Abstract
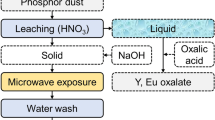
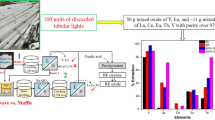
The current study examines the extraction of rare-earth elements from trichromatic phosphor of discarded tube lights via baking processes. The Y2O3:Eu3+ (YOX) and BaMgAl10O17:Eu2+ (BAM) phases completely decomposed during acid and alkali baking with > 90% Eu and Y extraction. Prior, selective acid leaching of the YOX phase enhanced the acid baking process at a lower temperature (< 230 ℃). Feed acid baking showed a maximum 87% Tb, 36% La, and 17% Ce extraction at 500 °C, 0.5 h, 1 ml/g H2SO4, and alkali baking yielded 16.1% Tb, 86% La, and 73% Ce at 300 ℃, 0.5 h, 50 wt.% NaOH. The acid baking of the acid-leached phosphor yielded 72% Tb, 95% La, 63% Ce, ~ 95% Y, and Eu extraction at 300 °C, 0.5 h, 1.05 mL/g H2SO4. Lower Tb extraction during the acid baking of acid-leached phosphor can be attributed to the formation of Ba3Tb(PO4)3 and BaTbO3 phases. Insoluble Si3Tb5 phase formation and partial decomposition of CeMgAl11O19:Tb3+ (CMAT) phase in the alkali baking route leads to lower Tb extraction. The acid baking process is suitable for extracting Tb, Eu, and Y, whereas the alkali baking process is suitable for La and Ce. The thermal analysis of phosphor and flux interaction depicts that alkali baking is less energy-intensive than acid baking. In comparison, the one-step acid baking process and the two-step alkali baking process yielded desirable extraction results (87% Tb, total RE (rare earth) 78.5% and 50.4% Tb, total RE 59.3%). The product yield in the acid and alkali baking route of feed, acid-leach phosphor is 36%, 29%, and 39%, 28.2%, respectively, with ~ 98% mixed rare-earth oxide purity.












Similar content being viewed by others
References
Anand A, Singh R (2021) Synthesis of Rare Earth Compounds from Phosphor Coating of Spent Fluorescent Lamps. Sep Purif Review 50(1):96–112
Innocenzi V (2018) Treatment of spent fluorescent lamps, cathode-ray tubes, and spent catalysts by hydrometallurgical procedures, WEEE 139–160. Woodhead Publishing
Zhang J, Zhang Z, Tang Z, Lin Y (2001) Mn2+ luminescence in (Ce, Tb) MgAl11O19 phosphor. Mater Chem Phys 72(1):81–84
Binnemans K, Jones P (2014) Perspectives for the recovery of rare earths from end-of-life fluorescent lamps. J Rare Earths 32(3):195–200
Belardi G, Ippolito N, Piga L, Serracino M (2014) Investigation on the status of rare earth elements contained in the powder of spent fluorescent lamps. Thermochim Acta 591:22–30
Dhawan N, Tanvar H (2022) A critical review of end-of-life fluorescent lamps recycling for recovery of rare earth values. Sustain Mater Technol 32:e00401. https://doi.org/10.1016/j.susmat.2022.e00401
Hirajima T, Sasaki K, Bissombolo A, Hirai H, Hamada M, Tsunekawa M (2005) Feasibility of an efficient recovery of rare earth-activated phosphors from waste fluorescent lamps through dense-medium centrifugation. Sep Purif Technol 44(3):197–204
Mei G, Rao P, Matsuda M, Fujita T (2009) Separation of red (Y2O3: Eu3+), blue (BaMgAl10O17: Eu2+) and green (CeMgAl10O17: Tb3+) rare earth phosphors by liquid/liquid extraction. J Wuhan Univ Technol Mater Sci Ed. 24(4): 603–607
Otsuki A, Dodbiba G, Shibayama A, Sadaki J, Mei G, Fujita T (2008) Separation of rare earth fluorescent powders by two-liquid flotation using organic solvents. Jpn J Appl Phys 47:5093
Mei G, Rao P, Mitsuaki M, Toyohisa F (2009) Separation of red (Y2O3: Eu3+), blue (Sr, Ca, Ba)10(PO4)6Cl2: Eu2+ and green (LaPO4: Tb3+, Ce3+) rare earth phosphors by liquid/liquid extraction. J Wuhan Univ Technol Mater Sci Ed. 24(3):418–423.
Yamashita M, Akai T, Murakami M, Oki T (2018) Recovery of LaPO4: Ce3+, Tb3+ from waste phosphors using high-gradient magnetic separation. Waste Manage 79:164
Eduafo P, Mishra B (2018) Leaching kinetics of yttrium and europium oxides from waste phosphor powder. J Sustain Metall 4(4):437–442
Tunsu C, Lapp J, Ekberg C, Retegan T (2016) Selective separation of yttrium and europium using Cyanex 572 for applications in fluorescent lamp waste processing. Hydrometallurgy 166:98–106
Tan Q, Deng C, Li J (2017) Enhanced recovery of rare earth elements from waste phosphors by mechanical activation. J Clean Prod 142:2187–2191
Van Loy S, Binnemans K, Van Gerven T (2017) Recycling of rare earths from lamp phosphor waste: Enhanced dissolution of LaPO4:Ce3+, Tb3+ by mechanical activation. J Clean Prod 156:226–234
United States Geological Survey Website (USGS) (2020) Commodity Statistics and Information. Available http://minerals.usgs.gov/minerals/pubs/commodity/ [Accessed 10 Feb 2021]
Binnemans K, Jones P, Blanpain B, Van Gerven T, Yang Y, Walton A, Buchert M (2013) Recycling of rare earths: a critical review. J Clean Prod 51:1–22
Tunsu C, Ekberg C, Retegan RT (2014) Characterization and leaching of real fluorescent lamp waste for the recovery of rare earth metals and mercury. Hydrometallurgy 144–145:91–98
De Carolis R, Fontana D, Pietrantonio M, Pucciarmati S, Torelli G (2015) A Hydrometallurgical Process for Recovering Rare Earths and Metals from Spent Fluorescent Lamps. Environ Eng Manag J 14(7):1603–1609
Innocenzi V, Ippolito N, De Michelis I, Medici F, Vegliò F (2016) A hydrometallurgical process for the recovery of terbium from fluorescent lamps: experimental design, optimization of acid leaching process and process analysis. J Environ Manage 184:552–559
Miskufova A, Kochmanova A, Havlik T, Horvathova H, Kuruc P (2018) Leaching of yttrium, europium and accompanying elements from phosphor coatings. Hydrometallurgy 176:216–228
Burgess WA, Keller MJ, Lekse JW, Howard BH, Roth EA, Granite EJ (2018) Effect of pre-reaction ball milling on kinetics of lanthanum phosphate roasting with sodium carbonate. Ind Eng Chem Res 57(18):6088–6096
Song G, Yuan W, Zhu X, Wang X, Zhang C, Li J, Bai J, Wang J (2017) Improvement in rare earth element recovery from waste trichromatic phosphors by mechanical activation. J Clean Prod 151:361–370
Liang Y, Liu Y, Lin R, Guo D, Liao C (2016) Leaching of rare earth elements from waste lamp phosphor mixtures by reduced alkali fusion followed by acid leaching. Hydrometallurgy 163:99–103
Zhang S, Liu H, Pan D, Tian J, Liu Y, Volinsky A (2015) Complete recovery of Eu from BaMgAl10 O17: Eu2+ by alkaline fusion and its mechanism. RSC Adv 5(2):1113–2111
Liu H, Zhang S, Pan D, Liu Y, Liu B, Tian J, Volinsky A (2015) Mechanism of CeMgAl11O19: Tb3+ alkaline fusion with sodium hydroxide. Rare Met 34(3):189–194
Ippolito N, Innocenzi V, De Michelis I, Medici F, Vegliò F (2017) Rare earth elements recovery from fluorescent lamps: A new thermal pretreatment to improve the efficiency of the hydrometallurgical process. J Clean Prod 153:287–298
Ippolito N, Ferella F, Innocenzi V, Trapasso F, Passeri D, Belardi G, Vegliò F (2021) Effect of mechanical activation on terbium dissolution from waste fluorescent powders. Miner Eng 167:106906
Braconnier J, Rollat A (2010) Process for recovery of rare earths starting from a solid mixture containing a halophosphate and a compound of one or more rare earths. Patent WO2010118967A1 (Rhodia Operations, France)
Liu H, Li S, Wang B, Wang K, Wu R, Ekberg C, Volinsky A (2019) Multiscale recycling rare earth elements from real waste trichromatic phosphors containing glass. J Clean Prod 238:117998
Yurramendi L, Gijsemans L, Forte F, Aldana J, del Río C, Binnemans K (2019) Enhancing rare-earth recovery from lamp phosphor waste. Hydrometallurgy 187:38–44
Yu M, Mei G, Chen X (2018) Recovering rare earths and aluminum from waste BaMgAl10O17: Eu2+ and CeMgAl11O19: Tb3+ phosphors using NaOH sub-molten salt method. Miner Eng 117:1–7
Liu H, Zhang S, Pan D, Tian J, Yang M, Wu M, Volinsky AA (2014) Rare earth elements recycling from waste phosphor by dual hydrochloric acid dissolution. J Hazard Mater 272:96–101
Innocenzi V, De Michelis I, Ferella F, Vegliò F (2017) Secondary yttrium from spent fluorescent lamps: recovery by leaching and solvent extraction. Int J Miner Process 168:87–94
Innocenzi V, Ippolito N, Pietrelli L, Centofanti M, Piga L, Vegliò F (2018) Application of solvent extraction operation to recover rare earths from fluorescent lamps. J Clean Prod 172:2840–2852
da Siva L, Nascimento M, de Oliveira E, de Queiroz A, Fernandes M, de Castro J (2020) Evaluation of the diffusional coefficient in the acid baking process using microwave energy to reduce phosphorus content in iron ore particles. Min Eng 157:106541
Kul M, Topkaya Y, Karakaya I (2008) Rare earth double sulfates from pre-concentrated bastnasite. Hydrometallurgy 93(3–4):129–135
Teixeira L, Silva R, Majuste D, Ciminelli V (2020) Stability of lanthanum in sulfate and phosphate systems and implications for selective rare earths extraction. Min Eng 155:106440
Demol J, Ho E, Senanayake G (2018) Sulfuric acid baking and leaching of rare earth elements, thorium and phosphate from a monazite concentrate: Effect of bake temperature from 200 to 800 °C. Hydrometallurgy 179:254–267
Zou D, Li H, Deng Y, Chen J, Bai Y (2021) Recovery of lanthanum and cerium from rare earth polishing powder wastes utilizing acid baking-water leaching-precipitation process. Sep Purific Technol 261:118244
Shukla N, Dhawan N (2020) Investigation of Different Processing Routes for Rare Earth Extraction from Discarded Tubular Lights. J Sustain Metall 6(2):269–280
Shukla N, Dhawan N (2020) Rapid microwave processing of discarded tubular lights for extraction of rare earth values. Pro Saf Environ Protect 142:238–249
Shukla N, Tanvar H, Dhawan N (2020) Evaluation of alkali processing for the recycling of rare earth values from spent fluorescent lamps. Physicochem Probl Miner Process 56(4):710–722
Shukla N, Agrawal S, Dhawan N (2021) Microwave acid baking process for recovery of rare-earth concentrate from phosphor of end-of-life fluorescent lamps. J Clean Prod 307:127235
Shukla N, Dhawan N (2021) Processing End-of-Life Tube Lights for Recovery of Rare Earth Oxides. JOM 73(4):1090–1102
Borra C, Mermans J, Blanpain B, Pontikes Y, Binnemans K, Van Gerven T (2016) Selective recovery of rare earths from bauxite residue by combination of sulfation, roasting and leaching. Min Eng 92:151–159
Liu Y, Zhang S, Liu H, Liu B, Volinsky AA, Chang C (2015) Free oxoanion theory for BaMgAl10O17: Eu2+ structure decomposition during alkaline fusion process. RSC Adv 5(62):50105–50112
Kumar U, Gaikwad V, Sahajwalla V (2018) Transformation of waste toner to iron using E-waste plastics as a carbon resource. J Clean Prod 192:244–251
Trinh H, Lee J, Kim S, Kim J (2020) Recovery of Cerium from Spent Autocatalyst by Sulfatizing–Leaching–Precipitation Process. ACS Sust Chem Eng 8(41):15630–15639
Paulenova A, Creager S, Navratil J, Wei Y (2002) Redox potentials and kinetics of the Ce3+/Ce4+ redox reaction and solubility of cerium sulfates in sulfuric acid solutions. J Power Sources 109:431–438
Pelczarska A, Radomińska E, Znamierowska T (2020) Eulytite-type solid solution in the LaPO4–Ba3(PO4)2 system. J Alloys Compd 822:153550
Kunzler J, Giauque W (1952) Aqueous Sulfuric Acid. Heat Capacity. Partial Specific Heat Content of Water at 25 and-20. J Am Chem Soc 74(14):3472–3476
Thiyagarajan T, Sreekumar K, Selvan V, Ramachandran K, Ananthapadmanabhan P (2010) Simulation studies to optimize the process of plasma spray deposition of yttrium oxide. In J Phys Conf Ser 208(1):012116
Acknowledgements
The authors acknowledge the funding received from the Indian Insitute of Technology, Roorkee (Faculty Initiation Grant; FIG-100714), and Shrey Agrawal for valuable discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shukla, N., Dhawan, N. Evaluation of Baking Process for Rare-Earth Recovery from Discarded Tube Lights Phosphor. Mining, Metallurgy & Exploration 39, 1571–1586 (2022). https://doi.org/10.1007/s42461-022-00598-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42461-022-00598-w