Abstract
Introduction
Preparation of self-microemulsifying drug delivery systems (SMEDDS) is a promising delivery approach for drugs with poor aqueous solubility to enhance their dissolution properties and hence oral bioavailability. The objective of the present study was to define the influence of the oily phase surface properties of meloxicam self-microemulsifying drug delivery systems on the physicochemical properties of the formulation.
Methods
Minitab software was applied for the definition of a response surface experimental design. The shake flask method was used to determine the solubility of Meloxicam in formulation ingredients and prepared formulations. Refractive index, transmittance percentage, particle size and zeta potential, and dissolution behaviour of each formulation were determined.
Results
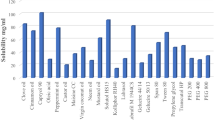
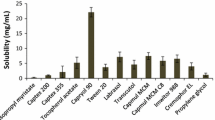
Meloxicam reveals higher solubility in Capryol PGMC (1.347 mg/ml) and PEG 400 (28.63 mg/ml) respectively. Surface tensions of pure Miglyol 812, Capryol PGMC, Labrasol, PEG 400, PG, and Transcutal P were 36.8, 36, 40.44, 40.22, 41, and 37.34 dyne/cm, and the interfacial tensions of Capryol PGMC and Miglyol 812 against the water were 7 and 12 dyne/cm respectively. In the majority of formulations, the solubility of meloxicam was higher in Capryol-containing formulations compared to Miglyol-containing ones. The lower surface tension of formulations associated with higher solubility of meloxicam, better dissolution, and lower emulsification times. Capryol-Labrasol-PEG 400 containing formulations show higher transmitted percentages. The size of droplets has been increased with an increase in the surface tension of formulations.
Conclusion
Lower surface tensions of SMEDDS excipients, especially oil phases, are associated with better physicochemical characteristics, i.e. higher solubilizing potential, emulsification ability, droplet size, transparency, refractive index, and dissolution behaviour.




Similar content being viewed by others
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Hecq J, Deleers M, Fanara D, Vranckx H, Amighi K. Preparation and characterization of nanocrystals for solubility and dissolution rate enhancement of nifedipine. Int J Pharm. 2005;299:167–77. https://doi.org/10.1016/j.ijpharm.2005.05.014.
Kazi M, Alhajri A, Alshehri SM, Elzayat EM, Al Meanazel OT, Shakeel F, et al. Enhancing oral bioavailability of apigenin using a bioactive self-nanoemulsifying drug delivery system (Bio-SNEDDS): in vitro, in vivo and stability evaluations. Pharmaceutics 2020; 12. https://doi.org/10.3390/pharmaceutics12080749.
Chaudhary A, Nagaich U, Gulati N, Sharma V, Khosa RL, editors. Enhancement of solubilization and bioavailability of poorly soluble drugs by physical and chemical modifications : A recent review 2012.
Kazi M, Shahba AA, Alrashoud S, Alwadei M, Sherif AY, Alanazi FK. Bioactive self-nanoemulsifying drug delivery systems (Bio-SNEDDS) for combined oral delivery of curcumin and piperine. Molecules 2020;25:1703.
Agrawal S, Giri TK, Tripathi DK, Ajazuddin, Alexander A. A review on novel therapeutic strategies for the enhancement of solubility for hydrophobic drugs through lipid and surfactant based self micro emulsifying drug delivery system: a novel approach. Am J Drug Disc Develop 2012;2: 143–83. https://doi.org/10.3923/ajdd.2012.143.183.
Alghananim A, Özalp Y, Mesut B, Serakinci N, Özsoy Y, Güngör S. A solid ultra fine self-nanoemulsifying drug delivery system (S-SNEDDS) of deferasirox for improved solubility: optimization, characterization, and in vitro cytotoxicity studies. Pharmaceuticals (Basel) 2020;13. https://doi.org/10.3390/ph13080162.
Cerpnjak K, Zvonar A, Gašperlin M, Vrečer F. Lipid-based systems as a promising approach for enhancing the bioavailability of poorly water-soluble drugs. Acta Pharm. 2013;63:427–45. https://doi.org/10.2478/acph-2013-0040.
Bannow J, Yorulmaz Y, Löbmann K, Müllertz A, Rades T. Improving the drug load and in vitro performance of supersaturated self-nanoemulsifying drug delivery systems (super-SNEDDS) using polymeric precipitation inhibitors. Int J Pharm. 2020;575: 118960. https://doi.org/10.1016/j.ijpharm.2019.118960.
Gershanik T, Benita S. Self-dispersing lipid formulations for improving oral absorption of lipophilic drugs. Eur J Pharm Biopharm. 2000;50:179–88. https://doi.org/10.1016/s0939-6411(00)00089-8.
Pouton CW. Lipid formulations for oral administration of drugs: non-emulsifying, self-emulsifying and “self-microemulsifying” drug delivery systems. Eur J Pharm Sci. 2000;11(Suppl 2):S93–8. https://doi.org/10.1016/s0928-0987(00)00167-6.
Kobayashi I, Mukataka S, Nakajima M. Effects of type and physical properties of oil phase on oil-in-water emulsion droplet formation in straight-through microchannel emulsification, experimental and CFD studies. Langmuir. 2005;21:5722–30. https://doi.org/10.1021/la050039n.
Turck D, Roth W, Busch U. A review of the clinical pharmacokinetics of meloxicam. Br J Rheumatol. 1996;35(Suppl 1):13–6. https://doi.org/10.1093/rheumatology/35.suppl_1.13.
Wisher D. Martindale: The complete drug reference. 37th ed. Journal of the Medical Library Association : JMLA 2012;100: 75–6. https://doi.org/10.3163/1536-5050.100.1.018.
Noble S, Balfour JA. Meloxicam. Drugs 1996;51: 424–30; discussion 31–32. https://doi.org/10.2165/00003495-199651030-00007.
Turck D, Busch U, Heinzel G, Narjes H. Clinical pharmacokinetics of meloxicam. Arzneimittelforschung 1997;47:253–8.
Gates BJ, Nguyen TT, Setter SM, Davies NM. Meloxicam: a reappraisal of pharmacokinetics, efficacy and safety. Expert Opin Pharmacother. 2005;6:2117–40. https://doi.org/10.1517/14656566.6.12.2117.
Pawlukianiec C, Gryciuk ME, Mil KM, Żendzian-Piotrowska M, Zalewska A, Maciejczyk M. A new insight into meloxicam: assessment of antioxidant and anti-glycating activity in in vitro studies. Pharmaceuticals 2020;13:240.
Hassan EM. Spectrophotometric and fluorimetric methods for the determination of meloxicam in dosage forms. J Pharm Biomed Anal. 2002;27:771–7. https://doi.org/10.1016/s0731-7085(01)00530-1.
Akula S, Gurram AK, Devireddy SR. Self-microemulsifying drug delivery systems: an attractive strategy for enhanced therapeutic profile. International Scholarly Research Notices. 2014;2014: 964051. https://doi.org/10.1155/2014/964051.
Balakrishnan P, Lee BJ, Oh DH, Kim JO, Lee YI, Kim DD, et al. Enhanced oral bioavailability of Coenzyme Q10 by self-emulsifying drug delivery systems. Int J Pharm. 2009;374:66–72. https://doi.org/10.1016/j.ijpharm.2009.03.008.
Mohamed AIA, Sultan AS, Hussein IA, Al-Muntasheri GA. Influence of surfactant structure on the stability of water-in-oil emulsions under high-temperature high-salinity conditions. J Chem. 2017;2017:5471376. https://doi.org/10.1155/2017/5471376.
Gurram AK, Deshpande PB, Kar SS, Nayak UY, Udupa N, Reddy MS. Role of components in the formation of self-microemulsifying drug delivery systems. Indian J Pharm Sci. 2015;77:249–57. https://doi.org/10.4103/0250-474x.159596.
Rahman MA, Hussain A, Hussain MS, Mirza MA, Iqbal Z. Role of excipients in successful development of self-emulsifying/microemulsifying drug delivery system (SEDDS/SMEDDS). Drug Dev Ind Pharm. 2013;39:1–19. https://doi.org/10.3109/03639045.2012.660949.
Wooster TJ, Golding M, Sanguansri P. Impact of oil type on nanoemulsion formation and Ostwald ripening stability. Langmuir. 2008;24:12758–65. https://doi.org/10.1021/la801685v.
Silva HD, Cerqueira MA, Vicente AA. Influence of surfactant and processing conditions in the stability of oil-in-water nanoemulsions. J Food Eng. 2015;167:89–98. https://doi.org/10.1016/j.jfoodeng.2015.07.037.
Limbani MD, Patel L. Studies on drug solubilization and role of lipid vehicle in pseudo ternary phase diagram in formulation development of SNEDDS containing poorly water soluble drug. Int J Pharm Sci Rev Res 2016;40: 228–37.
Alshamsan A, Kazi M, Badran MM, Alanazi FK. Role of alternative lipid excipients in the design of self-nanoemulsifying formulations for fenofibrate: characterization, in vitro dispersion, digestion and ex vivo gut permeation studies. Front Pharmacol. 2018;9:1219. https://doi.org/10.3389/fphar.2018.01219.
Parmar N, Singla N, Amin S, Kohli K. Study of cosurfactant effect on nanoemulsifying area and development of lercanidipine loaded (SNEDDS) self nanoemulsifying drug delivery system. Colloids Surf B Biointerfaces. 2011;86:327–38. https://doi.org/10.1016/j.colsurfb.2011.04.016.
Acknowledgements
Research reported in this publication was supported by Elite Researcher Grant Committee under award number [958770] from the National Institute for Medical Research Development (NIMAD), Tehran, Iran. This article is written as part of a Pharm D dissertation (No. 3744) registered at Faculty of Pharmacy, Tabriz University of Medical Sciences, Iran. This article is written as part of a PharmD dissertation (No. 3744) registered at Faculty of Pharmacy, Tabriz University of Medical Sciences, Iran.
Funding
Research reported in this publication was supported by Elite Researcher Grant Committee under award number [958770] from the National Institute for Medical Research Development (NIMAD), Tehran, Iran. This article is written as part of a Pharm D dissertation (No. 3744) registered at Faculty of Pharmacy, Tabriz University of Medical Sciences, Iran.
Author information
Authors and Affiliations
Contributions
Hadi Valizadeh and Parvin Zakeri-Milani conceived the original idea and designed the project. Keyhan Eskandarinia performed the experiments and collected the data. Ziba Islambulchilar presented the data and drafted the manuscript. Parvin Zakeri-Milani supervised and administered the project.
Corresponding author
Ethics declarations
Ethical Statement
None to be declared.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Valizadeh, H., Islambulchilar, Z., Eskandarinia, K. et al. Influence of Oil Phase Surface Properties on the Physicochemical Characteristics of Meloxicam Self-microemulsifying Drug Delivery Systems. J Pharm Innov 18, 381–390 (2023). https://doi.org/10.1007/s12247-021-09571-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-021-09571-4