseudo-second order kinetic model better fits the adsorption kinetic data of radionuclides. Langmuir models had the ability to well describe the equilibrium data of adsorption of radionuclides. Thermodynamic parameters were calculated and suggested the adsorption process of Co(II) was endothermic while exothermic in the case of Eu(III) adsorption and both adsorption systems were spontaneous in nature. Among the examined desorbing agents, both AlCl3 and HCl were succeeded to desorb most of the radionuclides.
Similar content being viewed by others
Introduction
Recently, many papers have shown an increasing interest in modifying the renewable source-based biodegradable polymers as a substitution of conventional synthetic materials because they are non-toxic, biodegradable, cheap, and easy to develop [1, 2]. Starch (St) is a polysaccharides polymer and has advantages above other biodegradable polymers such as its low cost, wide using and total composability without toxic remains. Because of the drawbacks of St such as lowly process capacity and poor long-standing stability, high sensitivity to water, and weak mechanical properties [3,4,5,6]. Many study attempts had been utilized both in industrial and academic organizations to solve these problems through chemical modification or cross-linking St. Also, modification of St was tested with various synthetic monomers, e.g., acrylamide, methacrylamide, acrylic acid, vinyl imidazole, acrylonitrile, vinyl alcohol, styrene [6,7,8]. El-hoshoudy and Desouky synthesized the acryloylated St then it's grafted by poly(acrylamide-vinylmethacrylate/1-vinyl-2-pyrrolidone) terpolymer in presence of diallylamine and dimethylphenylvinylsilane as a crosslinker through emulsified polymerization process [9]. Xiong et al., prepared St-based wood adhesive by grafting vinyl acetate and butyl acrylate with different ratios [10]. Abdelmonem et al., created and investigated a low-cost St-AA-VSA/f-MWCNTs nanocomposite [11]. Worzakowska prepared the St grafted-terpene acrylate by free radical polymerization [12]. Wang et al. synthesized a ternary flocculants based on St, acrylic acid, and chitosan by radical reaction [13]. Işıklan and Geyik synthesized novel temperature and pH-sensitive graft copolymer of κ-carrageenan with N,N-dimethylaminoethyl methacrylate as well as acrylic acid using 4,4-Azobis (4-cyanovaleric acid) under microwave irradiation [14]. Mittal et al. prepared polyvinyl alcohol/St and cellulosic material barley husk based composite films [15]. Superabsorbent polymers consisting of St, acrylic acid, acrylamide, poly(vinyl alcohol), 2-hydroxyethyl methacrylate, and 2-acrylamido-2-methylpropane sulfonic acid were produced and characterized by Czarnecka and Nowaczyk [5, 7].
Polyacrylic acid (PAA) is a high-absorbency polymer that can absorb and retain water while swelling to many times its original volume. It has been employed in water treatment as a result of this unique property [5, 7, 11, 16, 17]. Polyvinylsulfonic acid (PVSA) (as sodium salt) is a polyelectrolyte with negatively charged sulfonate groups. Vinylsulfonic acid (VSA) is a monomer used to make extremely acidic and anionic homopolymers and copolymers. These polymers are employed as photoresists and ion-conductive polymer electrolyte membranes for fuel cells in the industry. Polyvinylsulfonic acid, for example, can be used to make translucent membranes with high ion exchange capacity and proton conductivity [5, 7, 11, 16,17,18,19].
Radioactive materials are being used in a growing number of industrial investigations, including industrial radiography, nuclear medicine, agriculture, and oil production, as well as academic research. Therefore, radioactive waste management becomes a worldwide problem. 60Co(II) and 152+154Eu(III) radionuclides are among the most dangerous radionuclides. These isotopes emit γ-rays of high energies and have long half-lives. They may cause dangerous human disorders. As a result, removing such nuclides from radioactive wastewater is critical. Various technologies are existing to remove the radionuclides such as ion exchange, chemical precipitation, flocculation, coagulation, and reverse osmosis. Unfortunately, these technologies’ generality is expensive and/ or environmentally unfriendly. Adsorption has been popular as a method for eliminating radionuclides because of its many benefits, including high efficiency, environmental friendliness, and low cost [11, 20,21,22,23]. There is little research that has been interested in St and its derivatives as adsorbents for the removal of radionuclides and radioactive waste treatment [11, 24, 25]. The goal and novelty of this study are to develop and test a low-cost and new starch-grafted poly(acrylic acid-vinylsulfonic acid) (St-g-PAA-PVSA) graft copolymer for removing Co(II) and Eu(III) radionuclides from the radioactive waste under various experimental settings. The physical and chemical characterization of the St-g–PAA–PVSA can aid in determining the sorption mechanism as well as provide information on heat stability.
Experimental
Materials
St was obtained from Nice Chemical Pvt. Ltd. (India). Co(II) chloride, Eu(III) oxide. N, N′-Methylenebisacrylamide (NMBA), and acrylic acid (AA), were purchased from Merck (Germany). Vinyl sulfonic acid sodium salt solution monomer (VSA) was purchased from Aldrich. Methanol was obtained from ADWIC, Egypt. Bi-distilled water was used in all experiments, for preparation, dilution, and analytical purposes. all chemicals and reagents were of the highest purity grade.
Synthesis of St-g–PAA–PVSA Graft Copolymer
St-g–PAA–PVSA graft copolymer was synthesized by dispersing 2.50 g of St in 40 mL bi-distilled water and heated at 80 °C in a water bath until a homogeneous solution was obtained. Then, 8.75 g of AA, 3.75 g of VSA monomers, and 0.25 g of NMBA were added to the suspension via stirring and the mixture was completed to 60 mL and sonicated for 30 min. The mixture was subjected to gamma irradiation at dose of 20 KGy and room temperature using a 60Co –γ -ray field (Co -60 gamma cell of type MC -20, Cyclotron Project, Inshas, Egypt). The sample was then cut into small pieces and washed in a water–methanol solution. The grafted material was separated, filtered, and dried at 75 °C overnight to a constant weight.
Characterization
The structural features of the St-g-PAA-PVSA graft copolymer were released by Fourier transform infrared analysis (FT-IR). The FT-IR spectrum was recorded in the midinfrared range (4000–400 cm−1) with 4 cm−1 resolution using a Shimadzu infrared spectrometer (BOMEM, FT-IR, Japan) by KBr disc method with 98:2% of KBr: polymer concentration and the number of averaged scans equal 16. The morphological structure was obtained by scanning electron microscope (SEM) (JEOL -JSM 6510 LA, Japan) at high magnification and resolution. Thermogravimetric analysis (TGA) and differential thermal analysis (DTA) were performed at a heating rate of 20 °C/min using a Shimadzu DTG -60 thermal analyzer, Japan.
Adsorption Studies of Radioactive Isotopes
The adsorption behavior of Co(II) and Eu(III) radionuclides toward the St-g–PAA–PVSA graft copolymer was investigated using the batch technique. 0.01 g of the graft copolymer was equilibrated with 5 mL of the solution containing a definite amount of 60Co(II) or 152+154Eu(III) radionuclides stock solution, individually, by mechanical shaking at 25 ± 1 °C for 24 h. Aliquots of the solution before and after the equilibration were taken, and the radioactivity was assayed radiometrically by a single-channel analyzer (Spetech ST 360 to crystal, USA). Removal percentage of the radionuclides was calculated using the following equation [22]:
The removal capacity (q) (mg/g) was obtained from:
where Co and Ce (mg/L) are the beginning and the final radionuclides concentration, V is the volume of the radionuclide solution (L), and m is the adsorbent mass (g).
Desorption Experiments
Desorption investigations were carried out in the order to recover and reuse adsorbents in a variety of adsorption processes. 0.05 g of the St-g–PAA–PVSA graft copolymer was contacted with 5 mL of 250 mg/L of Co(II) and Eu(III) ion, individually, spiked with 60Co(II) and 152+154Eu(III) radionuclides at pH ~ 5 and ~ 4, respectively, for 24 h. The Co(II)-loaded St-g–PAA–PVSA graft copolymer and Eu(III) loaded St-g–PAA–PVSA graft copolymer were separated by centrifugation and dispersed into 5 mL of the desorbing agent (0.001–0.1 M of HCl, NaCl, MgCl2, and AlCl3) and leave under shaking at 120 rpm for 24 h. the Co(II) and Eu(III) radionuclides were measured radiometrically in the supernatant. The desorption percentage of the concerned radionuclide was calculated using the following equation.
Results and Discussion
Synthesis of St-g–PAA–PVSA Adsorbent
The starch grafted-vinyl monomers mechanism was discussed in [26,27,28,29,30,31]. In this study, the St-PAA-PVSA graft copolymer was synthesized by free radical polymerization using gamma irradiation in the presence of NMBA. When water is irradiated, three primary reactive species emerge: hydrated electrons, hydroxyl radicals, and hydrogen radicals. When the aqueous solution of the St, AA and VSA is irradiated by gamma radiation, the hydroxyl radicals are created from water. The hydroxyl radicals abstract hydrogen atoms from St backbone, resulting in the formation of macroradicals. The scheme shown in Fig. 1 is based on a fact that the C1–C2 (end groups) and C2–C3 are predominant sites for the graft copolymerization initiation [26].
Structural Analysis
FT-IR
FT-IR spectrum of the St and the St-g–PAA–PVSA graft copolymer is shown in Fig. 2. In the FT-IR spectrum of the St, the absorption band at 1386 cm−1 is the characteristic absorbance band of –C–OH, O–C–H and C–C–H. A broad absorption band at 3410 cm−1 for δ(OH) stretching vibration and a narrow absorption band at 2930 cm−1 is ascribed to C-H. The band at 1645 cm−1 is ascribed to the δ(OH) bending of tightly band water. Wavenumbers at 1158, 1082 and 1015 cm−1 describe C–O–C stretching (triplet for St). In the case of the St-g-PAA-PVSA graft copolymer, characteristic bands, assigned to PAA, appeared. The carboxyl acid group was detected by absorption of carbonyl C = O group at about 1740 cm−1 (C = O stretching). A strong band at 3443 cm−1 indicates the presence of OH groups. The absorption peaks at 1403 cm−1 could be assigned to asymmetric stretching vibrations of COO − groups. the peak at 1634 cm−1 is ascribed to the δ(OH) bending of tightly band water present in St. The absorption bands at 1032 cm−1 and 1167 cm−1 is characteristic absorbance band of symmetric stretching of SO2 of the PVSA.[11, 22, 26, 32, 33].
SEM
Figure 3 shows SEM images of the St and the St-g–PAA–PVSA graft copolymer. St micrographs show spherical or oval granules with smooth surfaces free of scratches or cracks. The SEM micrographs of St-g-PAA-PVSA graft copolymer contain irregular morphology with different size granules and appeared as a porous surface with interconnected pores. This irregular morphology may be caused by exposure to heat during the production of the graft copolymer. Heat and moisture can generate a minor gelatinization of the granule's surface, causing the granules to stick together and form aggregates [34]. In addition, irregular shape promotes the production of sufficient holes due to the binding of hydroxyl groups, which allows hydrogen and covalent connections to form between St chains [35]. Rough surfaces of the graft copolymer lead to enhancement of its surface area and increase the interaction between the graft copolymer and the radionuclides and decrease the diffusion limitations in radionuclides adsorption [11].
TGA
Figure 4 shows the TGA and the TDA of the St and the St-g–PAA–PVSA graft copolymer. The St shows three-step thermal degradation. The first step occurs in the temperature range 81–172ºC with 11.82% weight loss due to dehydration and pyrolytic volatilization processes. The second and the third stages are consecutive overlapping steps that occurred in the temperature range 275–490ºC with 72.53% weight loss. This is attributed to loss by depolymerization and oxidation of the organic matter. St-g–PAA–PVSA graft copolymer shows the fifth decomposition stage, the first stage continued up to the temperature of 200 °C and count for about 7.15% weight loss. This corresponds to a loss of moisture. The second stage of degradation was observed from 200 to 290 °C with weight loss of about 14.13%. It is mostly contributed to the decomposition of St. In the third stage, the decomposition is characterized by a weight loss of 16.97% due to the chain scission of the PAA and PVSA molecules, which has a maximum temperature of 385 °C. The fourth and fifth stages occur with the temperature range 385–470 °C with 21.7% weight loss due to the breaking up of covalent bonds. The DTA curves released the TGA results; St exhibited endothermic peaks at 142.38 ºC and exothermic at 300.92. However, St-g–PAA–PVSA showed endothermic decomposition at 156.90, 212.35, 313.50, 441.08, 525.12, and 567.05 ºC, and exothermic peaks at 247.84, 405.70, 546.69, and 581.87 ºC. The TGA-DTA studies revealed that the decomposition temperature of the St-g–PAA–PVSA graft copolymer was higher than that of native St [5, 6, 8, 11].
Batch Adsorption Optimization
Effect of pH
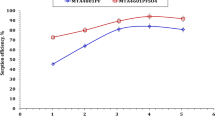
Figure 5 shows how the pH values influence the adsorption of Co(II) and Eu(III) radionuclides on the St-g-PAA-PVSA graft copolymer. The adsorption capacity for both radionuclides is very low at low pH values then increases with an increase in the pH values, clearly owing to the nature of the functional groups in the St-g-PAA-PVSA graft copolymer. At low pH, the surface charge of the graft copolymer will become positive and competition between H+ and Co(II) or Eu(III) radionuclides for occupancy of the active sites was increased. Whereas at higher pH values the surface charge of the graft copolymer becomes negative, increasing radionuclides adsorption. The adsorption capacity of Eu (III) is higher than that of Co(II), this may be attributed to the adsorption capacity values are proportional to the ionic potential, i.e., Z/r of ions; along with other factors, especially in the case of Eu(III) (as an f-block element) whose coordination bonds are predominantly electrostatic. Consequently, it can be expected that the adsorption capacity of Eu3+ ions should be higher than that of Co2+ ions. [22, 23, 36].
Adsorption Kinetics
Figure 6 shows the influence of the contact time on the adsorption of Co(II) and Eu(III) radionuclides. The results showed that adsorption has occurred rapidly. Then the adsorption nearly remains at a constant with increasing time, the adsorption was appeared to proceed rapidly when the numbers of available sites are larger than the number of adsorbed radionuclides.
Lagergren’s pseudo-first-order (Eq. 4), pseudo-second-order (Eq. 5), and Intra-particle diffusion models (Eq. 6) were applied to describe the adsorption process of Co(II) and Eu(III) radionuclides onto St-g-PAA-PVSA graft copolymer [37].
where qe (mg/g) is the amount of absorbed radionuclides at equilibrium, qt (mg/g) is the amount of absorbed radionuclides at t, t is the time. k1 (min−1), K2 (g/mg min) and Kdiff (mg/ g min0.5) are the constants of pseudo-first order, pseudo-second order and intra-particle diffusion models, respectively. m is denoting the adsorption mechanism for the intra-particle diffusion to be the rat-determine step, the value of m should be equal to 0.5.
Figure 7 depicted the nonlinear fitting of the experimental kinetics data. The calculated kinetic data of the adsorption of Co(II) and Eu(III) radionuclides were given in Table 1. According to the high correlation coefficient (R2), it can be observed that the experimental data for the adsorption of 60Co(II) was closed to pseudo-second-order model (R2 = 0.907) more than intra-particle diffusion model (R2 = 0.727) and pseudo-first-order (R2 = 0.701).
In the case of the Eu(III) adsorption, the pseudo-first-order model and the pseudo-second-order model both have high R2 values of 0.922 and 0.925, respectively, indicating the simultaneous occurrence of physical diffusion and chemical adsorption. A higher R2 (0.925) and lower stander error (SE) (0.146) confirmed that the adsorption process predominantly followed the pseudo-second-order kinetic model. It's worth noting that on the surface of an adsorbent, both physisorption and chemisorption can occur at the same time since a layer of molecules can be physically adsorbed on top of an underlying chemisorbed layer [38]. The intra-particle diffusion model for both radionuclides demonstrated the least fitting to experimental kinetic data. However, the m value, not equal to 0.5 for both systems implies that the intra-particle diffusion mechanism does not solely limit the overall adsorption process.
Adsorption Isotherms
The adsorption isotherm is helping in understanding the interaction between the graft copolymer and the radionuclides in the adsorption process. Figure 8 shows that the adsorption capacity of 60Co(II) and 152+154Eu(III) radionuclides increase by increasing the initial concentration of radionuclides. It is maybe a result of an increase of driving force for mass transfer at high initial concentration.
In this study, Langmuir (Eq. (7)), Freundlich (Eq. (8)), Temkin (Eq. (9)), and Dubinin–Radushkevich (Eq. (10, 11)) isotherm models were investigated to analysis of the equilibrium data of sorption of Co(II) and Eu(III) radionuclides onto the St-g-PAA-PVSA graft copolymer [25, 37].
where Ce is the concentration of radionuclides at equilibrium in solution (mg/L), qm is the maximum capacity of monolayer coverage (mg/g), and KL (L/mg), KF (mg1−n Ln/g), KT (L/g) and KDR (mol/J)2 are constants Langmuir, Freundlich, Temkin, and Dubinin–Radushkevich isotherm models. n represents a constant of the adsorption intensity. B(J/mol) = RT/b, b is related to the heat of adsorption, R is the universal gas constant (8.314 J /mol K), and T is the absolute temperature (K), ε (J mol−1) is the Polanyi potential which is related to equilibrium.
Figure 9 shows non-linear fits of the experimental isotherm data. The parameters of the studied isotherm models were summarized and are listed in Table 2. The high correlation coefficient (R2) value indicated that the experimental data for the adsorption of 60Co(II) and 152+154Eu (III) radionuclides by the St-g-PAA-PVSA graft copolymer follow the Langmuir model better than other models. According to the Langmuir model, adsorption happens by monolayer adsorption onto a homogenous surface.
Thermodynamic Studies
The effect of temperature on the adsorption of Co(II) and Eu(III) radionuclides onto the St-g–PAA–PVSA graft copolymer was determined by varying the temperature between 20 and 70 °C. Figure 10 shows that the removal capacities of Co(II) radionuclides slightly increase with the increasing temperature this may be attributed to decreases the viscosity of the solution and correspondingly increases the diffusion rate of the radionuclides within the pores of the graft copolymer by increasing temperature. While the removal capacities of Eu(III) radionuclides slightly decrease, showing that the sorption reaction is exothermic.
Thermodynamic parameters such as the adsorption standard free energy changes (ΔGo), the standard enthalpy change (ΔHo) and the standard entropy change (ΔSo) are obtained from experiments at various temperatures using the following equations: [39, 40, 40, 41]
where KL (L/mg) is the Langmuir constant. The values of ΔHo (kJ/mol) and ΔSo (kJ/mol K) can be calculated from the slopes and the intercepts of the linear straight lines by plotting lnKL against 1/T of Arrhenius reaction. The values of ΔGo (kJ/mol) can be calculated from Eq. (11). the values of ΔH°, ΔS°, and ΔG° are given in Table 3.
In case of the adsorption of Co(II) radionuclides:
-
The positive value of ΔH° indicated that the adsorption was endothermic.
-
The positive value of ΔSo for Co(II) radionuclides indicated that the randomness is increased at the solid–solute interface,
In case of the adsorption of Eu(III) radionuclides:
-
The negative value of ΔH° indicated that the adsorption was exothermic.
-
The negative value of ΔSo for Eu(III) radionuclides assumed is the arrangement of Eu(III) radionuclides is shaped more ordered onto the surface of the graft copolymer after adsorption
The negative value of ΔGo for the adsorption of both radionuclides indicated the feasibility of the reaction and spontaneous nature of the adsorption at a given temperature.
Desorption Study- Batch Method
Figure 11 illustrates the influence of desorbing agent concentrations (0.001–0.1 M of HCl, NaCl, MgCl2 and AlCl3) on the desorption percentage of Co(II) and Eu(III). the data in the figure show that the desorbing agent concentration played a significant role in the desorption of Co(II) and Eu(III) radionuclides. Increasing the desorbing agent concentration, resulting in an increase in the desorption percentage of Co(II) and Eu(III) radionuclides. The maximum desorption percentage of Co(II) radionuclides was ~ 59.60 and ~ 54.72% which was achieved by ≥ 0.1 M of AlCl3 and HCl, respectively. While The maximum desorption percentage of Eu(III) radionuclides was ~ 63.72 and ~ 63.05% which were achieved by ≥ 0.1 M of AlCl3 and HCl, respectively. These desorption results proposed that the radionuclides are adsorbed onto the active sites of the graft copolymer. While the fraction remained onto the adsorbent, 45–40% of radionuclides could not be desorbed, which clarified that an insignificant amount of radionuclides are adsorbed onto the internal adsorption sites [42, 43].
Conclusion
The St-g-PAA-PVSA graft copolymer was successfully synthesized and its chemical structure, morphology and thermal stability were investigated by FTIR, SEM, and TGA. The St-g-PAA-PVSA graft copolymer was applied as an adsorbent for Co(II) and Eu(III) radionuclides from their aqueous solutions. Modeling of the kinetic data showed that the pseudo-second-order model was the best one for describing the Co(II) radionuclides adsorption process. In the case of the adsorption of Eu(III) radionuclides, both pseudo-first order and pseudo-second order kinetic models better fit the adsorption kinetic data. But a higher R2 (0.925) and lower SE (0.146) confirmed that the adsorption process predominantly followed the pseudo-second-order kinetic model. Langmuir isotherm models had the ability to represent the equilibrium isotherms data. Thermodynamic parameters deduced that Co(II) radionuclides was endothermic while and Eu(III) radionuclides was exothermic and both adsorption systems were spontaneous in nature. At concentrations of 0.1 M, AlCl3 and HCl obtain a maximum desorption percentage of Co(II) radionuclides of about 59.60 and 54.72%, respectively, whereas AlCl3 and HCl achieve a maximum desorption percentage of Eu(III) ions of about 63.72 and 63.05%, respectively.
References
Ojogbo E, Ogunsona EO, Mekonnen TH (2020) Chemical and physical modifications of starch for renewable polymeric materials. Mater. Today Sustain. 7–8:100028
Jiang T, Duan Q, Zhu J et al (2020) Starch-based biodegradable materials: Challenges and opportunities. Adv. Ind. Eng. Polym. Res. 3:8–18
Cornejo-Ramírez YI, Martínez-Cruz O, Del Toro-Sánchez CL et al (2018) The structural characteristics of starches and their functional properties. CYTA – J Food. https://doi.org/10.1080/19476337.2018.1518343
Alcázar-Alay SC, Meireles MAA (2015) Physicochemical properties, modifications and applications of starches from different botanical sources. Food Sci Technol. https://doi.org/10.1590/1678-457X.6749
Czarnecka E, Nowaczyk J (2021) Synthesis and characterization superabsorbent polymers made of starch, acrylic acid, acrylamide, poly(Vinyl alcohol), 2-hydroxyethyl methacrylate, 2-acrylamido-2-methylpropane sulfonic acid. Int J Mol Sci. https://doi.org/10.3390/ijms22094325
Xun J, Lou T, Xing J et al (2019) Synthesis of a starch–acrylic acid–chitosan copolymer as flocculant for dye removal. J Appl Polym Sci. https://doi.org/10.1002/app.47437
Czarnecka E, Nowaczyk J (2020) Semi-Natural superabsorbents based on Starch-g-poly(acrylic acid): modification, synthesis and application. Polymers (Basel). https://doi.org/10.3390/polym12081794
Wijaya C, Do QD, Ju YH et al (2019) Isolation and characterization of starch from Limnophila aromatica. Heliyon 5:e01622. https://doi.org/10.1016/j.heliyon.2019.e01622
El-hoshoudy AN, Desouky SM (2018) Synthesis and evaluation of acryloylated starch-g-poly (Acrylamide/Vinylmethacrylate/1-Vinyl-2-pyrrolidone) crosslinked terpolymer functionalized by dimethylphenylvinylsilane derivative as a novel polymer-flooding agent. Int. J. Biol. Macromol. 116:434–442. https://doi.org/10.1016/j.ijbiomac.2018.05.056
Zia-ud-Din CL, Ullah I et al (2018) Synthesis and characterization of starch-g-poly(vinyl acetate-co-butyl acrylate) bio-based adhesive for wood application. Int. J. Biol. Macromol. 114:1186–1193. https://doi.org/10.1016/j.ijbiomac.2018.03.178
Abdelmonem IM, Metwally E, Siyam TE et al (2019) Radiation synthesis of starch-acrylic acid–vinyl sulfonic acid/multiwalled carbon nanotubes composite for the removal of 134 Cs and 152+154 Eu from aqueous solutions. J. Radioanal. Nucl. Chem. 319:1145–1157. https://doi.org/10.1007/s10967-018-6392-1
Worzakowska M (2019) Novel starch-g-copolymers obtained using acrylate monomers prepared from two geometric isomers of terpene alcohol. Eur. Polym. J. 110:265–275. https://doi.org/10.1016/j.eurpolymj.2018.11.024
Xun J, Lou T, Xing J et al (2019) Synthesis of a starch–acrylic acid–chitosan copolymer as flocculant for dye removal. J. Appl. Polym. Sci. 136:1–7. https://doi.org/10.1002/app.47437
Geyik G, Işıklan N (2020) Synthesis, characterization and swelling performance of a temperature/pH-sensitive κ-carrageenan graft copolymer. Int. J. Biol. Macromol. 152:359–370. https://doi.org/10.1016/j.ijbiomac.2020.02.129
Mittal A, Garg S, Bajpai S (2020) Fabrication and characteristics of poly (vinyl alcohol)-starch-cellulosic material based biodegradable composite film for packaging application. Mater Today Proc. 21:1577–1582. https://doi.org/10.1016/j.matpr.2019.11.210
Sennakesavan G, Mostakhdemin M, Dkhar LK et al (2020) Acrylic acid/acrylamide based hydrogels and its properties - A review. Polym Degrad Stab. https://doi.org/10.1016/j.polymdegradstab.2020.109308
Yilmaz SS, Yildirim N, Misir M et al (2020) Synthesis, characterization of a new polyacrylic acid superabsorbent, some heavy metal ion sorption, the adsorption isotherms, and quantum chemical investigation. Materials (Basel) 13:1–23. https://doi.org/10.3390/ma13194390
Kumar P, Ganure AL, Subudhi BB, Shukla S (2014) Synthesis and characterization of pH sensitive ampiphillic new copolymer of methyl methacrylate grafted on modified starch: influences of reaction variables on grafting parameters. Int. J. Pharm. Pharm. Sci. 6:868–880
Hussain T, Ansari M, Ranjha NM et al (2013) Chemically cross-Linked poly(acrylic- Co -Vinylsulfonic) acid hydrogel for the delivery of isosorbide mononitrate. Sci World J. https://doi.org/10.1155/2013/340737
Abo-Zahra SF, Abdelmonem IM, Siyam TE et al (2021) Radiation synthesis of polyacrylamide/functionalized multiwalled carbon nanotubes composites for the adsorption of Cu(II) metal ions from aqueous solution. Polym Bull. https://doi.org/10.1007/s00289-021-03726-6
Gizawy MA, Shamsel-Din HA, Abdelmonem IM et al (2020) Synthesis of chitosan-acrylic acid/multiwalled carbon nanotubes composite for theranostic 47Sc separation from neutron irradiated titanium target. Int. J. Biol. Macromol. 163:79–86. https://doi.org/10.1016/j.ijbiomac.2020.06.249
Abdelmonem IM, Metwally E, Siyam TE et al (2020) Adsorption of 60Co from aqueous solution onto alginate–acrylic acid–vinylsulfonic acid/multiwalled carbon nanotubes composite. Polym. Bull 77:4631–4653. https://doi.org/10.1007/s00289-019-02978-7
Abdelmonem IM, Metwally E, Siyam TE et al (2020) Gamma radiation-induced preparation of chitosan-acrylic acid-1-vinyl-2-vinylpyrrolidone/multiwalled carbon nanotubes composite for removal of 152+154Eu, 60Co and 134Cs radionuclides. Int. J. Biol. Macromol. 164:2258–2266. https://doi.org/10.1016/j.ijbiomac.2020.08.120
Sharaf El-Deen GE, Imam NG, Ayoub RR (2017) Preparation, characterization and application of superparamagnetic iron oxide nanoparticles modified with natural polymers for removal of 60Co-radionuclides from aqueous solution. Radiochim. Acta. 105:141–159. https://doi.org/10.1515/ract-2016-2595
Zou L, Liu Q, Rong J (2011) Removal of cesium from aqueous solutions using crosslinked amphoteric starch prepared by microwave treatment. Adv. Mater. Res. 221:95–98. https://doi.org/10.4028/www.scientific.net/AMR.221.95
Djordjevic S, Nikolic L, Kovacevic S et al (2013) Graft copolymerization of acrylic acid onto hydrolyzed potato starch using various initiators. Period Polytech Chem. Eng. 57:55–61. https://doi.org/10.3311/ppch.2171
Bhuyan MM, Dafader NC, Hara K et al (2016) Synthesis of potato starch-acrylic-acid hydrogels by gamma radiation and their application in dye adsorption. Int J Polym Sci. https://doi.org/10.1155/2016/9867859
Rosiak JM, Ulański P (1999) Synthesis of hydrogels by irradiation of polymers in aqueous solution. Radiat. Phys. Chem. 55:139–151. https://doi.org/10.1016/S0969-806X(98)00319-3
Said HM, Alla SGA, El-Naggar AWM (2004) Synthesis and characterization of novel gels based on carboxymethyl cellulose/acrylic acid prepared by electron beam irradiation. React. Funct. Polym. 61:397–404. https://doi.org/10.1016/j.reactfunctpolym.2004.07.002
Doba T, Rodehed C, Rånby B (1984) Mechanism of graft copolymerization onto polysaccharides initiated by metal ion oxidation reactions of model compounds for starch and cellulose. Macromolecules 17:2512–2519. https://doi.org/10.1021/ma00142a009
Witono JR, Noordergraaf IW, Heeres HJ, Janssen LPBM (2012) Graft copolymerization of acrylic acid to cassava starch - Evaluation of the influences of process parameters by an experimental design method. Carbohydr. Polym. 90:1522–1529. https://doi.org/10.1016/j.carbpol.2012.07.024
Rivas BL, Schiappacasse LN (2003) Poly(acrylic acid-co-vinylsulfonic acid): synthesis, characterization, and properties as polychelatogen. J. Appl. Polym. Sci. 88:1698–1704. https://doi.org/10.1002/app.11956
Oriakhi C, Farr IV, Lerner MM (1996) Within layered double hydroxides. J. Mater. Chem. 6:103–107
Casas M, Ferrero C, de Paz MV, Jiménez-Castellanos MR (2009) Synthesis and characterization of new copolymers of ethyl methacrylate grafted on tapioca starch as novel excipients for direct compression matrix tablets. Eur. Polym. J. 45:1765–1776. https://doi.org/10.1016/j.eurpolymj.2009.02.019
Soto D, Urdaneta J, Pernia K et al (2016) Itaconic acid grafted starch hydrogels as metal remover: capacity, selectivity and adsorption kinetics. J. Polym. Environ. 24:343–355. https://doi.org/10.1007/s10924-016-0780-9
El-Sweify FH, Abdel Fattah AA, El-Sheikh R et al (2018) Adsorption and separation of 152+154Eu(III) and 60Co(II) using Cerium(IV) tungstate. Radiochemistry 60:274–280. https://doi.org/10.1134/S1066362218030086
Mahmoud MR, Rashad GM, Elewa AM et al (2019) Optimization of adsorption parameters for removal of 152+154Eu(III) from aqueous solutions by using Zn-Cu-Ni ternary mixed oxide. J Mol Liq 291:111257. https://doi.org/10.1016/j.molliq.2019.111257
Jasper EE, Ajibola VO, Onwuka JC (2020) Nonlinear regression analysis of the sorption of crystal violet and methylene blue from aqueous solutions onto an agro-waste derived activated carbon. Appl. Water Sci. 10:1–11. https://doi.org/10.1007/s13201-020-01218-y
Gizawy MA, Aydia MI, Abdel Monem IM et al (2019) Radiochemical separation of reactor produced Sc-47 from natural calcium target using Poly(acrylamide-acrylic acid)/multi-walled carbon nanotubes composite. Appl. Radiat. Isot. 150:87–94. https://doi.org/10.1016/j.apradiso.2019.05.022
Şenol ZM, Şenol Arslan D, Şimşek S (2019) Preparation and characterization of a novel diatomite-based composite and investigation of its adsorption properties for uranyl ions. J. Radioanal. Nucl. Chem. 321:791–803. https://doi.org/10.1007/s10967-019-06662-y
Şenol ZM, Şimşek S, Ulusoy Hİ et al (2020) Insight from adsorption properties of Xylidyl Blue embedded hydrogel for effective removal of uranyl: experimental and theoretical approaches. Polym. Test 88:106566. https://doi.org/10.1016/j.polymertesting.2020.106566
Soliman MA, Rashad GM, Mahmoud MR (2019) Organo-modification of montmorillonite for enhancing the adsorption efficiency of cobalt radionuclides from aqueous solutions. Environ. Sci. Pollut. Res. 26:10398–10413. https://doi.org/10.1007/s11356-019-04478-7
Wołowicz A, Hubicki Z (2018) Comparison of ion-exchange resins for efficient cobalt(II) removal from acidic streams. Chem. Eng. Commun. 205:1207–1225. https://doi.org/10.1080/00986445.2018.1442332
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ghamry, M.A., Abdelmonem, I.M. Adsorption of 60Co and 154+152Eu Using Graft Copolymer of Starch-Polyacrylic Acid-Polyvinylsulfonic Acid. J Polym Environ 30, 3622–3632 (2022). https://doi.org/10.1007/s10924-022-02446-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-022-02446-w