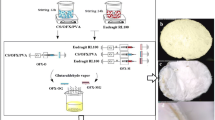
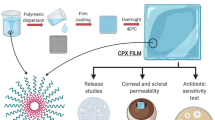
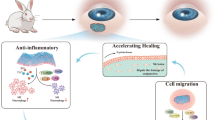
Abstract
Purpose
Dry eye can cause an increased risk of ocular infection due to loss of corneal epithelial integrity and changes to tear film homeostasis. Polymeric nanofibers with unique physicochemical and biological properties have attracted a lot of attention due to the improved in vivo performance of their conventional ocular formulations. The purpose of this present exposition was to assess the in vitro and in vivo efficacy of gatifloxacin (GAF)-loaded polyacrylonitrile (PAN) nanofiber in dry eye conditions.
Methods
The nanofibers were characterized for morphology, drug entrapment, swelling behavior, drug release, oxygen permeability, biodegradability, antimicrobial potential, and sterility. Draize test, in vivo antibacterial activity, corneal healing characteristics, and tear quality profiles were also used to determine in vivo safety and efficacy.
Results
Experimental findings suggested that electrospinning at defined conditions (applied voltage of 18 kV, flow rate of 0.5 mL/h, and tip to collector distance of 10 cm) produced smooth and continuous nanofibers with an average diameter of 480 nm. High drug entrapment of 95% combined with swelling indices of around 180% increased the controlled drug delivery capability of the prepared formulation. A slow degradation rate of 3±0.75% ensured a long-term cumulative drug release of 90% within 16 days. The inhibition diameter of the optimized formulation was found to be 15 ± 0.31 mm for E. coli and 13 ± 0.26 mm for S. aureus. Furthermore, after 3 days in an experimental dry eye animal model, the drug-loaded nanofiber showed better antibacterial activity, corneal healing, and tear film stability than the commercial eye drop.
Conclusion
Overall, the study concluded that the GAF-loaded PAN nanofiber has good application prospects in the treatment of dry eye due to its safety and controlled drug release profile.
Graphical Abstract











Similar content being viewed by others
References
Stapleton F, Alves M, Bunya VY, et al. Tfos dews ii epidemiology report. Ocul Surf. 2017;15(3):334–65.
Tsubota K, Yokoi N, Shimazaki J, et al. New perspectives on dry eye definition and diagnosis: a consensus report by the Asia Dry Eye Society. Ocul Surf. 2017;15(1):65–76.
Yu K, Bunya V, Maguire M, et al. Systemic conditions associated with severity of dry eye signs and symptoms in the dry eye assessment and management study. Ophthalmology. 2021.
Narayanan S, Redfern RL, Miller WL, et al. Dry eye disease and microbial keratitis: is there a connection? Ocul Surf. 2013;11(2):75–92.
Jegal U, Lee JH, Lee J, et al. Ultrasound-assisted gatifloxacin delivery in mouse cornea, in vivo. Sci Rep. 2019;9(1):1–11.
Agarwal P, Craig JP, Rupenthal ID. Formulation considerations for the management of dry eye disease. Pharmaceutics. 2021;13(2):207.
Al-Saedi Z, Zimmerman A, Devi Bachu R, et al. Dry eye disease: present challenges in the management and future trends. Curr Pharm Des. 2016;22(28):4470–90.
Phadatare SP, Momin M, Nighojkar P, et al. A comprehensive review on dry eye disease: diagnosis, medical management, recent developments, and future challenges. Adv Pharmaceut. 2015.
Bachu RD, Chowdhury P, Al-Saedi ZHF, et al. Ocular drug delivery barriers—role of nanocarriers in the treatment of anterior segment ocular diseases. Pharmaceutics. 2018;10(1):28.
Thakkar S, Misra M. Electrospun polymeric nanofibers: new horizons in drug delivery. Eur J Pharm Sci. 2017;107:148–67.
Feng X, Li J, Zhang X, et al. Electrospun polymer micro/nanofibers as pharmaceutical repositories for healthcare. J Control Rel. 2019.
Tawfik EA, Craig DQM, Barker SA. Dual drug-loaded coaxial nanofibers for the treatment of corneal abrasion. Int J Pharm. 2020;581:119296.
Singh H, Sharma R, Joshi M, et al. Transmucosal delivery of Docetaxel by mucoadhesive polymeric nanofibers. Artificial cells, nanomedicine, and biotechnology. 2015;43(4):263–9.
Garg T, Malik B, Rath G, et al. Development and characterization of nano-fiber patch for the treatment of glaucoma. Eur J Pharm Sci. 2014;53:10–6.
Omer S, Zelkó R. A systematic review of drug-loaded electrospun nanofiber-based ophthalmic inserts. Pharmaceutics. 2021;13(10):1637.
Naik JB, Pardeshi SR, Patil RP, et al. Mucoadhesive micro-/nano carriers in ophthalmic drug delivery: an overview. BioNanoScience. 2020;10(3):564–82.
Gupta SS, Mishra V, Mukherjee MD, et al. Amino acid derived biopolymers: recent advances and biomedical applications. Int J Biol Macromol. 2021;188:542–67.
Duxfield L, Sultana R, Wang R, et al. Development of gatifloxacin-loaded cationic polymeric nanoparticles for ocular drug delivery. Pharm Dev Technol. 2016;21(2):172–9.
Pang Y, Zong Z, Huo F, et al. In vitro drug susceptibility of bedaquiline, delamanid, linezolid, clofazimine, moxifloxacin, and gatifloxacin against extensively drug-resistant tuberculosis in Beijing. China Antimicrobial agents and chemotherapy. 2017;61(10):e00900-e17.
Pawar P, Katara R, Mishra S, et al. Topical ocular delivery of fluoroquinolones. Expert Opin Drug Deliv. 2013;10(5):691–711.
Maulvi FA, Parmar RJ, Desai AR, et al. Tailored gatifloxacin Pluronic® F-68-loaded contact lens: addressing the issue of transmittance and swelling. Int J Pharm. 2020;581:119279.
Ulubayram K, Calamak S, Shahbazi R, et al. Nanofibers based antibacterial drug design, delivery and applications. Curr Pharm Des. 2015;21(15):1930–43.
Kwatra D, Vadlapatla RK, Vadlapudi AD, et al. Interaction of gatifloxacin with efflux transporters: a possible mechanism for drug resistance. Int J Pharm. 2010;395(1–2):114–21.
Lagaron JM, Catalá R, Gavara R. Structural characteristics defining high barrier properties in polymeric materials. Mater Sci Technol. 2004;20(1):1–7.
Huang Z-M, Zhang YZ, Kotaki M, et al. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos Sci Technol. 2003;63(15):2223–53.
Semnani K, Shams-Ghahfarokhi M, Afrashi M, et al. Antifungal activity of eugenol loaded electrospun PAN nanofiber mats against Candida albicans. Curr Drug Deliv. 2018;15(6):860–6.
Sarwar MN, Ullah A, Haider M, et al. Evaluating antibacterial efficacy and biocompatibility of PAN nanofibers loaded with diclofenac sodium salt. Polymers. 2021;13(4):510.
Kharaghani D, Gitigard P, Ohtani H, et al. Design and characterization of dual drug delivery based on in-situ assembled PVA/PAN core-shell nanofibers for wound dressing application. Sci Rep. 2019;9(1):1–11.
Sirelkhatim N, Parveen A, LaJeunesse D, et al. Polyacrylonitrile nanofibrous mat from electrospinning: born with potential anti-fungal functionality. Eur Polymer J. 2019;119:176–80.
Rianjanu A, Roto R, Julian T, et al. Polyacrylonitrile nanofiber-based quartz crystal microbalance for sensitive detection of safrole. Sensors. 2018;18(4):1150.
Khan Z, Kafiah F, Shafi HZ, et al. Morphology, mechanical properties and surface characteristics of electrospun polyacrylonitrile (PAN) nanofiber mats. IJAENT. 2015;2(3):15–22.
Allwood MC. Factors influencing the stability of ascorbic acid in total parenteral nutrition infusions. J Clin Pharm Ther. 1984;9(2):75–85.
Taghe S, Mehrandish S, Mirzaeei S. Preparation of azithromycin nanofibers as controlled release ophthalmic drug carriers using electrospinning technique: in-vitro and in-vivo characterization. Adv Pharmaceut Bull. 2021.
Chen Y, et al. Sterility test method for gatifloxacin eye drops. Tianjin Pharmacy. 2009年04期.
Cullis CF, Waddington DJ. The colorimetric determination of secondary amines. Anal Chim Acta. 1956;15:158–63.
Cho S, Kim Y. Donor–acceptor Stenhouse adduct formation for the simple and rapid colorimetric detection of amphetamine-type stimulants. Sens Actuators, B Chem. 2022;355:131274.
Vaz VM, Jitta SR, Verma R, et al. Hesperetin loaded proposomal gel for topical antioxidant activity. Journal of Drug Delivery Science and Technology. 2021;66:102873.
Fernandes AV, Pydi CR, Verma R, et al. Design, preparation and in vitro characterizations of fluconazole loaded nanostructured lipid carriers. Brazilian J Pharmaceut Sci. 2020;56.
Li X, Wang C, Yang S, et al. Electrospun PCL/mupirocin and chitosan/lidocaine hydrochloride multifunctional double layer nanofibrous scaffolds for wound dressing applications. Int J Nanomed. 2018;13:5287.
Luechtefeld T, Maertens A, Russo DP, et al. Analysis of Draize eye irritation testing and its prediction by mining publicly available 2008–2014 REACH data. Altex. 2016;33(2):123.
Szermer-Olearnik B, Sochocka M, Zwolinska K, et al. Comparison of microbiological and physicochemical methods for enumeration of microorganisms. Postepy Hig Med Dosw. 2014;68:1392–6.
Thacker M, Tseng C-L, Chang C-Y, et al. Mucoadhesive Bletilla striata polysaccharide-based artificial tears to relieve symptoms and inflammation in rabbit with dry eyes syndrome. Polymers. 2020;12(7):1465.
Tseng Y-Y, Kao C-W, Liu K-S, et al. Treating intracranial abscesses in rats with stereotactic injection of biodegradable vancomycin-embedded microparticles. Pharmaceutics. 2020;12(2):91.
Shen X, Yu D, Zhu L, et al. Electrospun diclofenac sodium loaded Eudragit® L 100–55 nanofibers for colon-targeted drug delivery. Int J Pharm. 2011;408(1–2):200–7.
Arthanari S, Mani G, Jang JH, et al. Preparation and characterization of gatifloxacin-loaded alginate/poly (vinyl alcohol) electrospun nanofibers. Artificial cells, nanomedicine, and biotechnology. 2016;44(3):847–52.
Suteris NN, Yasin A, Misnon II, et al. Curcumin loaded waste biomass resourced cellulosic nanofiber cloth as a potential scaffold for regenerative medicine: an in-vitro assessment. Int J Biol Macromol. 2022;198:147–56.
Prabu D, Majdalawieh AF, Abu-Yousef IA, et al. Preparation and characterization of gatifloxacin-loaded sodium alginate hydrogel membranes supplemented with hydroxypropyl methylcellulose and hydroxypropyl cellulose polymers for wound dressing. International Journal of Pharmaceutical Investigation. 2016;6(2):86.
Rohde F, Walther M, Wächter J, et al. In-situ tear fluid dissolving nanofibers enable prolonged viscosity-enhanced dual drug delivery to the eye. Int J Pharmaceut. 2022:121513.
Adegbola TA, Agboola O, Fayomi OSI. Review of polyacrylonitrile blends and application in manufacturing technology: recycling and environmental impact. Results in Engineering. 2020;7:100144.
Yang H, Gao PF, Wu WB, et al. Antibacterials loaded electrospun composite nanofibers: release profile and sustained antibacterial efficacy. Polym Chem. 2014;5(6):1965–75.
Lala NL, Ramaseshan R, Bojun L, et al. Fabrication of nanofibers with antimicrobial functionality used as filters: protection against bacterial contaminants. Biotechnol Bioeng. 2007;97(6):1357–65.
Lantyer-Araujo NL, Lacerda ADJ, Mendonça MA, et al. Rabbit as an animal model for ocular surface disease, tear osmolarity, electrolyte, and tear ferning profiles. Optom Vis Sci. 2020;97(10):847–51.
Acknowledgements
The authors are thankful to the SOA Institute and IMS-SUM Hospital for providing the necessary facilities and encouragement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Statement
The Institutional Animal Ethical Committee (IAEC/ SPS/SOA/26/2020) SPS, SOA University authorized all animal-related experimental protocols, which were carried out in compliance with CPCSEA guidelines for the care and use of laboratory animals. All efforts were made to use the smallest number of animals possible in order to obtain valid scientific data.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sahu, D.K., Pradhan, D., Halder, J. et al. Preparation and Characterization of Gatifloxacin-Loaded Polyacrylonitrile Nanofiber for the Management of Dry Eye Infection. J Pharm Innov 18, 391–403 (2023). https://doi.org/10.1007/s12247-022-09650-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-022-09650-0